HIV and STI Testing Preferences for Men Who Have Sex with Men in High-Income Countries: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Search Strategy and Inclusion Criteria
2.2. Data Analysis
3. Results
3.1. Study Characteristics
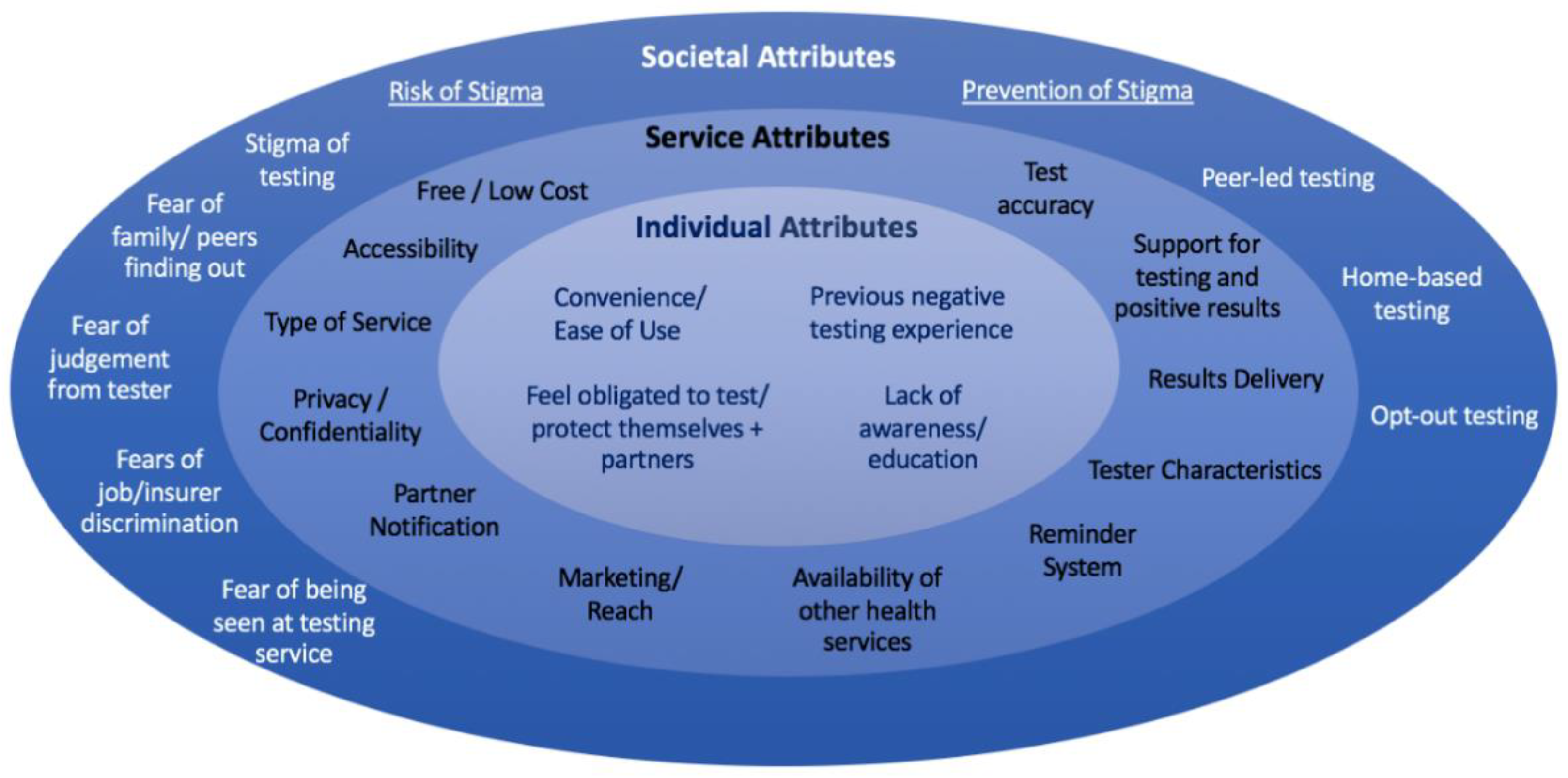
3.2. Socioecological Framework
3.3. Individual Attributes
3.3.1. Convenience
| Attribute | Attribute Examples |
|---|---|
| Convenience/Ease of Use of Testing | Self-testing (+) [16,17,18,19,20,21,22,23,24,25,26,27] |
| Self-testing (−) [28,29,30] | |
| Self-sampling (+) [14,15,21] | |
| Barriers | Previous negative experience (−) [28,31,32,33,34,35] |
| Lack of awareness/education (−) [14,36,37] | |
| Confidentiality concerns in community-based settings (−) [38] | |
| Perceived low risk (−) [36,39] | |
| Lack of priority/lifestyle too busy (−) [33,39] | |
| Fear of positive result (−) [34] | |
| Medical mistrust (−) [35] | |
| Individual Attitudes/Perceptions | Lack of testing among peers (−) [40] |
| Feel obligated to test/protect themselves + partners (+) [14,15,19,22,26,40,41] | |
| Testing in response to risk incidents, unexpected symptoms or part of a sexual health routine (+) [42] |
3.3.2. Previous Testing Experience
3.3.3. Attitudes and Perceptions
3.4. Service Attributes
3.4.1. Type of Service
3.4.2. Type of Testing
3.4.3. Rapid Testing
3.4.4. Cost
3.4.5. Tester Characteristics
3.4.6. Results and Support
3.5. Societal Attributes
Stigma Associated with HIV/STI Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2018. [Google Scholar]
- Cawley, C.; Marcus, U. Review of HIV and Sexually Transmitted Infections among Men Who Have Sex with Men (MSM) in Europe; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Australian Government Department of Health. Fourth National Sexually Transmissible Infections Strategy. Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-bbvs-1//$File/STI-Fourth-Nat-Strategy-2018-22.pdf (accessed on 25 February 2022).
- STIGMA. Australian Sexually Transmitted Infection & HIV Testing Guidelines 2019 for Asymptomatic Men Who Have Sex with Men. Available online: https://stipu.nsw.gov.au/wp-content/uploads/STIGMA_Guidelines2019_Final-1.pdf (accessed on 25 February 2022).
- DiNenno, E.A.; Prejean, J.; Irwin, K.; Delaney, K.P.; Bowles, K.; Martin, T.; Tailor, A.; Dumitru, G.; Mullins, M.M.; Hutchinson, A.B. Recommendations for HIV screening of gay, bisexual, and other men who have sex with men—United States, 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, X.; Brecht, M.-L.; Koniak-Griffin, D. Can self-testing increase HIV testing among men who have sex with men: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0188890. [Google Scholar]
- Yilu, Q.; Larry, H.; Andrew BABBITT, J.S.W.; Fengying, L.; Harsha THIRUMURTHY, W.T.; Joseph, D.T. Experiences using and organizing HIV self-testing: A global qualitative systematic review. AIDS 2018, 32, 371. [Google Scholar]
- Golden, S.D.; Earp, J.A. Social ecological approaches to individuals and their contexts: Twenty years of health education & behavior health promotion interventions. Health Educ. Behav. 2012, 39, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.; Pollard, A.; Smith, H.; Fisher, M. Are home sampling kits for sexually transmitted infections acceptable among men who have sex with men? J. Health Serv. Res. Policy 2009, 14, 35–43. [Google Scholar] [CrossRef]
- Wayal, S.; Llewellyn, C.; Smith, H.; Fisher, M. Home sampling kits for sexually transmitted infections: Preferences and concerns of men who have sex with men. Cult. Health Sex. 2011, 13, 343–353. [Google Scholar] [CrossRef]
- Balan, I.; Frasca, T.; Ibitoye, M.; Dolezal, C.; Carballo-Dieguez, A. Fingerprick Versus Oral Swab: Acceptability of Blood-Based Testing Increases If Other STIs Can Be Detected. AIDS Behav. 2017, 21, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.A.; Golden, M.R.; Hughes, J.P.; Farquhar, C.; Stekler, J.D. HIV Self-testing increases HIV testing frequency in high-risk men who have sex with men: A randomized controlled trial. J. Acquir. Immune Defic. Syndr. 2018, 78, 505–512. [Google Scholar] [CrossRef]
- Skolnik, H.S.; Phillips, K.A.; Binson, D.; Dilley, J.W. Deciding where and how to be tested for HIV: What matters most? J. Acquir. Immune Defic. Syndr. (1999) 2001, 27, 292–300. [Google Scholar] [CrossRef]
- Spielberg, F.; Branson, B.M.; Goldbaum, G.M.; Lockhart, D.; Kurth, A.; Celum, C.L.; Rossini, A.; Critchlow, C.W.; Wood, R.W. Overcoming barriers to HIV testing: Preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J. Acquir. Immune Defic. Syndr. 2003, 32, 318–327. [Google Scholar] [CrossRef]
- Witzel, T.C.; Rodger, A.J.; Burns, F.M.; Rhodes, T.; Weatherburn, P. HIV Self-Testing among Men Who Have Sex with Men (MSM) in the UK: A Qualitative Study of Barriers and Facilitators, Intervention Preferences and Perceived Impacts. PLoS ONE 2016, 11, e0162713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamil, M.S.; Prestage, G.; Fairley, C.K.; Grulich, A.E.; Smith, K.S.; Chen, M.; Holt, M.; McNulty, A.M.; Bavinton, B.R.; Conway, D.P. Effect of availability of HIV self-testing on HIV testing frequency in gay and bisexual men at high risk of infection (FORTH): A waiting-list randomised controlled trial. Lancet HIV 2017, 4, e241–e250. [Google Scholar] [CrossRef]
- Zhang, Y.; Guy, R.J.; Smith, K.S.; Jamil, M.S.; Prestage, G.; Applegate, T.L.; Conway, D.P.; Holt, M.; Keen, P.; Bavinton, B.; et al. Sustaining success: A qualitative study of gay and bisexual men’s experiences and perceptions of HIV self-testing in a randomized controlled trial. BMC Public Health 2021, 21, 2048. [Google Scholar] [CrossRef] [PubMed]
- Witzel, T.C.; Bourne, A.; Burns, F.M.; Rodger, A.J.; McCabe, L.; Gabriel, M.M.; Gafos, M.; Ward, D.; Collaco-Moraes, Y.; Dunn, D.T.; et al. HIV self-testing intervention experiences and kit usability: Results from a qualitative study among men who have sex with men in the SELPHI (Self-Testing Public Health Intervention) randomized controlled trial in England and Wales. HIV Med. 2020, 21, 189–197. [Google Scholar] [CrossRef]
- Raffe, S.; Pollard, A.; Vera, J.H.; Soni, S.; Peralta, C.; Rodriguez, L.; Dean, G.; Llewellyn, C.D. HIV self-tests for men who have sex with men, accessed via a digital vending machine: A qualitative study of acceptability. Int. J. STD AIDS 2020, 31, 420–425. [Google Scholar] [CrossRef]
- Balán, I.C.; Rios, J.L.; Lentz, C.; Arumugam, S.; Dolezal, C.; Kutner, B.; Rael, C.T.; Ying, A.W.; Macar, O.U.; Sia, S.K. Acceptability and Use of a Dual HIV/Syphilis Rapid Test and Accompanying Smartphone App to Facilitate Self- and Partner-Testing Among Cisgender Men and Transgender Women Who Have Sex with Men. AIDS Behav. 2022, 26, 35–46. [Google Scholar] [CrossRef]
- Biello, K.B.; Horvitz, C.; Mullin, S.; Mayer, K.H.; Scott, H.; Coleman, K.; Dormitzer, J.; Norelli, J.; Hightow-Weidman, L.; Sullivan, P.; et al. HIV self-testing and STI self-collection via mobile apps: Experiences from two pilot randomized controlled trials of young men who have sex with men. Mhealth 2021, 7, 26. [Google Scholar] [CrossRef]
- Iribarren, S.; Lentz, C.; Sheinfil, A.Z.; Giguere, R.; Lopez-Rios, J.; Dolezal, C.; Frasca, T.; Balán, I.C.; Tagliaferri Rael, C.; Brown, W., 3rd; et al. Using an HIV Self-test Kit to Test a Partner: Attitudes and Preferences Among High-Risk Populations. AIDS Behav. 2020, 24, 3232–3243. [Google Scholar] [CrossRef]
- Hubach, R.D.; O’Neil, A.M.; Stowe, M.; Hamrick, J.; Giano, Z.; Currin, J.M. Preferred Methods of HIV and Sexually Transmissible Infection Screening Delivery Among a Rural Sample of Men Who Have Sex with Men. AIDS Patient Care STDS 2020, 34, 470–476. [Google Scholar] [CrossRef]
- Eaton, E.F.; Austin, E.L.; Dodson, C.K.; Heudebert, J.P.; Jackson, D.; Muzny, C.A. Do young black men who have sex with men in the deep south prefer traditional over alternative STI testing? PLoS ONE 2018, 13, e0209666. [Google Scholar] [CrossRef] [Green Version]
- Medline, A.; Daniels, J.; Marlin, R.; Young, S.; Wilson, G.; Huang, E.; Klausner, J.D. HIV Testing Preferences Among MSM Members of an LGBT Community Organization in Los Angeles. J. Assoc. Nurses AIDS Care JANAC 2017, 28, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Cushman, T.A.; Graves, S.K.; Little, S.J. Attitudes and preferences regarding the use of rapid self-testing for sexually transmitted infections and HIV in San Diego area men who have sex with men. Open Forum Infect. Dis. 2019, 6. [Google Scholar] [CrossRef]
- Datta, J.; Reid, D.; Hughes, G.; Mercer, C.H.; Wayal, S.; Weatherburn, P. Places and people: The perceptions of men who have sex with men concerning STI testing: A qualitative study. Sex. Transm. Infect. 2018, 94, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, B.A.; Dellucci, T.V.; Graham, S.; Parsons, J.T.; Mustanski, B. Sexually transmitted infections among young men who have sex with men: Experiences with diagnosis, treatment, and reinfection. Sex. Res. Soc. Policy A J. NSRC 2018, 15, 172–182. [Google Scholar] [CrossRef]
- Frye, V.; Wilton, L.; Hirshfield, S.; Chiasson, M.A.; Lucy, D.; Usher, D.; McCrossin, J.; Greene, E.; Koblin, B. Preferences for HIV test characteristics among young, Black Men Who Have Sex with Men (MSM) and transgender women: Implications for consistent HIV testing. PLoS ONE 2018, 13, e0192936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheim, A.I.; Travers, R. Barriers and facilitators to HIV and sexually transmitted infections testing for gay, bisexual, and other transgender men who have sex with men. AIDS Care Psychol. Soc.-Med. Asp. AIDS/HIV 2017, 29, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Heijman, T.; Zuure, F.; Stolte, I.; Davidovich, U. Motives and barriers to safer sex and regular STI testing among MSM soon after HIV diagnosis. BMC Infect. Dis. 2017, 17, 194. [Google Scholar] [CrossRef] [Green Version]
- Hoyos, J.; Koutentakis, K.; Maté, T.; Pulido, J.; Sordo, L.; Guerras, J.M.; Belza, M.J. High risk men who have sex with men in Spain are reporting low intentions of actively seeking HIV testing: Results from a cross-sectional study. BMC Public Health 2020, 20, 398. [Google Scholar] [CrossRef]
- Balan, I.C.; Lopez-Rios, J.; Nayak, S.; Lentz, C.; Arumugam, S.; Kutner, B.; Dolezal, C.; Macar, O.U.; Pabari, T.; Wang Ying, A.; et al. SMARTtest: A Smartphone App to Facilitate HIV and Syphilis Self- and Partner-Testing, Interpretation of Results, and Linkage to Care. AIDS Behav. 2019, 24, 1560–1573. [Google Scholar] [CrossRef]
- Barnard, S.; Free, C.; Bakolis, I.; Turner, K.M.E.; Looker, K.J.; Baraitser, P. Comparing the characteristics of users of an online service for STI self-sampling with clinic service users: A cross-sectional analysis. Sex. Transm. Infect. 2018, 94, 377–383. [Google Scholar] [CrossRef]
- Chen, M.Y.; Bilardi, J.E.; Lee, D.; Cummings, R.; Bush, M.; Fairley, C.K. Australian men who have sex with men prefer rapid oral HIV testing over conventional blood testing for HIV. Int. J. STD AIDS 2010, 21, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Lea, T.; Anning, M.; Wagner, S.; Owen, L.; Howes, F.; Holt, M. Barriers to accessing hiv and sexual health services among gay men in Tasmania, Australia. J. Gay Lesbian Soc. Serv. Q. J. Community Clin. Pract. 2019, 31, 153–165. [Google Scholar] [CrossRef]
- Tan, R.K.J.; Kaur, N.; Kumar, P.A.; Tay, E.; Leong, A.; Chen, M.I.; Wong, C.S. Clinics as spaces of costly disclosure: HIV/STI testing and anticipated stigma among gay, bisexual and queer men. Cult. Health Sex. 2020, 22, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S. General Practitioners’ views and experiences on the barriers and facilitators that men who have sex with men have when accessing primary care for HIV testing and sexual health screening. Prim. Health Care Res. Dev. 2018, 19, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohall, A.; Dini, S.; Nye, A.; Dye, B.; Neu, N.; Hyden, C. HIV testing preferences among young men of color who have sex with men. Am. J. Public Health 2010, 100, 1961–1966. [Google Scholar] [CrossRef]
- Clark, H.A.; Oraka, E.; DiNenno, E.A.; Wesolowski, L.G.; Chavez, P.R.; Pitasi, M.A.; Delaney, K.P. Men Who Have Sex with Men (MSM) Who Have Not Previously Tested for HIV: Results from the MSM Testing Initiative, United States (2012–2015). AIDS Behav. 2019, 23, 359–365. [Google Scholar] [CrossRef]
- Dodge, B.; Van Der Pol, B.; Rosenberger, J.G.; Reece, M.; Roth, A.M.; Herbenick, D.; Fortenberry, J.D. Field collection of rectal samples for sexually transmitted infection diagnostics among men who have sex with men. Int. J. STD AIDS 2010, 21, 260–264. [Google Scholar] [CrossRef]
- Mustanski, B.; Moskowitz, D.A.; Moran, K.O.; Rendina, H.J.; Newcomb, M.E.; Macapagal, K. Factors Associated with HIV Testing in Teenage Men Who Have Sex with Men. Pediatrics 2020, 145. [Google Scholar] [CrossRef]
- den Daas, C.; Geerken, M.B.R.; Bal, M.; de Wit, J.; Spijker, R.; Op de Coul, E.L.M. Reducing health disparities: Key factors for successful implementation of social network testing with HIV self-tests among men who have sex with men with a non-western migration background in the Netherlands. AIDS Care Psychol. Socio-Med. Asp. AIDS/HIV 2020, 32, 50–56. [Google Scholar] [CrossRef]
- Flowers, P.; Riddell, J.; Park, C.; Ahmed, B.; Young, I.; Frankis, J.; Davis, M.; Gilbert, M.; Estcourt, C.; Wallace, L.; et al. Preparedness for use of the rapid result HIV self-test by gay men and other men who have sex with men (MSM): A mixed methods exploratory study among MSM and those involved in HIV prevention and care. HIV Med. 2017, 18, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Knight, R.E.; Chabot, C.; Carson, A.; Thomson, K.; Haag, D.; Gilbert, M.; Shoveller, J. Qualitative analysis of the experiences of gay, bisexual and other men who have sex with men who use GetCheckedOnline.com: A comprehensive internet-based diagnostic service for HIV and other STIs. Sex. Transm. Infect. 2019, 95, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Fairley, C.; Cummings, R.; Bush, M.; Read, T.; Chen, M. Men who have sex with men prefer rapid testing for syphilis and may test more frequently using it. Sex. Transm. Dis. 2010, 37, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Witzel, T.C.; Melendez-Torres, G.J.; Hickson, F.; Weatherburn, P. HIV testing history and preferences for future tests among gay men, bisexual men and other MSM in England: Results from a cross-sectional study. BMJ Open 2016, 6, e011372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wray, T.B.; Chan, P.A.; Simpanen, E.; Operario, D. A pilot, randomized controlled trial of HIV self-testing and real-time post-test counseling/referral on screening and preventative care among men who have sex with men. AIDS Patient Care STDs 2018, 32, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; De Abreu Lourenco, R.; Street, D.; Smith, K.; Jamil, M.S.; Terris-Prestholt, F.; Fairley, C.K.; McNulty, A.; Hynes, A.; Johnson, K.; et al. The Preferred Qualities of Human Immunodeficiency Virus Testing and Self-Testing Among Men Who Have Sex with Men: A Discrete Choice Experiment. Value Health 2020, 23, 870–879. [Google Scholar] [CrossRef]
- John, S.A.; Starks, T.J.; Rendina, H.J.; Parsons, J.T.; Grov, C. High willingness to use novel HIV and bacterial sexually transmitted infection partner notification, testing, and treatment strategies among gay and bisexual men. Sex. Transm. Infect. 2020, 96, 173–176. [Google Scholar] [CrossRef]
- Johnson, M.C.; Chung, R.; Leung, S.J.; Edelstein, Z.; Yuan, Y.; Flavin, S.M. Combating Stigma Through HIV Self-Testing: New York State’s HIV Home Test Giveaway Program for Sexual Minorities. J. Public Health Manag. Pract. 2022, 28, 174–183. [Google Scholar] [CrossRef]
- Leenen, J.; Hoebe, C.; Ackens, R.P.; Posthouwer, D.; van Loo, I.H.M.; Wolffs, P.F.G.; Dukers-Muijrers, N. Pilot implementation of a home-care programme with chlamydia, gonorrhoea, hepatitis B, and syphilis self-sampling in HIV-positive men who have sex with men. BMC Infect. Dis. 2020, 20, 925. [Google Scholar] [CrossRef]
- Maté, T.; Hoyos, J.; Guerras, J.M.; Agustí, C.; Chanos, S.; Kuske, M.; Fuertes, R.; Stefanescu, R.; Pulido, J.; Sordo, L.; et al. Potential of HIV Self-Sampling to Increase Testing Frequency Among Gay, Bisexual, and Other Men Who Have Sex with Men, and the Role of Online Result Communication: Online Cross-Sectional Study. J. Med. Internet Res. 2020, 22, e21268. [Google Scholar] [CrossRef]
- Nash, S.G.; Maffeo, M.; Likatavicius, G.; Cosmaro, L.; Rudaitis, K.; Lapsinov, A.; Enayat, Q.; Delpech, V.; Kall, M. Acceptability and usability of HIV self-tests in two European countries: Findings from surveys of clients at non-governmental organisations in Lithuania and Italy. BMC Infect. Dis. 2021, 21, 844. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Sullivan, P.S.; Stephenson, R. Acceptability and Feasibility of a Telehealth Intervention for STI Testing Among Male Couples. AIDS Behav. 2021, 25, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, J.; Maté, T.; Guerras, J.M.; Donat, M.; Agustí, C.; Kuske, M.; Fuertes, R.; Chanos, S.; Pichon, F.; Sordo, L.; et al. Preference towards HIV Self-Testing above Other Testing Options in a Sample of Men Who Have Sex with Men from Five European Countries. Int. J. Environ. Res. Public Health 2021, 18, 4804. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.P.; Guy, R.; Davies, S.C.; Couldwell, D.L.; McNulty, A.; Smith, D.E.; Keen, P.; Cunningham, P.; Holt, M. Rapid HIV Testing Is Highly Acceptable and Preferred among High-Risk Gay and Bisexual Men after Implementation in Sydney Sexual Health Clinics. PLoS ONE 2015, 10, e0123814. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.E.; Pedrana, A.; Leitinger, D.; Wilkinson, A.L.; Locke, P.; Hellard, M.E.; Stoove, M. Trial and error: Evaluating and refining a community model of HIV testing in Australia. BMC Health Serv. Res. 2017, 17, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strömdahl, S.; Liljeros, F.; Thorson, A.E.; Persson, K.I.; Forsberg, B.C. HIV testing and prevention among foreign-born Men Who have Sex with Men: An online survey from Sweden. BMC Public Health 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miners, A.; Nadarzynski, T.; Witzel, C.; Phillips, A.N.; Cambiano, V.; Rodger, A.J.; Llewellyn, C.D. Preferences for HIV testing services among men who have sex with men in the UK: A discrete choice experiment. PLoS Med. 2019, 16, e1002779. [Google Scholar] [CrossRef]
- Holt, M.; Bernard, D.; Race, K. Gay men’s perceptions of sexually transmissible infections and their experiences of diagnosis: ‘Part of the way of life’ to feeling ‘dirty and ashamed’. Sexual Health 2010, 7, 411–416. [Google Scholar] [CrossRef]
- Hoyos, J.; Belza, M.J.; Fernandez-Balbuena, S.; Rosales-Statkus, M.E.; Pulido, J.; de la Fuente, L. Preferred HIV testing services and programme characteristics among clients of a rapid HIV testing programme. BMC Public Health 2013, 13, 791. [Google Scholar] [CrossRef] [Green Version]
- Hottes, T.S.; Farrell, J.; Bondyra, M.; Haag, D.; Shoveller, J.; Gilbert, M.; Hottes, T.S.; Farrell, J.; Bondyra, M.; Haag, D.; et al. Internet-based HIV and sexually transmitted infection testing in British Columbia, Canada: Opinions and expectations of prospective clients. J. Med. Internet Res. 2012, 14, e41. [Google Scholar] [CrossRef]
- Leitinger, D.; Ryan, K.E.; Brown, G.; Pedrana, A.; Wilkinson, A.L.; Ryan, C.; Hellard, M.; Stoove, M. Acceptability and HIV prevention benefits of a peer-based model of rapid point of care HIV testing for Australian gay, bisexual and other men who have sex with men. AIDS Behav. 2018, 22, 178–189. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, A.B.; Morrison, C.A.; Lopez-Rios, J.; MacCrate, C.J.; Pantalone, D.W.; Stief, M.; Grov, C. Experiences Receiving HIV-Positive Results by Phone: Acceptability and Implications for Clinical and Behavioral Research. AIDS Behav. 2021, 25, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Knight, V.; Read, P.J.; McNulty, A. Clients’ preferred methods of obtaining sexually transmissable infection or HIV results from Sydney Sexual Health Centre. Sex. Health 2013, 10, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Tobin, K.; Edwards, C.; Flath, N.; Lee, A.; Tormohlen, K.; Gaydos, C.A. Acceptability and feasibility of a Peer Mentor program to train young Black men who have sex with men to promote HIV and STI home-testing to their social network members. AIDS Care Psychol. Socio-Med. Asp. AIDS/HIV 2018, 30, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Contesse, M.G.; Fredericksen, R.J.; Wohlfeiler, D.; Hecht, J.; Kachur, R.; Strona, F.V.; Katz, D.A. Acceptability of Using Geosocial Networking Applications for HIV/Sexually Transmitted Disease Partner Notification and Sexual Health Services. Sex. Transm. Dis. 2020, 47, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pant Pai, N.; Smallwood, M.; Desjardins, L.; Goyette, A.; Birkas, K.G.; Vassal, A.F.; Joseph, L.; Thomas, R. An Unsupervised Smart App-Optimized HIV Self-Testing Program in Montreal, Canada: Cross-Sectional Study. J. Med. Internet Res. 2018, 20, e10258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfeiler, D.; Hecht, J.; Volk, J.; Fisher Raymond, H.; Kennedy, T.; McFarland, W. How can we improve online HIV and STD prevention for men who have sex with men? Perspectives of hook-up website owners, website users, and HIV/STD directors. AIDS Behav. 2013, 17, 3024–3033. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; King, C.; Gilson, R.; Richardson, D.; Burns, F.; Rodger, A.; Clark, L.; Miners, A.; Pollard, A.; Desai, S.; et al. Healthcare provider and service user perspectives on STI risk reduction interventions for young people and MSM in the UK. Sex. Transm. Infect. 2020, 96, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.; Hottes, T.S.; Kerr, T.; Taylor, D.; Fairley, C.K.; Lester, R.; Wong, T.; Trussler, T.; Marchand, R.; Shoveller, J.; et al. Factors associated with intention to use internet-based testing for sexually transmitted infections among men who have sex with men. J. Med. Internet Res. 2013, 15, e254. [Google Scholar] [CrossRef]
- Frank, T.D.; Carter, A.; Jahagirdar, D.; Biehl, M.H.; Douwes-Schultz, D.; Larson, S.L.; Arora, M.; Dwyer-Lindgren, L.; Steuben, K.M.; Abbastabar, H. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019, 6, e831–e859. [Google Scholar] [CrossRef] [Green Version]
- UNAIDS. UNAIDS Data 2020. Available online: https://www.unaids.org/en/resources/documents/2020/unaids-data (accessed on 25 February 2022).
- Mathews, A.; Farley, S.; Conserve, D.F.; Knight, K.; Le’Marus, A.; Blumberg, M.; Rennie, S.; Tucker, J. “Meet people where they are”: A qualitative study of community barriers and facilitators to HIV testing and HIV self-testing among African Americans in urban and rural areas in North Carolina. BMC Public Health 2020, 20, 494. [Google Scholar] [CrossRef] [Green Version]
- Pollard, A.; Llewellyn, C.; Smith, H.; Richardson, D.; Fisher, M. Opt-out testing for HIV: Perspectives from a high prevalence community in south-east England, UK. Int. J. STD AIDS 2013, 24, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, J.; Owczarzak, J.T.; Petroll, A.E. Marketing the HIV test to MSM: Ethnic differences in preferred venues and sources. Health Promot. Pract. 2013, 14, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadatmand, H.J.; Bernstein, K.T.; McCright, J.; Gallaread, A.; Philip, S.S.; Lippman, S.A. Young men’s preferences for sexually transmitted disease and reproductive health services in San Francisco, California. Sex. Transm. Dis. 2012, 39, 421–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, E.P.; Grulich, A.E.; Fairley, C.K. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV 2019, 6, e396–e405. [Google Scholar] [CrossRef]
- UNAIDS. Fact Sheet-Latest Global and Regional Statistics on the Status of the AIDS Epidemic. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 15 November 2021).
- Martin, L.; Knight, V.; Ryder, N.; Lu, H.; Read, P.J.; McNulty, A. Client feedback and satisfaction with an express sexually transmissible infection screening service at an inner-city sexual health center. Sex. Transm. Dis. 2013, 40, 70–74. [Google Scholar] [CrossRef]
- Gilbert, M.; Thomson, K.; Salway, T.; Haag, D.; Grennan, T.; Fairley, C.K.; Buchner, C.; Krajden, M.; Kendall, P.; Shoveller, J.; et al. Differences in experiences of barriers to STI testing between clients of the internet-based diagnostic testing service GetCheckedOnline.com and an STI clinic in Vancouver, Canada. Sex. Transm. Infect. 2019, 95, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Lau, J.T.F.; Tsui, H. Psychological factors in association with uptake of voluntary counselling and testing for HIV among men who have sex with men in Hong Kong. Public Health 2011, 125, 275–282. [Google Scholar] [CrossRef]
- Baytop, C.; Royal, S.; Hubbard McCree, D.; Simmons, R.; Tregerman, R.; Robinson, C.; Johnson, W.D.; McLaughlin, M.; Price, C. Comparison of strategies to increase HIV testing among African-American gay, bisexual, and other men who have sex with men in Washington, DC. AIDS Care Psychol. Socio-Med. Asp. AIDS/HIV 2014, 26, 608–612. [Google Scholar] [CrossRef]

| Attribute | Attribute Examples |
|---|---|
| Type of Service | Self-testing/Self-sampling (+) [14,15,16,17,18,19,20,22,23,24,26,29,31,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] |
| Self-collection (+) [21,31] | |
| Self-collection (−) [18,21] | |
| Mobile testing (+) [62] | |
| Mobile testing (−) [32] | |
| Online testing service (+) [43] | |
| Rapid testing (+) [18,19,30,31,34,36,45,49,51,63,64] | |
| Express service (+) [65] | |
| Accessibility | Appointment system availability (−) [28] |
| Appointment system ease of use (+) [66] | |
| Walk-in Service (+) [62] | |
| Waiting times (−) [66] | |
| Self-testing kits available at a variety of locations (+) [22,54,59] | |
| Non-specialist setting (−) [67] | |
| Type of Test | Oral (+) [16] |
| Rectal (+) [68] | |
| Urine (+) [28] | |
| Blood (+) [25,34] | |
| Venepuncture (−) [19] | |
| Other attributes (e.g., cost, speed) > type of test (+) [16,27] | |
| Accuracy | High accuracy (+) [26,34] |
| Accuracy > convenience of sample collection (+) [14] | |
| Concerns about accuracy/reliability of self-testing and rapid testing (−) [22,28] | |
| Cost | Free/low cost (+) [17,18,27,49,54,55,69,70] |
| No health insurance (−) [28] | |
| Cost (−) [27,37,59] | |
| Privacy, Confidentiality & Anonymity | Fears of disclosing sexual identity or behaviour in an unfamiliar environment (−) [48] |
| Privacy/anonymity when testing + receiving results (+) [28] | |
| Open waiting room (−) [14] | |
| Non-specialist setting (−) [32] | |
| Picking up of self-testing kit from pharmacy/clinic (−) [29] | |
| Named reporting (−) [19] | |
| Providing personal information online (−) [71] | |
| Tester Characteristics | Credibility of tester and legitimacy (+) [18,28,32,35,48] |
| Tester attitude (+) [28,32,33,42,71,72,73] | |
| Risk of being recognised (−) [64] | |
| Familiarity with tester/comfortable environment (+) [18,27,28,29,64] | |
| Skill/knowledge of tester (+) [18,32,34,37,71,72] | |
| Healthcare professional (+) [28] | |
| Peer-testing (+) [51] | |
| Lack of trans knowledge (−) [35] | |
| Results Delivery | In person/via phone call if positive (+) [15,19,20,31,71,73,74] |
| Online results/via phone app (+) [27,43,58,73] | |
| Online results (−) [59,75] | |
| Quick/immediate results (+) [27,59] | |
| Through text if negative (+) [31] | |
| Support for Testing and Positive Results | Education/counselling (+) [18,36,48,59,71,76,77,78] |
| Availability of immediate treatment (+) [27,28] | |
| Face to face counselling (−) [19] | |
| Linkage to care (+) [79] | |
| Linkage of results to other health professionals (+) [18,27,73] | |
| Home-based testing: support offered (+) [45] | |
| Partner delivered partner therapy (+) [55] | |
| Anonymous partner notification (+) [55] | |
| Self-testing: lack of support (−) [14,20,24,38,49,59,75] | |
| Reminder System | From local health department (+) [28] |
| From online service (+) [50] | |
| From STI testing service (+) [36] | |
| Partner Notification | Via phone app (+) [43,80] |
| Anonymous e-card (+) [70] | |
| Availability of Other Health Services | Offering other health services (+) [36] |
| Self-testing: lack of availability of other health services (−) [49] | |
| Lack of co-testing of other STIs (−) [64] | |
| Integrating testing with ongoing monitoring for hormone therapy (+) [35] | |
| Availability of condoms and lubricants (+) [36] | |
| Reach/Marketing | Use of apps/internet (+) [70] |
| Attribute | Attribute Examples |
|---|---|
| Risk of Being Stigmatised | Fear of being seen/community finding out (−) [32,83] |
| Fears of family/peers finding out (−) [15,83] | |
| Fears of job/insurer discrimination (−) [19] | |
| Stigma of STI testing (−) [19,28,32,34,37,41,48,49,71,83] | |
| Fear of judgement from tester/negative treatment (−) [38,66] | |
| Preventing Stigma | Peer-led testing (+) [64,72] |
| Home-self testing (+) [14,30] | |
| Opt-out testing (+) [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kularadhan, V.; Gan, J.; Chow, E.P.F.; Fairley, C.K.; Ong, J.J. HIV and STI Testing Preferences for Men Who Have Sex with Men in High-Income Countries: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 3002. https://doi.org/10.3390/ijerph19053002
Kularadhan V, Gan J, Chow EPF, Fairley CK, Ong JJ. HIV and STI Testing Preferences for Men Who Have Sex with Men in High-Income Countries: A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(5):3002. https://doi.org/10.3390/ijerph19053002
Chicago/Turabian StyleKularadhan, Varsicka, Joscelyn Gan, Eric P. F. Chow, Christopher K. Fairley, and Jason J. Ong. 2022. "HIV and STI Testing Preferences for Men Who Have Sex with Men in High-Income Countries: A Scoping Review" International Journal of Environmental Research and Public Health 19, no. 5: 3002. https://doi.org/10.3390/ijerph19053002
APA StyleKularadhan, V., Gan, J., Chow, E. P. F., Fairley, C. K., & Ong, J. J. (2022). HIV and STI Testing Preferences for Men Who Have Sex with Men in High-Income Countries: A Scoping Review. International Journal of Environmental Research and Public Health, 19(5), 3002. https://doi.org/10.3390/ijerph19053002






