Age, Cognitive Factors, and Acceptance of Living with the Disease in Rheumatoid Arthritis: The Short-Term Perspective
Abstract
:1. Introduction
- Research objective 1: Describe the relationship between age, duration of the disease, and levels of ALD in RA patients.
- Research objective 2: Describe the relationships between modifiable cognitive variables and levels of ALD.
- Research objective 3: Assess the stability of IRBs and CAs in the short term.
- Research objective 4: Identify ALD predictors in RA in the short-term perspective and compare them between different medical diagnoses.
2. Method
2.1. Participants and Procedure
2.2. Questionnaires
Sociodemographic Variables: Gender (Male/Female/Other); Age in Years
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations and Further Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Scharn, M.; Hengel, K.O.; Boot, C.R.L.; Burdorf, A.; Schuring, M.; Van Der Beek, A.J.; Robroek, S.J.W. Influence of chronic diseases on societal participation in paid work, volunteering and informal caregiving in Europe: A 12-year follow-up study. J. Epidemiol. Community Health 2018, 73, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Birk, J.L.; Kronish, I.M.; Moise, N.; Falzon, L.; Yoon, S.; Davidson, K.W. Depression and multimorbidity: Considering temporal characteristics of the associations between depression and multiple chronic diseases. Health Psychol. 2019, 38, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Lotfaliany, M.; Bowe, S.J.; Kowal, P.; Orellana, L.; Berk, M.; Mohebbi, M. Depression and chronic diseases: Co-occurrence and communality of risk factors. J. Affect. Disord. 2018, 241, 461–468. [Google Scholar] [CrossRef]
- de Ridder, D.; Geenen, R.; Kuijer, R.; van Middendorp, H. Psychological adjustment to chronic disease. Lancet 2008, 372, 246–255. [Google Scholar] [CrossRef]
- Beşirli, A.; Alptekin, J.Ö.; Kaymak, D.; Özer, Ö.A. The Relationship Between Anxiety, Depression, Suicidal Ideation and Quality of Life in Patients with Rheumatoid Arthritis. Psychiatr. Q. 2020, 91, 53–64. [Google Scholar] [CrossRef]
- Jang, S.M.; Kim, K.U.; Na, H.J.; Song, S.E.; Lee, S.H.; Lee, H.; Kim, Y.S.; Lee, M.K.; Park, H.-K. Depression is a major determinant of both disease-specific and generic health-related quality of life in people with severe COPD. Chronic Respir. Dis. 2018, 16, 1–8. [Google Scholar] [CrossRef]
- Turan, B.; Rice, W.; Crockett, K.B.; Johnson, M.; Neilands, T.B.; Ross, S.N.; Kempf, M.-C.; Konkle-Parker, D.; Wingood, G.; Tien, P.C.; et al. Longitudinal association between internalized HIV stigma and antiretroviral therapy adherence for women living with HIV. AIDS 2019, 33, 571–576. [Google Scholar] [CrossRef]
- Obiegło, M.; Siennicka, A.; Jankowska, E.A.; Danel, D.P. Direction of the Relationship Between Acceptance of Illness and Health-Related Quality of Life in Chronic Heart Failure Patients. J. Cardiovasc. Nurs. 2017, 32, 348–356. [Google Scholar] [CrossRef]
- Bień, A.; Rzońca, E.; Kańczugowska, A.; Iwanowicz-Palus, G. Factors Affecting the Quality of Life and the Illness Acceptance of Pregnant Women with Diabetes. Int. J. Environ. Res. Public Health 2015, 13, 68. [Google Scholar] [CrossRef]
- Guzek, Z.; Kowalska, J. Analysis of the Degree of Acceptance of Illness Among Patients After a Stroke: An Observational Study. Clin. Interv. Aging 2020, 15, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Bąk, E.; Marcisz, C.; Krzemińska, S.; Dobrzyn-Matusiak, D.; Foltyn, A.; Drosdzol-Cop, A. Relationships of Sexual Dysfunction with Depression and Acceptance of Illness in Women and Men with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2017, 14, 1073. [Google Scholar] [CrossRef] [PubMed]
- Janowski, K.; Steuden, S.; Kuryłowicz, J. Factors accounting for psychosocial functioning in patients with low back pain. Eur. Spine J. 2009, 19, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Paloș, R.; Vîșcu, L. Anxiety, Automatic Negative Thoughts, and Unconditional Self-Acceptance in Rheumatoid Arthritis: A Preliminary Study. Int. Sch. Res. Not. 2014, 2014, 317259. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, D.; Mil, A.H.V.D.H.-V. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2018, 32, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Steer, S. The course of established rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2007, 21, 943–967. [Google Scholar] [CrossRef]
- Welsing, P.M.J.; Fransen, J.; van Riel, P.L.C.M. Is the disease course of rheumatoid arthritis becoming milder?: Time trends since 1985 in an inception cohort of early rheumatoid arthritis. Arthritis Care Res. 2005, 52, 2616–2624. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, X.; An, Y.; Zhang, X.; Han, S.; Li, X.; Wang, L.; Wang, C.; Wang, Y.; Yang, R. Disability and health-related quality of life in Chinese patients with rheumatoid arthritis: A cross-sectional study. Int. J. Rheum. Dis. 2018, 21, 1709–1715. [Google Scholar] [CrossRef]
- Pu, D.; Luo, J.; Wang, Y.; Ju, B.; Lv, X.; Fan, P.; He, L. Prevalence of depression and anxiety in rheumatoid arthritis patients and their associations with serum vitamin D level. Clin. Rheumatol. 2018, 37, 179–184. [Google Scholar] [CrossRef]
- Machin, A.R.; Babatunde, O.; Haththotuwa, R.; Scott, I.; Blagojevic-Bucknall, M.; Corp, N.; Chew-Graham, C.A.; Hider, S.L. The association between anxiety and disease activity and quality of life in rheumatoid arthritis: A systematic review and meta-analysis. Clin. Rheumatol. 2020, 39, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Berner, C.; Erlacher, L.; Fenzl, K.H.; Dorner, T.E. A cross-sectional study on self-reported physical and mental health-related quality of life in rheumatoid arthritis and the role of illness perception. Health Qual. Life Outcomes 2018, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, N.; Wang, L. Moderating role of self-efficacy on the associations of social support with depressive and anxiety symptoms in Chinese patients with rheumatoid arthritis. Neuropsychiatr. Dis. Treat. 2017, 13, 2141–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostova, Z.; Caiata-Zufferey, M.; Schulz, P.J. The process of acceptance among rheumatoid arthritis patients in Switzerland: A qualitative study. Pain Res. Manag. 2014, 19, 61–68. [Google Scholar] [CrossRef]
- Maes, S.; Leventhal, H.; de Ridder, D.T. Coping with chronic diseases. In Handbook of Coping: Theory, Research, Applications; Zeidner, M., Endler, N.S., Eds.; John Wiley: Hoboken, NJ, USA, 1996; pp. 221–251. [Google Scholar]
- Leventhal, H.; Meyer, D.; Nerenz, D. The common sense representation of illness danger. In Contributions to Medical Psychology; Rachman, S., Ed.; Psychology Press: Hove, UK, 1980; Volume 2, pp. 7–30. [Google Scholar]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal and Coping, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Leventhal, H.; Phillips, L.A.; Burns, E. The Common-Sense Model of Self-Regulation (CSM): A dynamic framework for understanding illness self-management. J. Behav. Med. 2016, 39, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Weinman, J.; Petrie, K.J.; Moss-Morris, R.; Horne, R. The illness perception questionnaire: A new method for assessing the cognitive representation of illness. Psychol. Health 1996, 11, 431–445. [Google Scholar] [CrossRef]
- Groeneveld, I.; van der Pas, S.; Meesters, J.; Schuurman, J.; van Meijeren-Pont, W.; Jagersma, E.; Goossens, P.; Kaptein, A.; Vlieland, T.V. Illness perceptions of stroke survivors: Predictors and changes over time—A 1 year follow-up study. J. Psychosom. Res. 2019, 116, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsay, S.-L. Self-efficacy training for patients with end-stage renal disease. J. Adv. Nurs. 2003, 43, 370–375. [Google Scholar] [CrossRef]
- Cooper, V.; Gellaitry, G.; Hankins, M.; Fisher, M.; Horne, R. The influence of symptom experiences and attributions on adherence to highly active anti-retroviral therapy (HAART): A six-month prospective, follow-up study. AIDS Care 2009, 21, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Pankowski, D.; Wytrychiewicz, K.; Janowski, K. Illness-Related Beliefs Scale. 2021. Available online: https://doi.org/10.17605/OSF.IO/JCP2U (accessed on 22 February 2022). [CrossRef]
- Pankowski, D.; Wytrychiewicz, K.; Janowski, K. Illness-Related Appraisal Scale. 2021. Available online: https://doi.org/10.17605/OSF.IO/BYAXS (accessed on 18 February 2022). [CrossRef]
- Janowski, K.; Steuden, S.; Pietrzak, A.; Krasowska, D.; Kaczmarek, Łukasz; Gradus, I.; Chodorowska, G. Social support and adaptation to the disease in men and women with psoriasis. Arch. Dermatol. Res. 2012, 304, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Long, J. Analysis and Presentation of Social Scientific Data. 2021. Available online: https://CRAN.R-project.org/package=jtools (accessed on 7 January 2022).
- Theis, K.A.; Roblin, D.W.; Helmick, C.G.; Luo, R. Prevalence and causes of work disability among working-age U.S. adults, 2011–2013, NHIS. Disabil. Health J. 2018, 11, 108–115. [Google Scholar] [CrossRef]
- Wolf, E.J.; Harrington, K.M.; Clark, S.L.; Miller, M.W. Sample size requirements for structural equation models: An evaluation of power, bias, and solution propriety. Educ. Psychol. Meas. 2013, 73, 913–934. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Soliman, M.M. Arabic Translation and Validation of the Acceptance of Illness Scale and Person-Centered Dermatology Self-care Index. Adv. Ski. Wound Care 2021, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wytrychiewicz, K.; Pankowski, D.; Janowski, K.; Benoit, C.E.; Bargiel-Matusiewicz, K.; Pisula, E.; Walicka, M. The role of beliefs about the impact of illness on fertility and close relationships for psychopathological symptoms in women treated for hypothyroidism. J. Clin. Psychol. 2020, 76, 2314–2328. [Google Scholar] [CrossRef]
- Seligman, M.E.P. Positive Health. Appl. Psychol. 2008, 57, 3–18. [Google Scholar] [CrossRef]
- D’Aloisio, A.A.; Nichols, H.B.; Hodgson, M.E.; Deming-Halverson, S.L.; Sandler, D.P. Validity of self-reported breast cancer characteristics in a nationwide cohort of women with a family history of breast cancer. BMC Cancer 2017, 17, 692. [Google Scholar] [CrossRef] [Green Version]
- Leeb, B.F.; Andel, I.; Sautner, J.; Bogdan, M.; Maktari, A.; Nothnagl, T.; Rintelen, B. Disease activity measurement of rheumatoid arthritis: Comparison of the simplified disease activity index (SDAI) and the disease activity score including 28 joints (DAS28) in daily routine. Arthritis Care Res. 2005, 53, 56–60. [Google Scholar] [CrossRef]
- Tackling the Burden of Chronic Diseases in the USA. Lancet 2009, 373, 185. Available online: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(09)60048-9/fulltext (accessed on 15 January 2022). [CrossRef]
- Raghupathi, W.; Raghupathi, V. An Empirical Study of Chronic Diseases in the United States: A Visual Analytics Approach to Public Health. Int. J. Environ. Res. Public Health 2018, 15, 431. [Google Scholar] [CrossRef] [Green Version]
- Maresova, P.; Javanmardi, E.; Barakovic, S.; Husic, J.B.; Tomsone, S.; Krejcar, O.; Kuca, K. Consequences of chronic diseases and other limitations associated with old age—A scoping review. BMC Public Health 2019, 19, S170–S182. [Google Scholar] [CrossRef]
- Benyamini, Y. Health and illness perceptions. In The Oxford Handbook of Health Psychology; Friedman, H.S., Ed.; Oxford University Press: Oxford, UK, 2011; pp. 281–314. [Google Scholar]
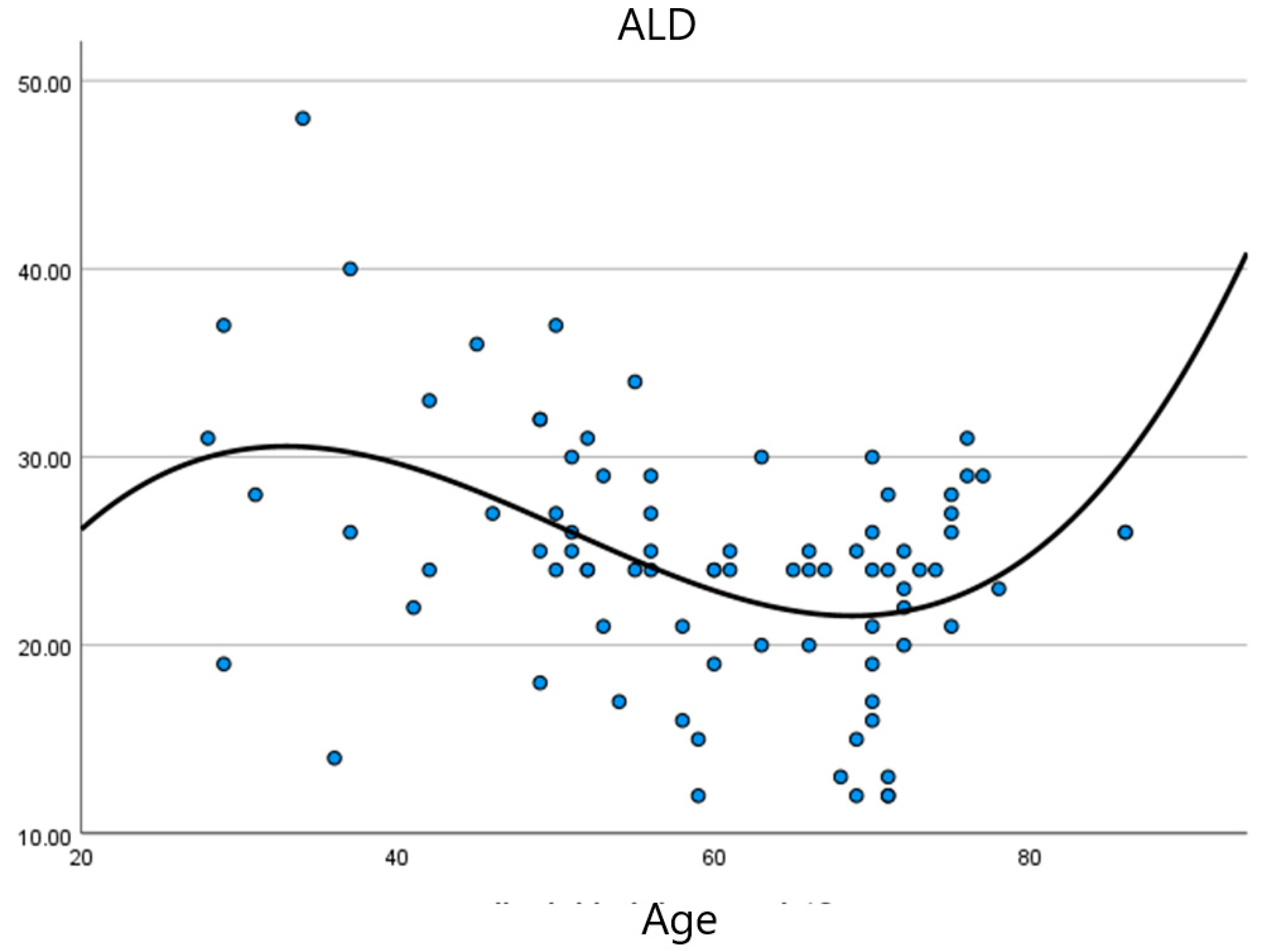
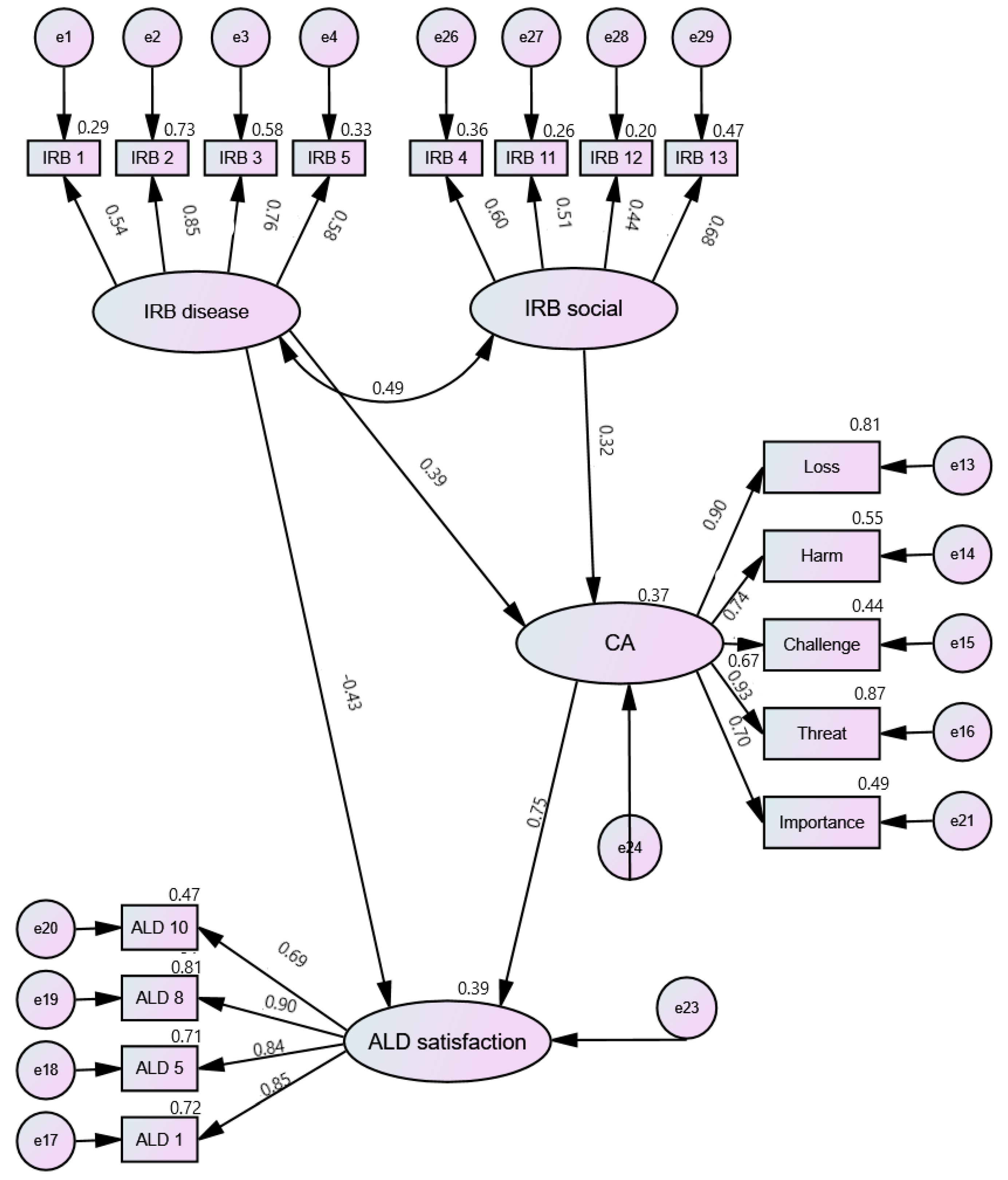
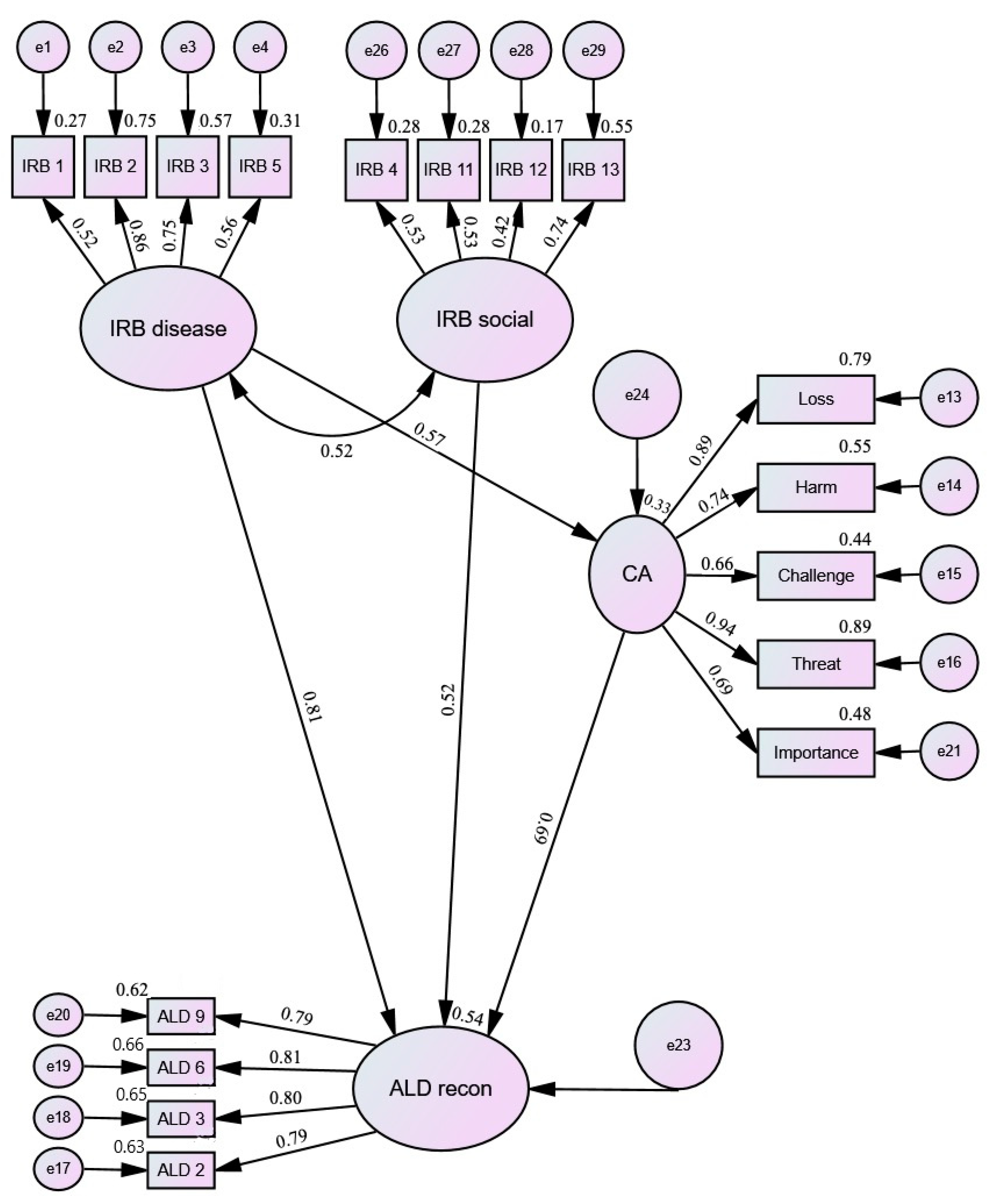
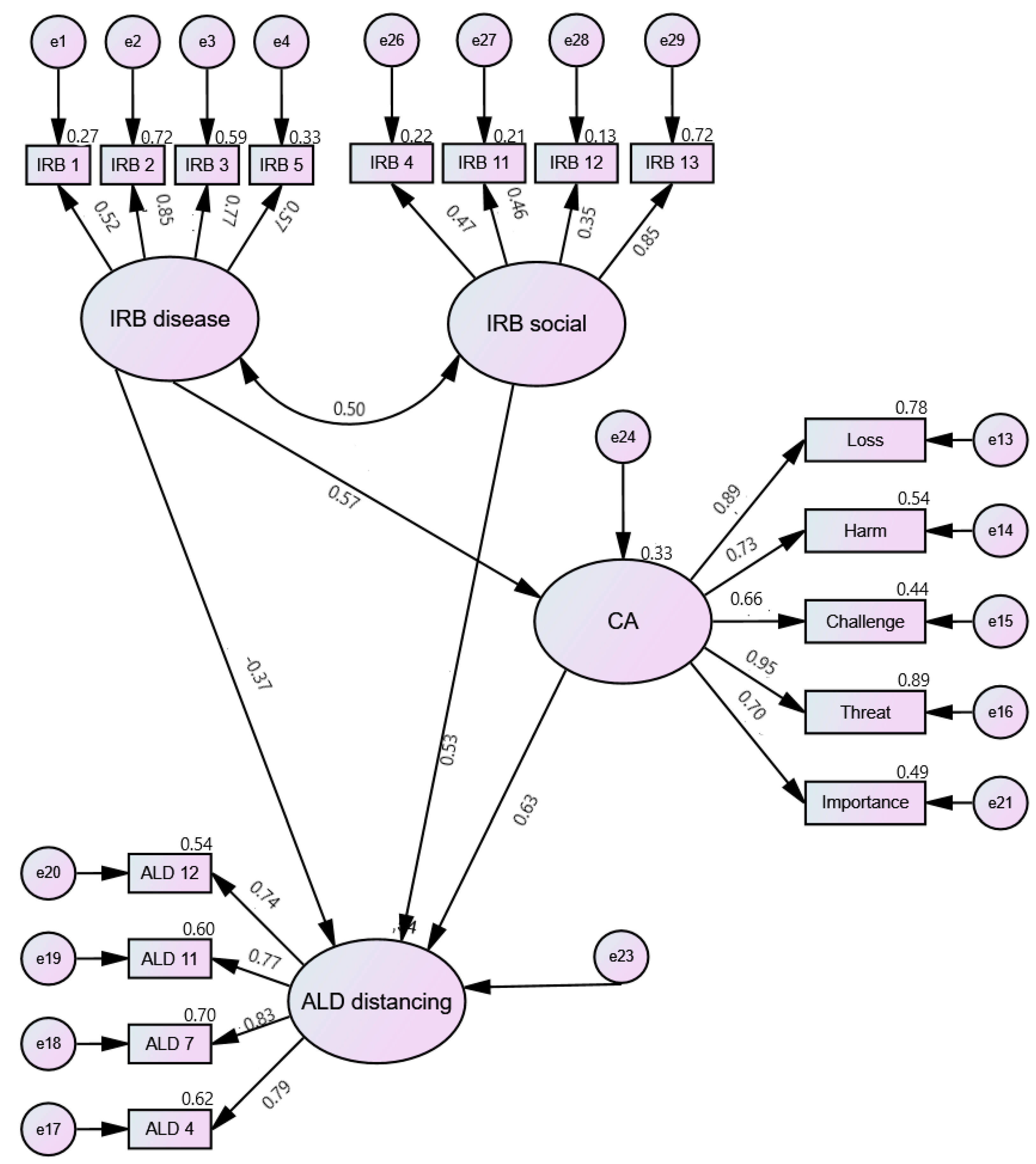
| Latent Variable | χ2 | p | GFI | CFI | TLI | RMSEA |
|---|---|---|---|---|---|---|
| IRB-Disease | 0.86 | >0.05 | 0.995 | 1000 | 1.038 | 0.000 |
| IRB-Social | 4.587 | >0.05 | 0.975 | 0.934 | 0.803 | 0.126 |
| IRB-Control | 3.939 | >0.05 | 0.982 | 1000 | 1.426 | 0.000 |
| CA | 1.102 | >0.05 | 0.995 | 1000 | 1.032 | 0.000 |
| ALD-Reconcilement | 3.228 | >0.05 | 0.982 | 0.993 | 0.979 | 0.087 |
| ALD-Satisfaction | 0.502 | >0.05 | 0.997 | 1000 | 1.024 | 0.000 |
| ALD-Self-distancing | 1.741 | >0.05 | 0.989 | 1000 | 1.005 | 0.000 |
| IRB-Disease | IRB-Control | IRB-Social | CA | ALD-Satisfaction | ALD-Reconcilement | ALD-Distancing | |
|---|---|---|---|---|---|---|---|
| IRB 1 | 0.58 | ||||||
| IRB 2 | 0.77 | ||||||
| IRB 3 | 0.83 | ||||||
| IRB 5 | 0.58 | ||||||
| IRB 6 | −0.02 | ||||||
| IRB 7 | 0.26 | ||||||
| IRB 8 | 0.25 | ||||||
| IRB 9 | 0.6 | ||||||
| IRB 10 | 0.44 | ||||||
| IRB 4 | 0.55 | ||||||
| IRB 11 | 0.56 | ||||||
| IRB 12 | 0.53 | ||||||
| IRB 13 | 0.64 | ||||||
| CA: Loss | 0.91 | ||||||
| CA: Harm | 0.75 | ||||||
| CA: Challenge | 0.67 | ||||||
| CA: Threat | 0.92 | ||||||
| CA: Importance | 0.68 | ||||||
| ALD 1 | 0.86 | ||||||
| ALD 5 | 0.85 | ||||||
| ALD 8 | 0.89 | ||||||
| ALD 10 | 0.68 | ||||||
| ALD 2 | 0.81 | ||||||
| ALD 3 | 0.82 | ||||||
| ALD 6 | 0.84 | ||||||
| ALD 9 | 0.78 | ||||||
| ALD 4 | 0.78 | ||||||
| ALD 7 | 0.85 | ||||||
| ALD 11 | 0.78 | ||||||
| ALD 12 | 0.75 | ||||||
| ALD Subscales | χ2 | df | p | Normed χ2 Value | CFI | TLI | AIC | BIC |
|---|---|---|---|---|---|---|---|---|
| ALD-Reconcilement | 198.769 | 114 | <0.001 | 1.744 | 0.877 | 0.854 | 276.769 | 371.103 |
| ALD-Satisfaction | 186.682 | 114 | <0.001 | 1.638 | 0.896 | 0.876 | 264.682 | 359.017 |
| ALD-Distancing | 177.89 | 114 | <0.001 | 1.56 | 0.903 | 0.884 | 255.89 | 350.225 |
| Variables | T1 | T2 | t | p | Cohen’s d | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| IRB 1 | 8.76 | 1.65 | 8.32 | 2.03 | 2.137 | 0.035 | 0.194 |
| IRB 2 | 7.83 | 1.97 | 7.54 | 2.15 | 1.424 | 0.157 | 0.129 |
| IRB 3 | 7.36 | 2.06 | 7.06 | 2.18 | 1.700 | 0.092 | 0.155 |
| IRB 4 | 4.76 | 2.70 | 4.58 | 2.81 | 0.800 | 0.425 | 0.073 |
| IRB 5 | 6.40 | 2.42 | 6.39 | 2.47 | 0.079 | 0.937 | 0.007 |
| IRB 6 | 7.83 | 1.75 | 7.93 | 1.81 | −0.696 | 0.488 | −0.063 |
| IRB 7 | 4.34 | 2.86 | 4.69 | 2.76 | −1.772 | 0.079 | −0.161 |
| IRB 8 | 5.07 | 2.69 | 5.06 | 2.74 | 0.061 | 0.952 | 0.006 |
| IRB 9 | 5.34 | 2.66 | 5.12 | 2.52 | 0.880 | 0.380 | 0.080 |
| IRB 10 | 4.88 | 2.29 | 5.07 | 2.30 | −0.745 | 0.458 | −0.068 |
| IRB 11 | 3.82 | 2.28 | 4.09 | 2.44 | −1.328 | 0.187 | −0.121 |
| IRB 12 | 2.69 | 2.43 | 3.12 | 2.77 | −2.298 | 0.023 | −0.209 |
| IRB 13 | 4.31 | 2.47 | 4.53 | 2.38 | −1.036 | 0.302 | −0.094 |
| Loss | 14.31 | 5.09 | 13.95 | 5.25 | 1.060 | 0.291 | 0.096 |
| Harm | 12.06 | 5.09 | 12.03 | 5.43 | 0.077 | 0.938 | 0.007 |
| Benefit | 7.79 | 3.15 | 8.55 | 4.16 | −2.577 | 0.011 | −0.234 |
| Challenge | 16.01 | 4.57 | 15.55 | 4.71 | 1.433 | 0.154 | 0.130 |
| Value | 13.35 | 4.51 | 13.64 | 4.66 | −1.025 | 0.308 | −0.093 |
| Threat | 15.56 | 4.25 | 14.88 | 4.93 | 2.134 | 0.035 | 0.194 |
| Importance | 16.07 | 4.27 | 15.93 | 4.59 | 0.381 | 0.704 | 0.035 |
| ALD-Satisfaction | 7.88 | 2.25 | 8.12 | 2.38 | −1.745 | 0.084 | −0.159 |
| ALD-Reconcilement | 6.99 | 1.88 | 7.06 | 1.92 | −0.487 | 0.627 | −0.044 |
| ALD-Distancing | 8.75 | 2.35 | 9.04 | 2.72 | −1.434 | 0.154 | −0.130 |
| ALD Global Score | 23.63 | 5.77 | 24.22 | 6.20 | −1.517 | 0.132 | −0.138 |
| Statistically Significant Predictors | R2 for the Model | Adjusted R2 for the Model | β | t | p | |
|---|---|---|---|---|---|---|
| Acceptance: Global score | ||||||
| All: (B = 0.943; F = 7.465; p < 0.001) | Importance (T2-T1) | 0.161 | 0.139 | 0.289 | 3.359 | 0.001 |
| Belief about control over the onset of the disease (T2-T1) | −0.193 | −2.251 | 0.026 | |||
| Benefit (T2-T1) | −0.169 | −1.982 | 0.050 | |||
| RA: (B = 0.578; F = 10.829; p = 0.002) | Threat (T2-T1) | 0.139 | 0.126 | 0.373 | 3.291 | 0.002 |
| VD: (B = 3.405; F = 8.120; p = 0.002) | Belief regarding one’s condition improving (T1) | 0.414 | 0.363 | −0.656 | −3.824 | 0.001 |
| Threat (T1) | 0.442 | 2.580 | 0.017 | |||
| D: (B = 1.068; F = 18.738; p < 0.001) | Importance (T2-T1) | 0.438 | 0.415 | 0.662 | 4.329 | 0.000 |
| Satisfaction | ||||||
| All (B = 0.551; F = 5.405; p < 0.001) | Belief regarding the possibility of predicting the course of the disease (T2-T1) | 0.157 | 0.128 | 0.152 | 1.732 | 0.086 |
| Belief about control over the onset of the disease (T2-T1) | −0.177 | −2.043 | 0.043 | |||
| Female gender | −0.204 | −2.355 | 0.020 | |||
| Importance (T2-T1) | 0.175 | 2.004 | 0.047 | |||
| RA (B = −3.251; F = 5.899; p < 0.001) | Benefit (T1) | 0.214 | 0.178 | 0.379 | 3.290 | 0.002 |
| The belief about the duration of the disease (T1) | 0.309 | 2.660 | 0.010 | |||
| Threat (T2-T1) | 0.260 | 2.343 | 0.022 | |||
| Vascular (B = 0.615; F = 18.915; p < 0.001) | Belief regarding the possibility of predicting the course of the disease (T2-T1) | 0.622 | 0.589 | 0.715 | 5.574 | 0.000 |
| Female gender | −0.358 | −2.791 | 0.010 | |||
| Diabetes (B = −0.015; F = 9.963; p < 0.001) | Belief regarding how embarrassing the disease is (T1) | 0.576 | 0.518 | 0.535 | 3.850 | 0.001 |
| Number of hospitalizations | −0.438 | −3.094 | 0.005 | |||
| Belief about control over the onset of the disease (T2-T1) | −0.407 | −2.876 | 0.009 | |||
| Reconcilement | ||||||
| All (B = 0.141; F = 12.732; p < 0.001) | Belief about control over the onset of the disease (T2-T1) | 0.097 | 0.089 | −0.311 | −3.568 | 0.001 |
| RA (B = 0.126; F = 14.543; p < 0.001) | Threat (T2-T1) | 0.178 | 0.166 | 0.422 | 3.814 | 0.000 |
| Vascular (B = 0.089; F = 4.875; p = 0.037) | Belief about control over the onset of the disease (T2-T1) | 0.169 | 0.134 | −0.411 | −2.208 | 0.037 |
| Diabetes (B = −0.193; F = 12.408; p < 0.001) | Importance (T2-T1) | 0.519 | 0.477 | 0.658 | 4.549 | 0.000 |
| Duration of the disease | 0.324 | 2.236 | 0.035 | |||
| Distancing | ||||||
| All (B = −0.096; F = 8.361; p < 0.001) | Importance (T2-T1) | 0.177 | 0.155 | 0.352 | 4.112 | 0.000 |
| Benefit (T2-T1) | −0.224 | −2.658 | 0.009 | |||
| Duration of the disease | 0.189 | 2.216 | 0.029 | |||
| RA (B = −2.247; F = 6.101; p < 0.001) | Importance (T2-T1) | 0.371 | 0.310 | 0.308 | 2.614 | 0.011 |
| Number of hospitalizations | −0.276 | −2.695 | 0.009 | |||
| Female gender | −0.277 | −2.694 | 0.009 | |||
| Belief about knowledge about a disease (T1) | 0.272 | 2.611 | 0.011 | |||
| Benefit (T1) | 0.247 | 2.375 | 0.021 | |||
| Threat (T2-T1) | 0.253 | 2.197 | 0.032 | |||
| Vascular (B = −1.480; F = 6.535; p = 0.006) | Belief regarding one’s condition improving (T1) | 0.362 | 0.307 | −0.432 | −2.595 | 0.016 |
| Age | 0.417 | 2.505 | 0.020 | |||
| Diabetes (B = 0.357; F = 16.051; p < 0.001) | Importance (T2-T1) | 0.583 | 0.546 | 0.596 | 4.236 | 0.000 |
| Loss (T2-T1) | 0.333 | 2.367 | 0.027 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pankowski, D.; Wytrychiewicz-Pankowska, K.; Pisula, E.; Fal, A.; Kisiel, B.; Kamińska, E.; Tłustochowicz, W. Age, Cognitive Factors, and Acceptance of Living with the Disease in Rheumatoid Arthritis: The Short-Term Perspective. Int. J. Environ. Res. Public Health 2022, 19, 3136. https://doi.org/10.3390/ijerph19053136
Pankowski D, Wytrychiewicz-Pankowska K, Pisula E, Fal A, Kisiel B, Kamińska E, Tłustochowicz W. Age, Cognitive Factors, and Acceptance of Living with the Disease in Rheumatoid Arthritis: The Short-Term Perspective. International Journal of Environmental Research and Public Health. 2022; 19(5):3136. https://doi.org/10.3390/ijerph19053136
Chicago/Turabian StylePankowski, Daniel, Kinga Wytrychiewicz-Pankowska, Ewa Pisula, Andrzej Fal, Bartłomiej Kisiel, Ewa Kamińska, and Witold Tłustochowicz. 2022. "Age, Cognitive Factors, and Acceptance of Living with the Disease in Rheumatoid Arthritis: The Short-Term Perspective" International Journal of Environmental Research and Public Health 19, no. 5: 3136. https://doi.org/10.3390/ijerph19053136






