Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus faecalis and Human Gingival Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
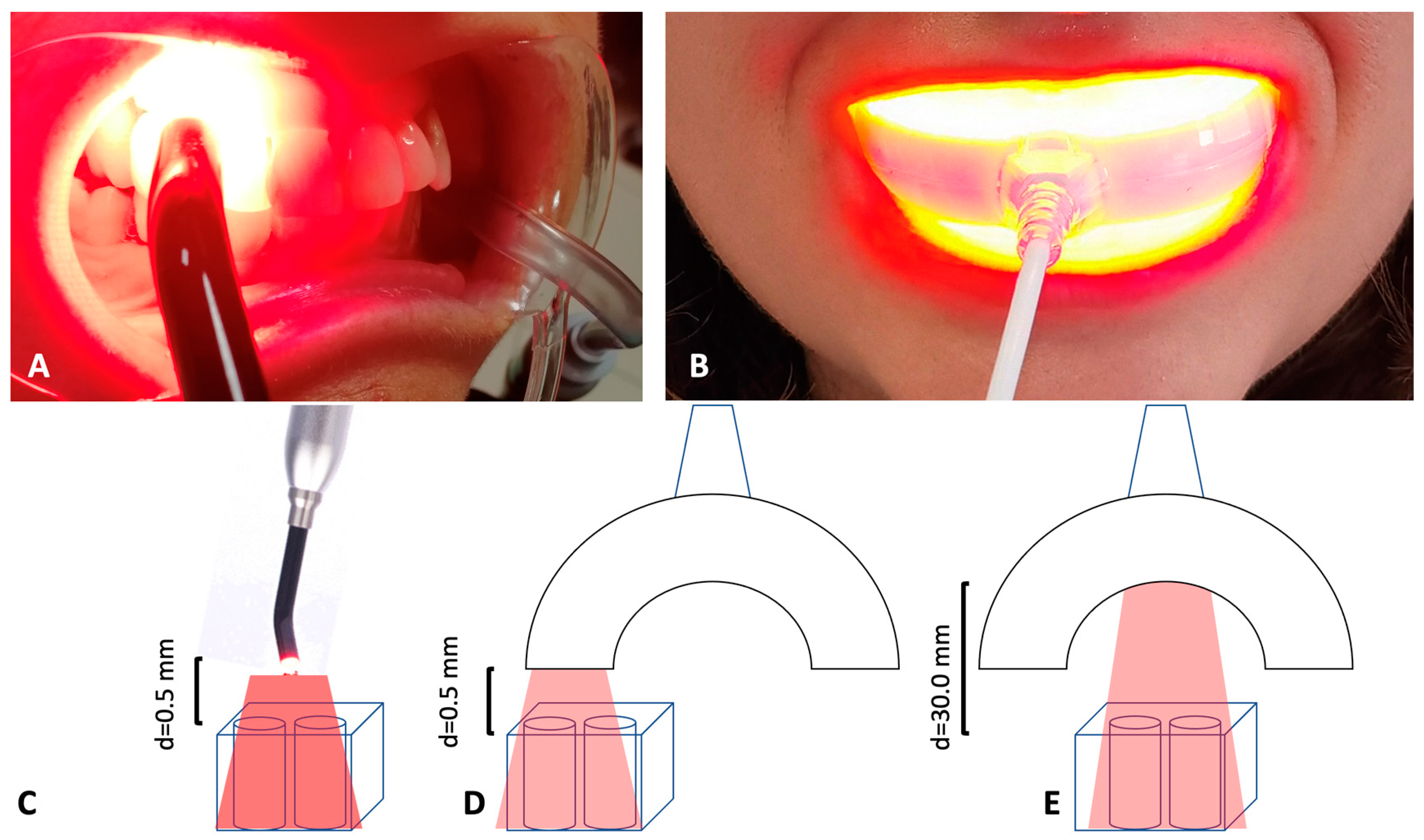
2.1. Light Sources
2.2. Experimental Design
- TL-01 N (near) = 0.5 mm distance between the samples and the light source, 7 min of irradiation (Figure 1C).
- TL-03 N (near) = 0.5 mm distance between the samples and the light source, 15 min of irradiation (Figure 1D).
- TL-03 F (far) = 30.0 mm distance between the samples and the light source, 15 min of irradiation (Figure 1E).
2.3. Strain Culture Condition
2.4. Colony Forming Units (CFU) Determination
2.5. Live/Dead Assay
2.6. Intracellular Detection of PpIX
2.7. Viability and Proliferation Assay
2.8. Morphological Characterization of Adherent Cells
2.9. Statistical Analysis
3. Results
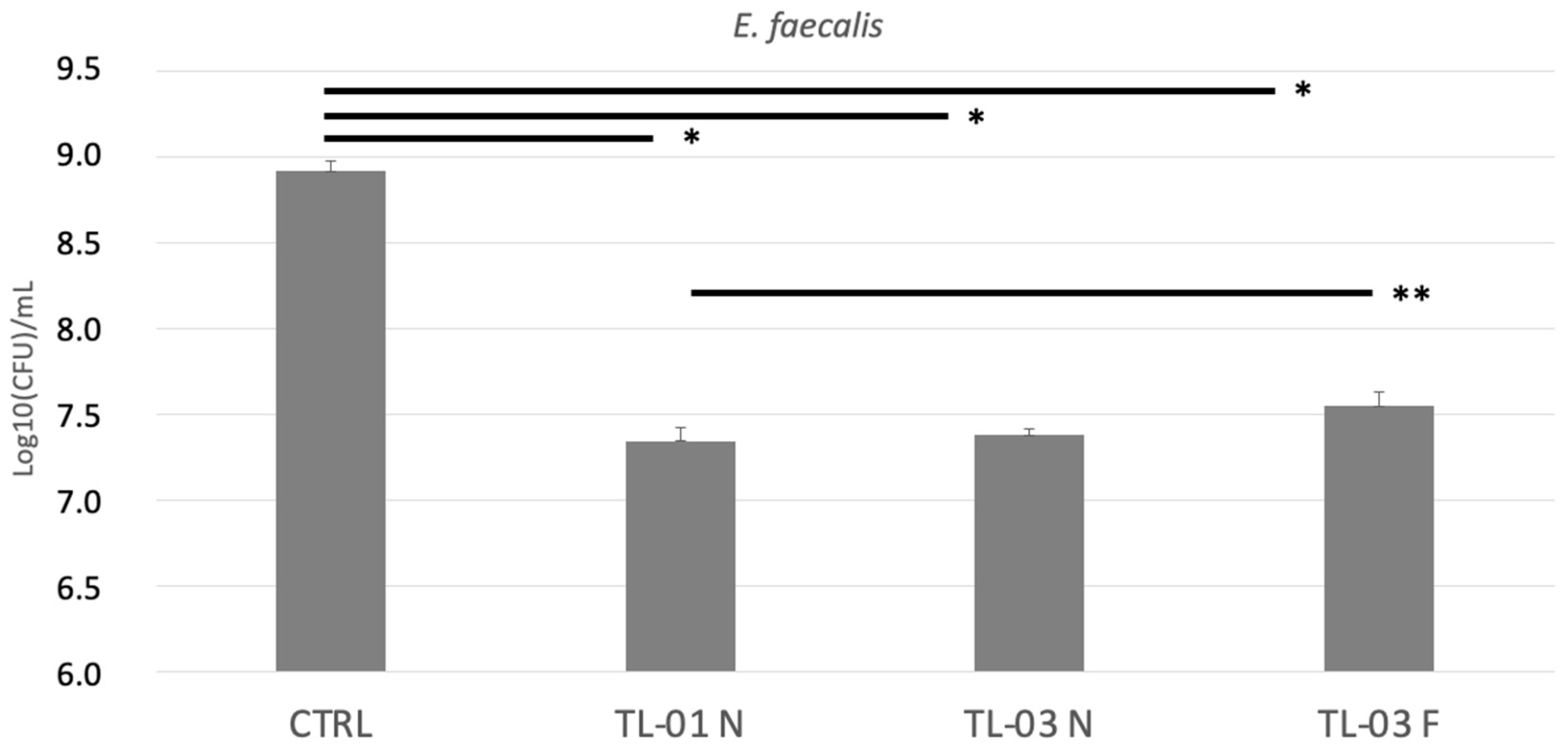
3.1. Colony Forming Units (CFUs) Determination
3.2. Live/Dead Assay
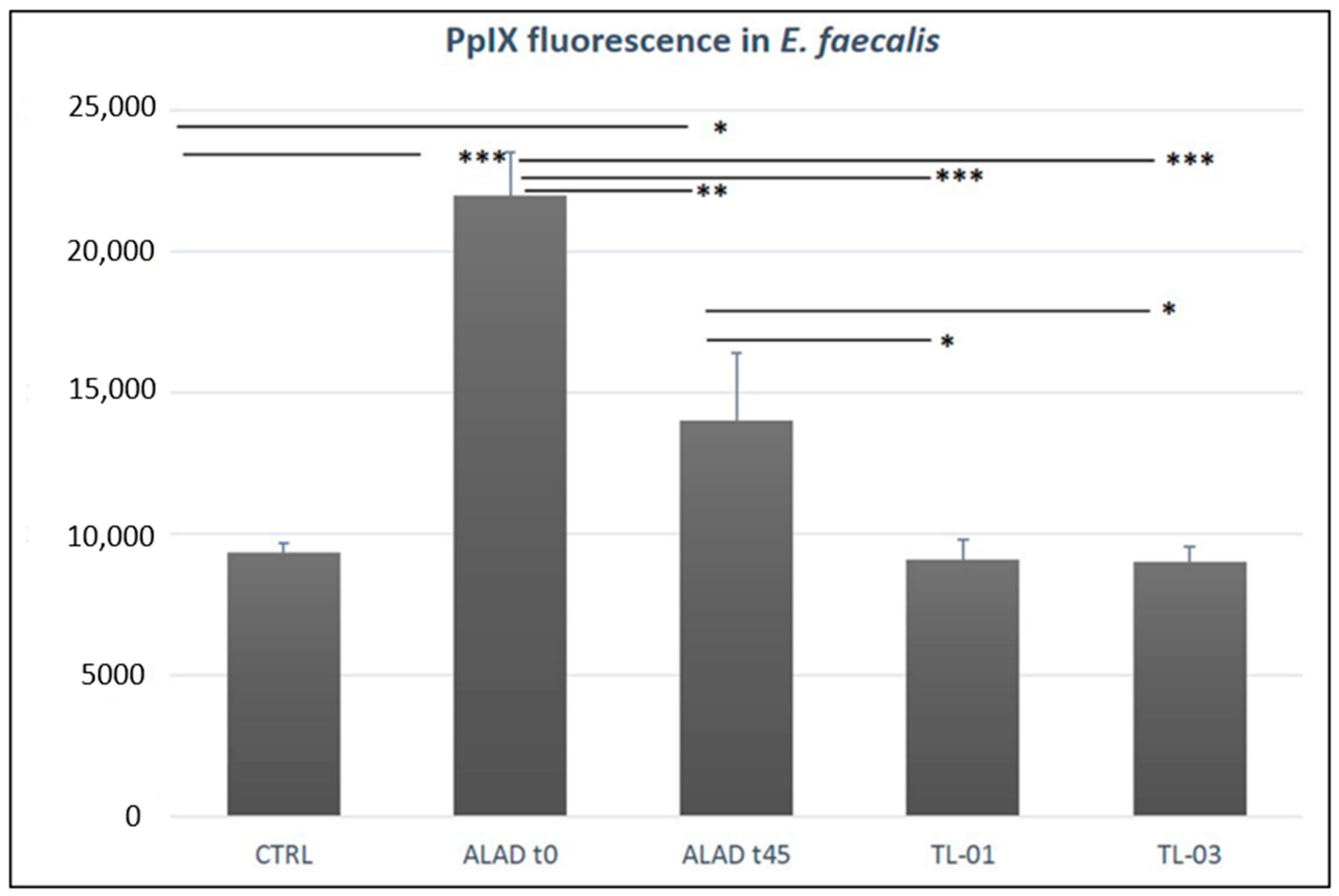
3.3. Intracellular Detection of PpIX
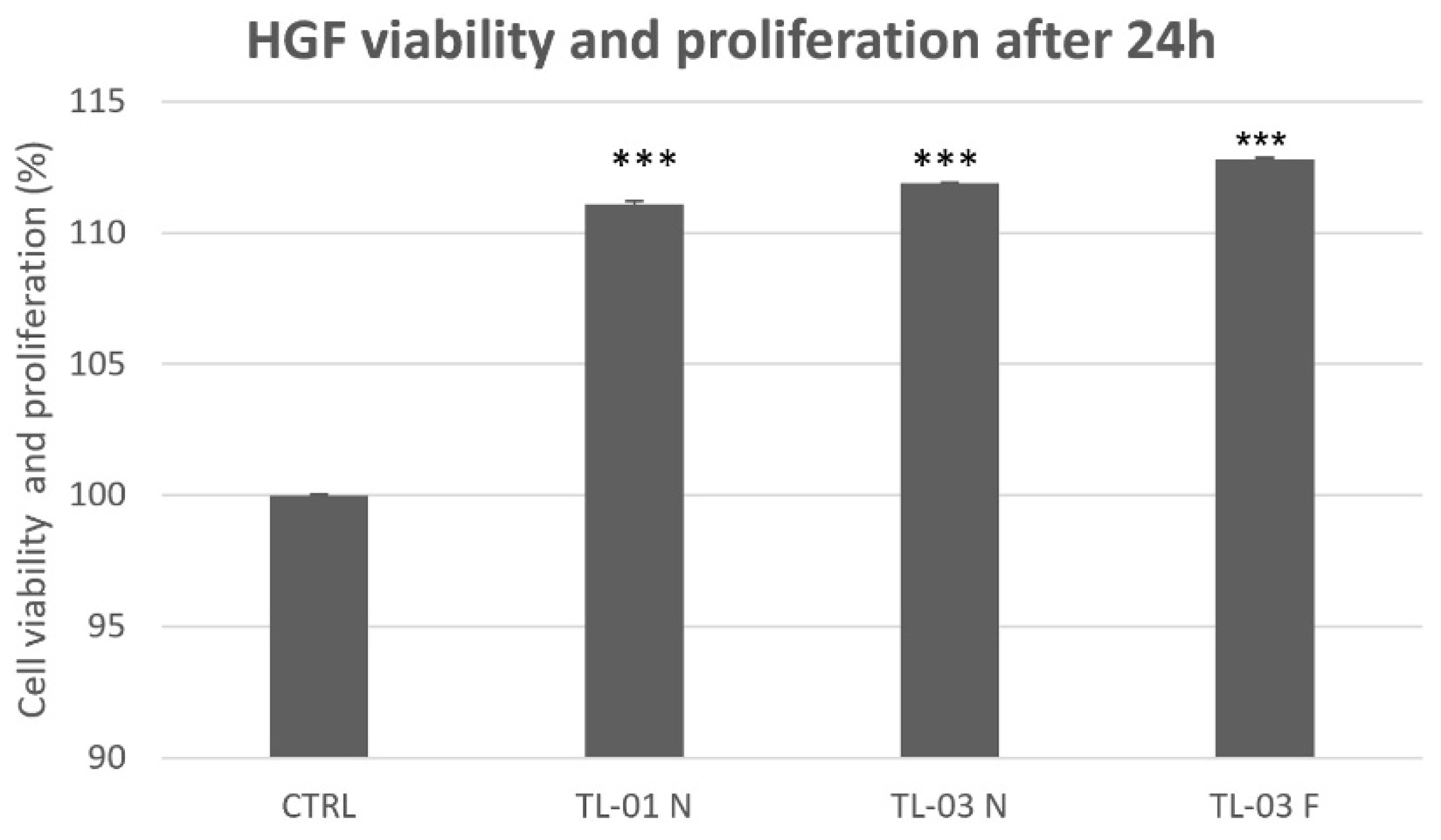
3.4. Viability and Proliferation Assay
3.5. Cellular Morphology Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Praveen, S.; Boppudi, B.S.; Gorre, A.; Bhukya, C.; Abdul, S.N.; Mamillapalli, S.L.; Padmalatha, K. A review on recent advancements in treatment of periodontitis. World J. Pharm. Pharm. Sci. 2020, 9, 940–961. [Google Scholar] [CrossRef]
- Fernandes, T.; Bhavsar, C.; Sawarkar, S.; D’souza, A. Current and novel approaches for control of dental biofilm. Int. J. Pharm. 2018, 536, 199–210. [Google Scholar] [CrossRef]
- Gmurek, M.; Borowska, E.; Schwartz, T.; Horn, H. Does Light-Based Tertiary Treatment Prevent the Spread of Antibiotic Resistance Genes? Perform. Regrowth Future Direction. Sci. Total Environ. 2022, 817, 153001. [Google Scholar] [CrossRef]
- D’Ercole, S.; Spoto, G.; Trentini, P.; Tripodi, D.; Petrini, M. In Vitro Inactivation of Enterococcus Faecalis with a Led Device. J. Photochem. Photobiol. B Biol. 2016, 160, 172–177. [Google Scholar] [CrossRef]
- D’Ercole, S.; di Fermo, P.; di Giulio, M.; Di Lodovico, S.; di Campli, E.; Scarano, A.; Tripodi, D.; Cellini, L.; Petrini, M. Near-Infrared NIR Irradiation and Sodium Hypochlorite: An Efficacious Association to Counteract the Enterococcus Faecalis Biofilm in Endodontic Infections. J. Photochem. Photobiol. B Biol. 2020, 210, 111989. [Google Scholar] [CrossRef]
- Petrini, M.; Spoto, G.; Scarano, A.; D’Arcangelo, C.; Tripodi, D.; di Fermo, P.; D’Ercole, S. Near-Infrared LEDS Provide Persistent and Increasing Protection against E. Faecalis. J. Photochem. Photobiol. B Biol. 2019, 197, 111527. [Google Scholar] [CrossRef]
- Cieplik, F.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Hiller, K.A.; Maisch, T.; Karygianni, L. Antimicrobial photodynamic therapy as an adjunct for treatment of deep carious lesions-A systematic review. Photodiagnosis Photodyn. Ther. 2017, 18, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Meimandi, M.; Talebi Ardakani, M.R.; Esmaeil Nejad, A.; Yousefnejad, P.; Saebi, K.; Tayeed, M.H. The Effect of Photodynamic Therapy in the Treatment of Chronic Periodontitis: A Review of Literature. J. Lasers Med. Sci. 2017, 8, S7–S11. [Google Scholar] [CrossRef]
- Harris, F.; Pierpoint, L. Photodynamic Therapy Based on 5-Aminolevulinic Acid and Its Use as an Antimicrobial Agent. Med. Res. Rev. 2012, 32, 1292–1327. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Evolution of Phthalocyanine Structures as Photodynamic Agents for Bacteria Inactivation. Chem. Rec. 2022, e202100292. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a Novel Gel Containing 5-Aminolevulinic Acid and Red LED against Bacteria Involved in Peri-Implantitis and Other Oral Infections. J. Photochem. Photobiol. B Biol. 2020, 205, 111826. [Google Scholar] [CrossRef]
- Petrini, M.; Mancini, M.; Iezzi, G.; Piattelli, A.; di Campli, E.; D’Ercole, S. Peri-Implantiti: Efficacia Di Un Nuovo Gel a Base Di Acido Delta Aminolevulinico Contro i Batteri Gram Negativi. Dent. Cadmos 2021, 89, 44–51. [Google Scholar] [CrossRef]
- Greco, G.; di Piazza, S.; Chan, J.; Zotti, M.; Hanna, R.; Gheno, E.; Zekiy, A.O.; Pasquale, C.; de Angelis, N.; Amaroli, A. Newly Formulated 5% 5-Aminolevulinic Acid Photodynamic Therapy on Candida Albicans. Photodiagnosis Photodyn. Ther. 2020, 29, 101575. [Google Scholar] [CrossRef]
- Moan, J.; Bech, Ø.; Gaullier, J.-M.; Stokke, T.; Steen, H.B.; Ma, L.; Berg, K. Protoporphyrin IX Accumulation in Cells Treated with 5-Aminolevulinic Acid: Dependence on Cell Density, Cell Size and Cell Cycle. Int. J. Cancer 1998, 75, 134–139. [Google Scholar] [CrossRef]
- Stájer, A.; Kajári, S.; Gajdács, M.; Musah-Eroje, A.; Baráth, Z. Utility of Photodynamic Therapy in Dentistry: Current Concepts. Dent. J. 2020, 8, 43. [Google Scholar] [CrossRef]
- Moreno, I.; Viveros-Méndez, P.X. Modeling the Irradiation Pattern of LEDs at Short Distances. Opt. Express 2021, 29, 6845. [Google Scholar] [CrossRef]
- Nitzan, Y.; Salmon-Divon, M.; Shporen, E.; Malik, Z. ALA Induced Photodynamic Effects on Gram Positive and Negative Bacteria. Photochem. Photobiol. Sci. 2004, 3, 430–435. [Google Scholar] [CrossRef]
- Ramstad, S.; Futsaether, C.M.; Johnsson, A. Porphyrin Sensitization and Intracellular Calcium Changes in the Prokaryote Propionibacterium Acnes. J. Photochem. Photobiol. B Biol. 1997, 40, 141–148. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.; Ridgway, T.D.; Higbee, R.G.; Howard, E.W.; Lucroy, M.D. Effect of Wavelength on Low-Intensity Laser Irradiation-Stimulated Cell Proliferation in Vitro. Lasers Surg. Med. 2005, 36, 8–12. [Google Scholar] [CrossRef]
- Haddad, R. Effect of Photodynamic Therapy on Normal Fibroblasts and Colon Anastomotic Healing in Mice. J. Gastrointest. Surg. 1999, 3, 602–606. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrini, M.; Pierfelice, T.V.; D’Amico, E.; Carlesi, T.; Iezzi, G.; D’Arcangelo, C.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus faecalis and Human Gingival Fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 3048. https://doi.org/10.3390/ijerph19053048
Petrini M, Pierfelice TV, D’Amico E, Carlesi T, Iezzi G, D’Arcangelo C, Di Lodovico S, Piattelli A, D’Ercole S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus faecalis and Human Gingival Fibroblasts. International Journal of Environmental Research and Public Health. 2022; 19(5):3048. https://doi.org/10.3390/ijerph19053048
Chicago/Turabian StylePetrini, Morena, Tania Vanessa Pierfelice, Emira D’Amico, Teocrito Carlesi, Giovanna Iezzi, Camillo D’Arcangelo, Silvia Di Lodovico, Adriano Piattelli, and Simonetta D’Ercole. 2022. "Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus faecalis and Human Gingival Fibroblasts" International Journal of Environmental Research and Public Health 19, no. 5: 3048. https://doi.org/10.3390/ijerph19053048
APA StylePetrini, M., Pierfelice, T. V., D’Amico, E., Carlesi, T., Iezzi, G., D’Arcangelo, C., Di Lodovico, S., Piattelli, A., & D’Ercole, S. (2022). Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus faecalis and Human Gingival Fibroblasts. International Journal of Environmental Research and Public Health, 19(5), 3048. https://doi.org/10.3390/ijerph19053048










