Modelling and Determination of Parameters Influencing the Transfer of Microorganisms from Food Contact Materials
Abstract
:1. Introduction
Aim of the Study
2. Materials and Methods
2.1. Materials Studied
2.2. Determination of the Total Number of Colony-Forming Units per 100 Square Centimeters (CFU/100 cm2) for Each Sample
2.3. Transfer Evaluation of Microorganisms to Agar Plates under Increasing Incubation Time, Increasing Applied Weight and from Outer and Inner Layers from Tested Samples
2.4. Data Analysis
3. Results
3.1. Subsection
3.1.1. Characteristics of Tested Samples
3.1.2. Transfer Analysis Comparing Front and Back of Tested Samples
3.1.3. Transfer Analysis with Different Applied Weights
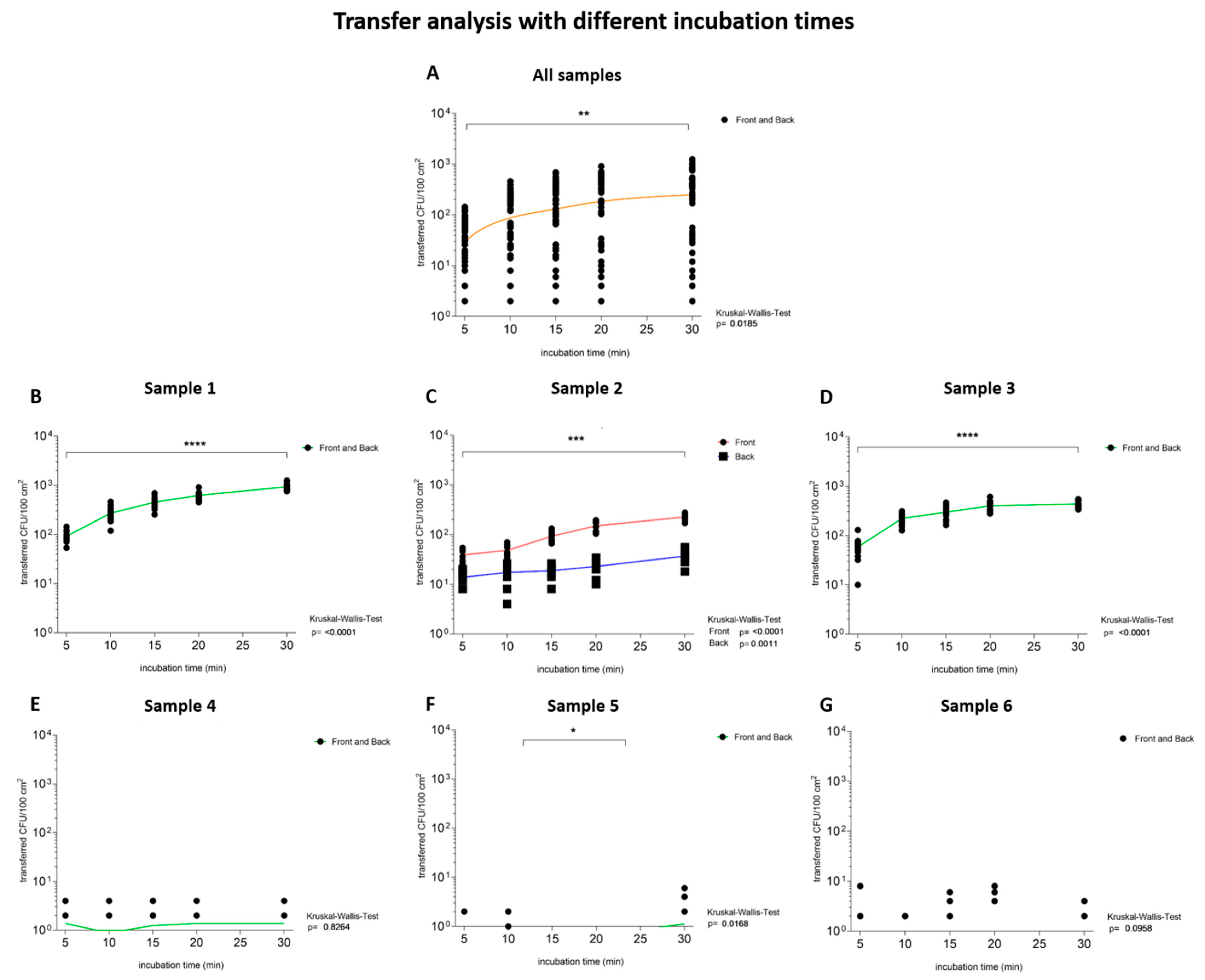
3.1.4. Transfer Analysis with Increasing Incubation Time
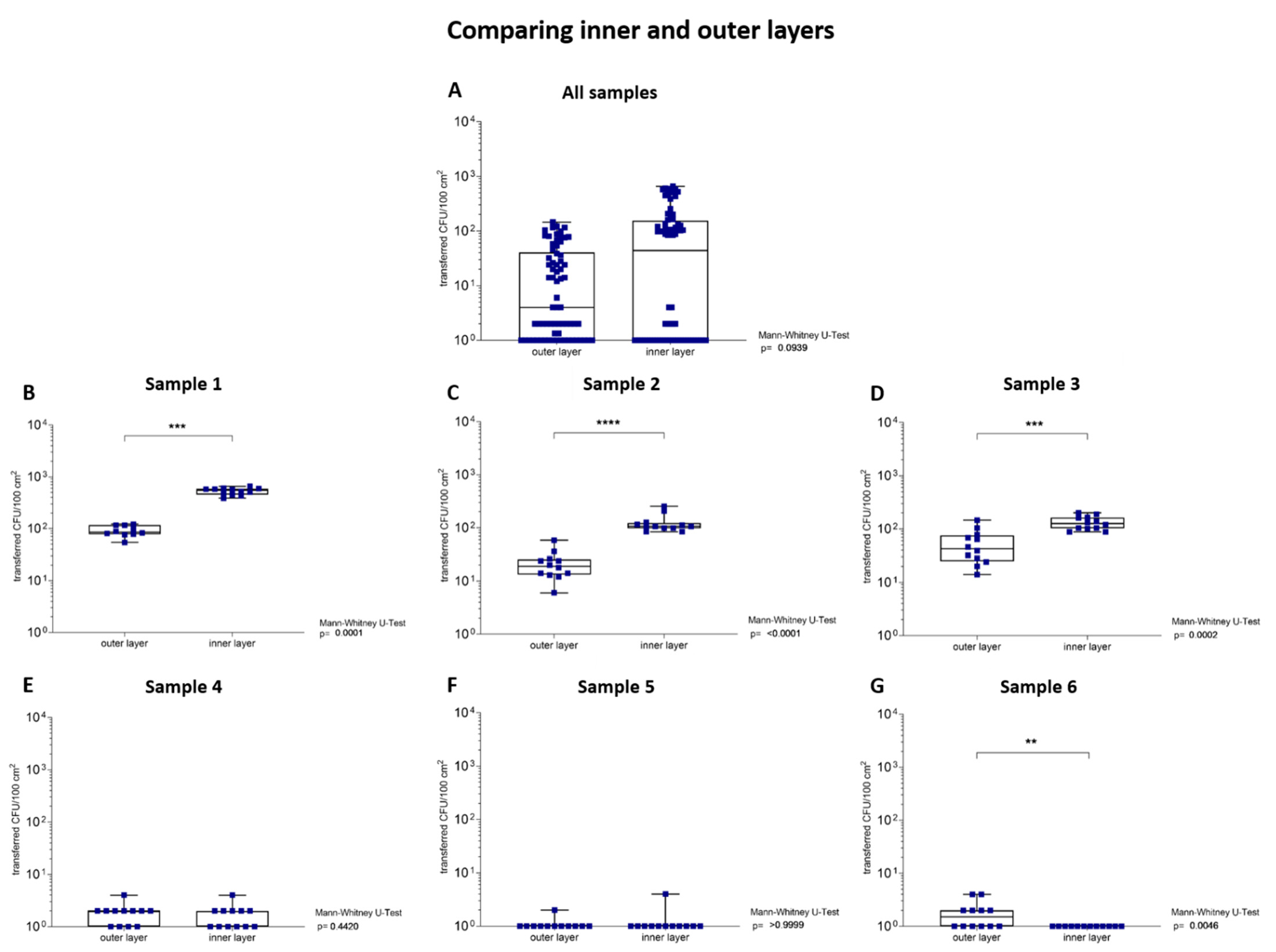
3.1.5. Transfer Analysis of Inner and Outer Layers of the Samples
3.1.6. Transfer Analysis Considering Transfer Ratios and the Total Number of CFU/100 cm2 of Tested Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klaiman, K.; Ortega, D.L.; Garnache, C. Consumer preferences and demand for packaging material and recyclability. Resour. Conserv. Recycl. 2016, 115, 1–8. [Google Scholar] [CrossRef]
- Simoneau, V.R.C.; Raffael, B.; Garbin, S.; Hoekstra, E.; Mieth, A.; Lopes, J.A. Non-harmonised food contact materials in the EU: Regulatory and market situation. JRC Sci. Policy Rep. EUR 2016, 28357. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues: Scientific status summary. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Regulation (EC) No 1935/2004 of the European Parliament and the Council. Off. J. Eur. Union2004, 338/4–338/17. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R1935&from=de (accessed on 27 October 2004).
- Suihko, M.L.; Sinkko, H.; Partanen, L.; Mattila-Sandholm, M.; Salkinoja-Salonen, M.; Raaska, L. Description of heterotrophic occurring in paper mills and paper products. J. Appl. Microbiol. 2004, 96, 1228–1235. [Google Scholar] [CrossRef]
- Pirttijärvi, T.S.M.; Graeffe, T.H.; Salkinoja-Salonen, M.S. Bacterial contaminants in liquid packaging boards: Assessment of potential for food spoilage. J. Appl. Bacteriol. 1996, 81, 445–458. [Google Scholar] [CrossRef]
- Väisänen, O.M.; Weber, A.; Bennasar, A.; Rainey, F.A.; Busse, H.J.; Salkinoja-Salonen, M.S. Microbial communities of printing paper machines. J. Appl. Microbiol. 1998, 84, 1069–1084. [Google Scholar] [CrossRef] [Green Version]
- Hladíková, Z.; Kejlova, K.; Sosnovcova, J.; Jirova, D.; Vavrouš, A.; Janoušek, A.; Syčová, M.; Špelina, V. Microbial contamination of paper-based food contact materials with different contents of recycled fiber. Czech J. Food Sci. 2015, 33, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Välsänen, O.M.; Mentu, J.; Salkinoja-Salonen, M.S. Bacteria in food packaging paper and board. J. Appl. Bacteriol. 1991, 71, 130–133. [Google Scholar] [CrossRef]
- Suominen, I.; Suihko, M.L.; Salkinoja-Salonen, M. Microscopic study of migration of microbes in food-packaging paper and board. J. Ind. Microbiol. Biotechnol. 1997, 19, 104–119. [Google Scholar] [CrossRef]
- Chen, Y.; Jackson, K.M.; Chea, F.P.; Schaffner, D.W. Quantification and variability analysis of bacterial cross-contamination rates in common food service tasks. J. Food Prot. 2001, 64, 72–80. [Google Scholar] [CrossRef]
- Montville, R.; Chen, Y.; Schaffner, D.W. Glove barriers to bacterial cross-contamination between hands to food. J. Food Prot. 2001, 64, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Haysom, I.W.; Sharp, A.K. Bacterial contamination of domestic kitchens over a 24-h period. Br. Food J. 2005, 107, 453–466. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Gardini, F.; Lanciotti, R. Contribution of two different packaging material to microbial contamination of peaches: Implications in their microbiological quality. Front. Microbiol. 2016, 7, 938. [Google Scholar] [CrossRef] [Green Version]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Chiavari, C.; Benevelli, M.; Grazia, L.; Lanciotti, R. Survival of spoilage and pathogenic microorganisms on cardboard and plastic packaging materials. Front. Microbiol. 2017, 8, 2606. [Google Scholar] [CrossRef] [Green Version]
- Aviat, F.; le Bayon, I.; Federighi, M.; Montibus, M. Comparative study of microbiological transfer from four materials used in direct contact with apples. Int. J. Food Microbiol. 2020, 333, 108–780. [Google Scholar] [CrossRef] [PubMed]
- Jaakko, E.; Irina, T.; Assi, W.; Christina, N.L.; Didier, L.; Mirja, S.S. Transfer of Bacillus cereus spores from packaging paper into food. J. Food Prot. 2009, 72, 2236–2242. [Google Scholar]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- Knotzer, U.; Wiltsche, M.; Stark, H. Papierstruktur in z-Richtung. Wochenbl. Fuer Pap. 2003, 11–12, 688–699. [Google Scholar]
- Hirn, U.; Bauer, W. Evaluating an Improved Method to Determine Layered Fibre Orientation by Sheet Splitting. In Proceedings of the 61st Annual APPITA Conference and 2007 Paper Physics Conference, Gold Coast, Australia, 7–10 May 2007; pp. 71–80. [Google Scholar]
- Vorst, K.L.; Todd, E.C.D.; Ryser, E.T. Transfer of Listeria monocytogenes during mechanical slicing of turkey breast, bologna, and salami. J. Food Prot. 2006, 69, 619–626. [Google Scholar] [CrossRef]
- Moore, G.; Griffith, C. Factors Influencing Recovery of Micro- organisms from Surfaces by Use of Traditional Hygiene Swabbing. Dairy Food Environ. Sanit. 2002, 22, 410–421. [Google Scholar]
- Bower, C.K.; McGuire, J.; Daeschel, M.A. The adhesion and detachment of bacteria and spores on food-contact surfaces. Trends Food Sci. Technol. 1996, 7, 152–157. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Valero, A.; Carrasco, E.; García, R.M.; Zurera, G. Understanding and modelling bacterial transfer to foods: A review. Trends Food Sci. Technol. 2008, 19, 131–144. [Google Scholar] [CrossRef]
- Dawson, P.; Han, I.; Cox, M.; Black, C.; Simmons, L. Residence time and food contact time effects on transfer of Salmonella Typhimurium from tile, wood and carpet: Testing the five-second rule. J. Appl. Microbiol. 2007, 102, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Cholodowski, D.; Bompeix, G. Adhesion and germination of waterborne and airborne conidia of Penicillium expansum to apple and inert surfaces. Physiol. Mol. Plant Pathol. 2005, 67, 40–48. [Google Scholar] [CrossRef]
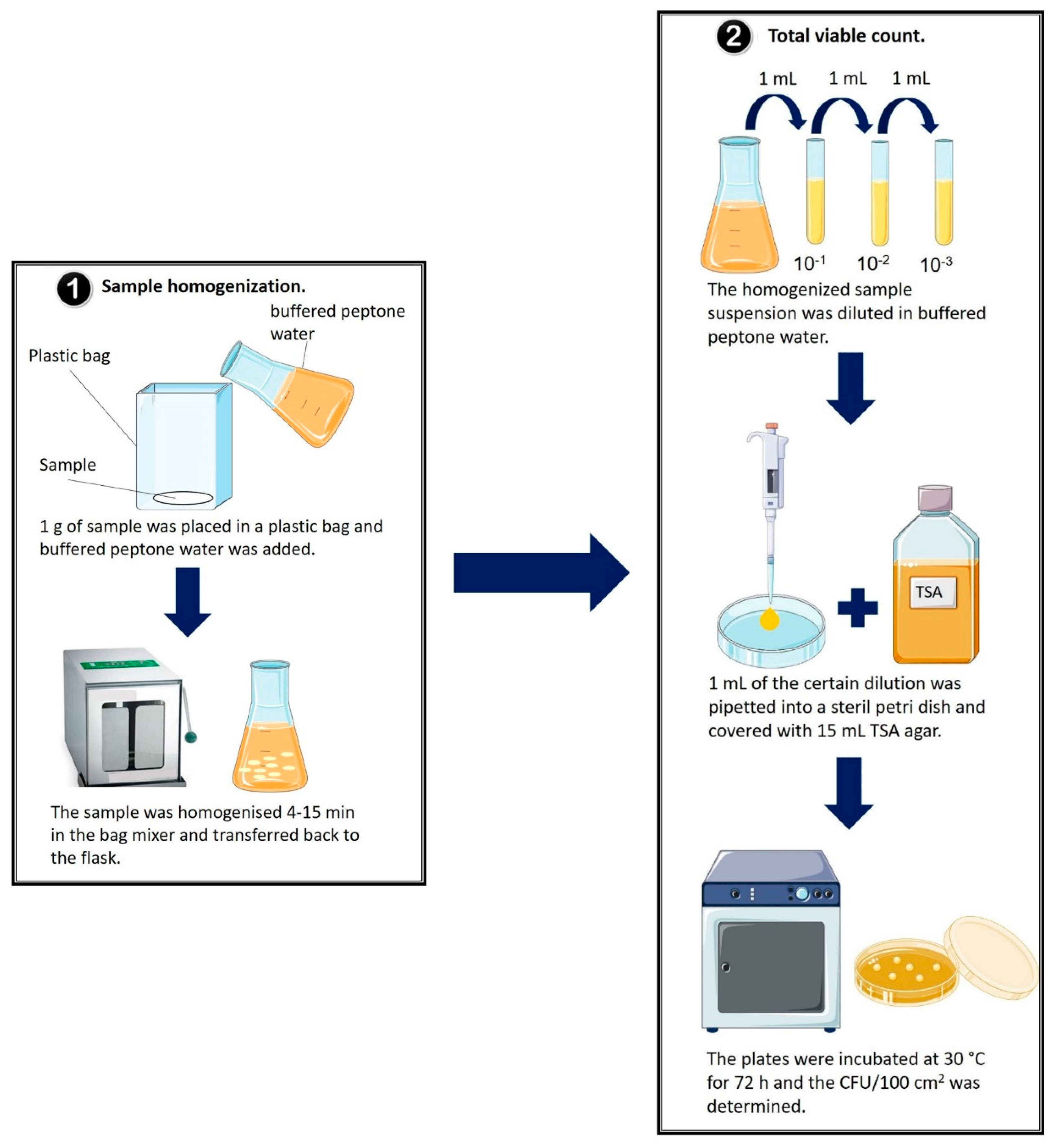
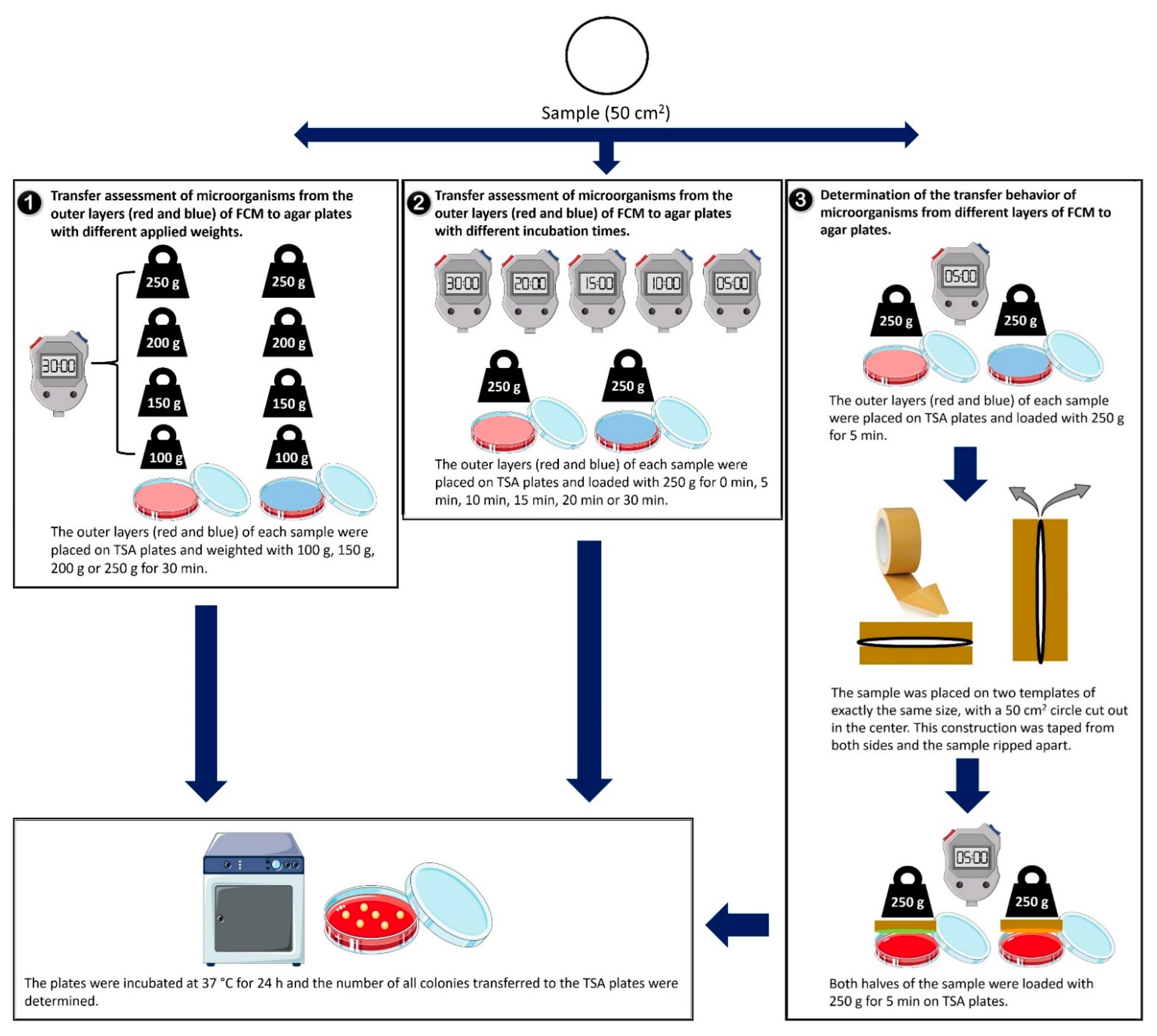


| Classification of Samples | Sample ID | Total Number of CFU/100 cm2 | Food Packaging Applications | g/cm2 |
|---|---|---|---|---|
| Samples with high numbers of CFU/100 cm2 | 1 | 8.63 × 105 | secondary food packaging: dry, non-fatty, peeled or washed | 9.60 × 10−3 |
| 2 | 5.64 × 105 | secondary food packaging: dry, non-fatty, peeled or washed | 1.34 × 10−2 | |
| 3 | 1.32 × 105 | dry, moist, fatty | 1.10 × 10−2 | |
| Samples with low numbers of CFU/100 cm2 | 4 | 5.00 × 102 | dry, moist, fatty (baking up to 220 °C) | 3.60 × 10−3 |
| 5 | 3.00 × 102 | dry, moist, fatty | 7.00 × 10−3 | |
| 6 | 1.00 × 101 | dry, moist, fatty | 1.18 × 10−2 |
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|---|---|---|
| Transfer of microorganisms to TSA plates | outer layers | CFU/100 cm2 | 8.63 × 105 | 5.64 × 105 | 1.32 × 105 | 5.44 × 102 | 2.62 × 102 | 6.49 × 101 | |
| front | transferred CFU/100 cm2 | 7.91 × 102 ± 5.28 × 102 | 1.19 × 102 ± 8.07 × 101 | 3.48 × 102 ± 1.40 × 102 | 1 × 100 ± 2 × 100 | 1 × 100 ± 4 × 100 | 2 × 100 ± 3 × 100 | ||
| transfer ratio (%) | 0.09 ± <0.06 | 0.02 ± 0.01 | 0.26 ± 0.11 | 0.26 ± 0.32 | 0.48 ± 1.36 | 2.48 ± 4.55 | |||
| back | transferred CFU/100 cm2 | 7.69 × 102 ± 4.64 × 102 | 6.56 × 101 ± 7.02 × 101 | 3.35 × 102 ± 1.43 × 102 | 2 × 100 ± 2 × 100 | 1 × 100 ± 1 × 100 | 1 × 100 ± 2 × 100 | ||
| transfer ratio (%) | 0.09 ± 0.05 | 0.01 ± 0.01 | 0.25 ± 0.11 | 0.30 ± 0.33 | 0.21 ± 0.57 | 1.75 ± 2.90 |
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|
| Transfer of microorganisms to TSA plates | outer layers | CFU/100 cm2 | 8.63 × 105 | 5.64 × 105 | 1.32 × 105 | 5.44 × 102 | 2.62 × 102 | 6.49 × 101 |
| transferred CFU/100 cm2 | 1.16 × 103 ± 4.31 × 102 | front: 1.45 × 102 ± 6.33 × 100 back: 8.3 × 101 ± 3 × 100 | 4.05 × 102 ± 6.40 × 101 | 4 × 100 ± 5 × 100 | 2 × 100 ± 4 × 100 | 1 × 100 ± 3 × 100 | ||
| transfer ratio (%) | 0.13 ± 0.05 | front: 0.03 ± <0.01 back: 0.02 ± <0.01 | 0.31 ± 0.05 | 0.67 ± 0.87 | 0.60 ± 1.44 | 2.12. ± 4.49 | ||
| Kruskal–Wallis test | 0.80 | front: 0.80 back: 0.08 | 0.08 | 0.67 | 0.38 | 0.48 |
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|---|---|---|
| Transfer of microorganisms to TSA plates | outer layers | CFU/100 cm2 | 8.63 × 105 | 5.64 × 105 | 1.32 × 105 | 5.44 × 102 | 2.62 × 102 | 6.49 × 101 | |
| 5 min | transferred CFU/100 cm2 | 9.30 × 101 ± 2.20 × 101 | front: 3.90 × 101 ± 1.00 × 101 back: 1.40 × 101 ± 4 × 100 | 5.90 × 101 ± 2.50 × 101 | 1 × 100 ± 2 × 100 | <1 × 100 ± <1 × 100 | 1 × 100 ± 2 × 100 | ||
| transfer ratio (%) | 0.01 ± <0.01 | front: 0.01 ± <0.01 back: <0.01 ± <0.01 | 0.04 ± <0.02 | 0.25 ± 0.31 | 0.05 ± 0.18 | 1.54 ± 3.08 | |||
| 30 min | transferred CFU/100 cm2 | 9.30 × 102 ± 1.70 × 102 | front: 1.65 × 102 ± 9.73 × 101 back: 9.55 × 101 ± 8.15 × 101 | 4.36 × 102 ± 6.80 × 101 | 1 × 100 ± 1 × 100 | 2 × 100 ± 4 × 100 | 1 × 100 ± 1 × 100 | ||
| transfer ratio (%) | 0.11 ± 0.02 | front: 0.03 ± 0.02 back: 0.02 ± <0.01 | 0.33 ± 0.05 | 0.25 ± 0.21 | 0.67 ± 1.35 | 0.96 ± 1.80 | |||
| comparing 5 min and 30 min | Kruskal–Wallis test | <0.01 | front: <0.01 back: <0.01 | <0.01 | 0.83 | 0.02 | 0.10 |
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|---|---|---|
| Transfer of microorganism to TSA plates | 5 min | CFU/100 cm2 | 8.63 × 105 | 5.64 × 105 | 1.32 × 105 | 5.44 × 102 | 2.62 × 102 | 6.49 × 101 | |
| outer layer | transferred CFU/100 cm2 | 9.10 × 101 ± 2.06 × 101 | 2.20 × 101 ± 1.30 × 101 | 5.50 × 101 ± 3.80 × 101 | 2 × 100 ± 1 × 100 | <1 × 100 ± 1 × 100 | 1 × 100 ± 1 × 100 | ||
| transfer ratio (%) | 0.01 ± <0.01 | <0.01 ± <0.01 | 0.03 ± 0.02 | 0.32 ± 0.20 | <0.01 ± <0.01 | 2.36 ± 2.30 | |||
| inner layer | transferred CFU/100 cm2 | 5.28 × 102 ± 7.80 × 101 | 1.25 × 102 ± 5.00 × 101 | 1.32 × 102 ± 3.70 × 101 | 1 × 100 ± 1 × 100 | <1 × 100 ± 1 × 100 | <1 × 100 ± <1 × 100 | ||
| transfer ratio (%) | 0.06 ± 0.01 | 0.02 ± <0.01 | 0.11 ± 0.02 | 0.18 ± 0.26 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| comparing layers | Mann–Whitney U test | <0.01 | <0.01 | <0.01 | 0.44 | <0.99 | <0.01 |
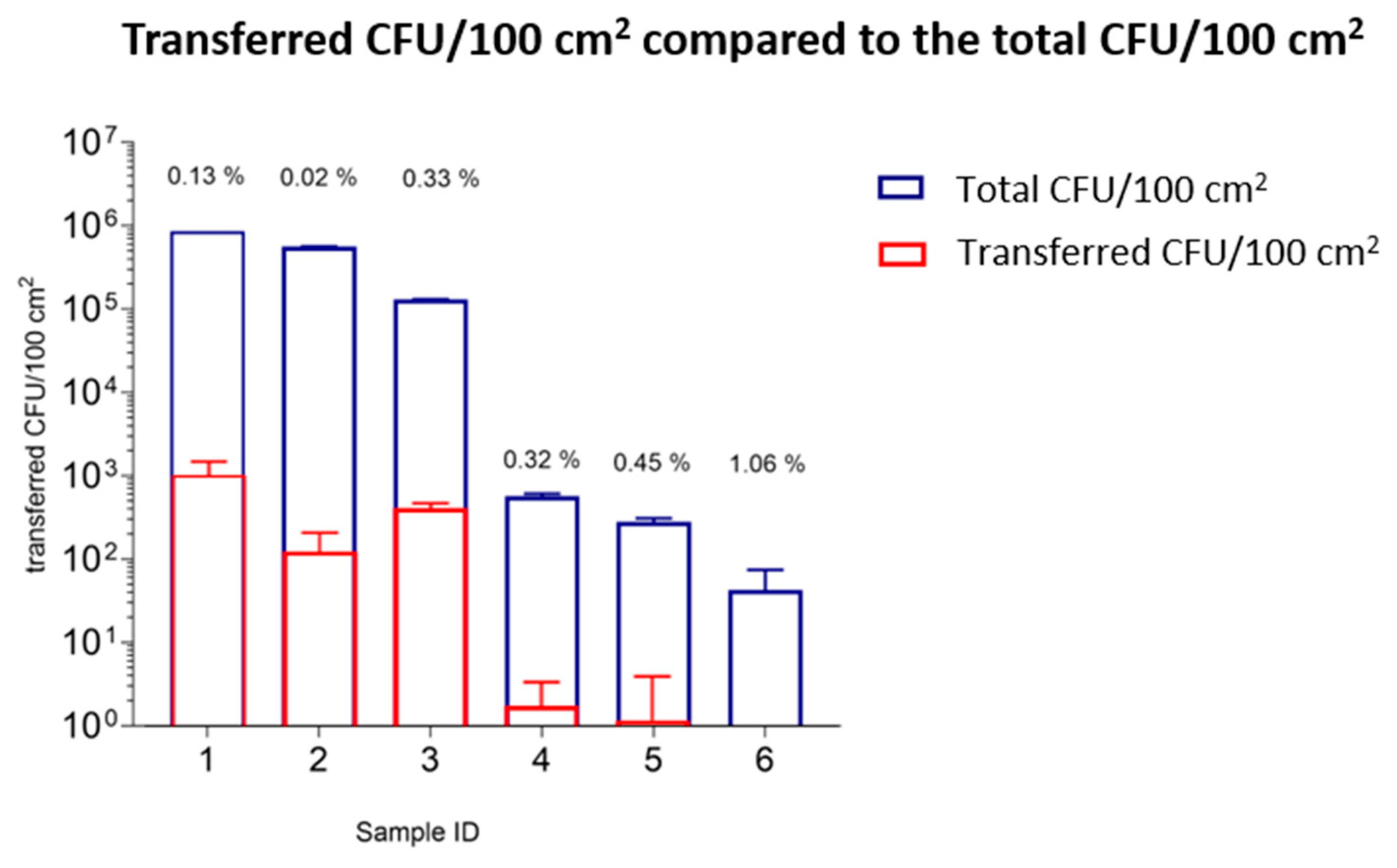
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|---|---|---|
| Transfer of microorganisms to TSA plates | CFU/100 cm2 | 8.63 × 105 | 5.64 × 105 | 1.32 × 105 | 5.44 × 102 | 2.62 × 102 | 6.49 × 101 | ||
| 30 min | outer layers | transferred CFU/100 cm2 | 1.10 × 103 ± 4.80 × 102 | 1.30 × 102 ± 8.90 × 101 | 4.37 × 102 ± 6.20 × 101 | 2 × 100 ± 2 × 100 | 2 × 100 ± 3 × 100 | <1 × 101 ± 1 × 101 | |
| transfer ratio (%) | 0.13 ± 0.06 | 0.02 ± 0.02 | 0.33 ± 0.05 | 0.32 ± 0.30 | 0.45 ± 1.06 | 1.06 ± 1.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maitz, S.; Schmid, P.J.; Kittinger, C. Modelling and Determination of Parameters Influencing the Transfer of Microorganisms from Food Contact Materials. Int. J. Environ. Res. Public Health 2022, 19, 2996. https://doi.org/10.3390/ijerph19052996
Maitz S, Schmid PJ, Kittinger C. Modelling and Determination of Parameters Influencing the Transfer of Microorganisms from Food Contact Materials. International Journal of Environmental Research and Public Health. 2022; 19(5):2996. https://doi.org/10.3390/ijerph19052996
Chicago/Turabian StyleMaitz, Stephanie, Paul Jakob Schmid, and Clemens Kittinger. 2022. "Modelling and Determination of Parameters Influencing the Transfer of Microorganisms from Food Contact Materials" International Journal of Environmental Research and Public Health 19, no. 5: 2996. https://doi.org/10.3390/ijerph19052996







