Liver Disease Screening and Hepatitis C Virus Elimination in Taiwan Rural Indigenous Townships: Village-By-Village Screening and Linking to Outreach Hepatology Care
Abstract
:1. Introduction
2. Materials and Methods
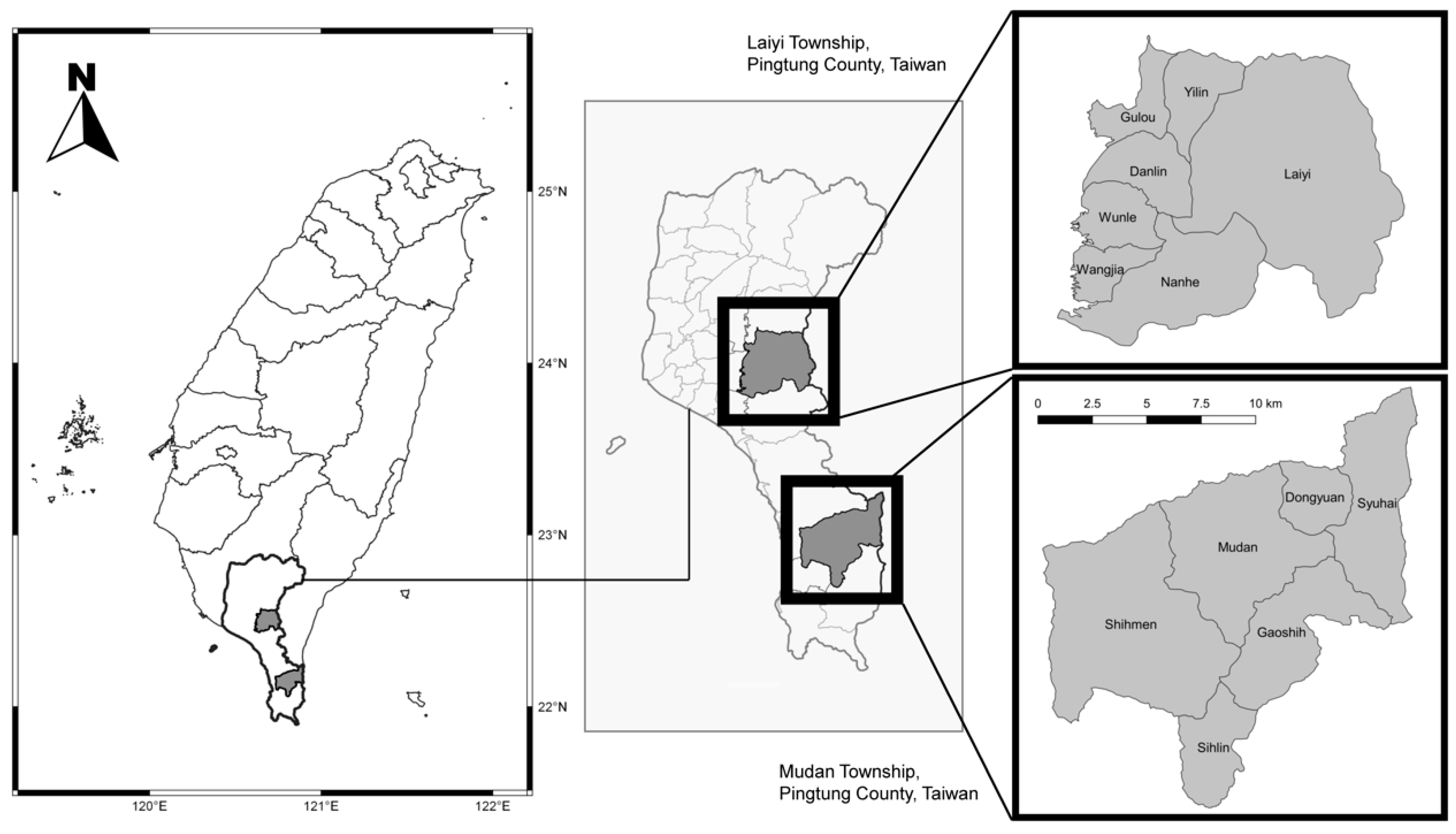
2.1. Study Sites and Participants
2.2. Community-Based Screening and Links to Accessible Care
2.3. Statistics
3. Results
3.1. Resident and Screen Coverage Rate
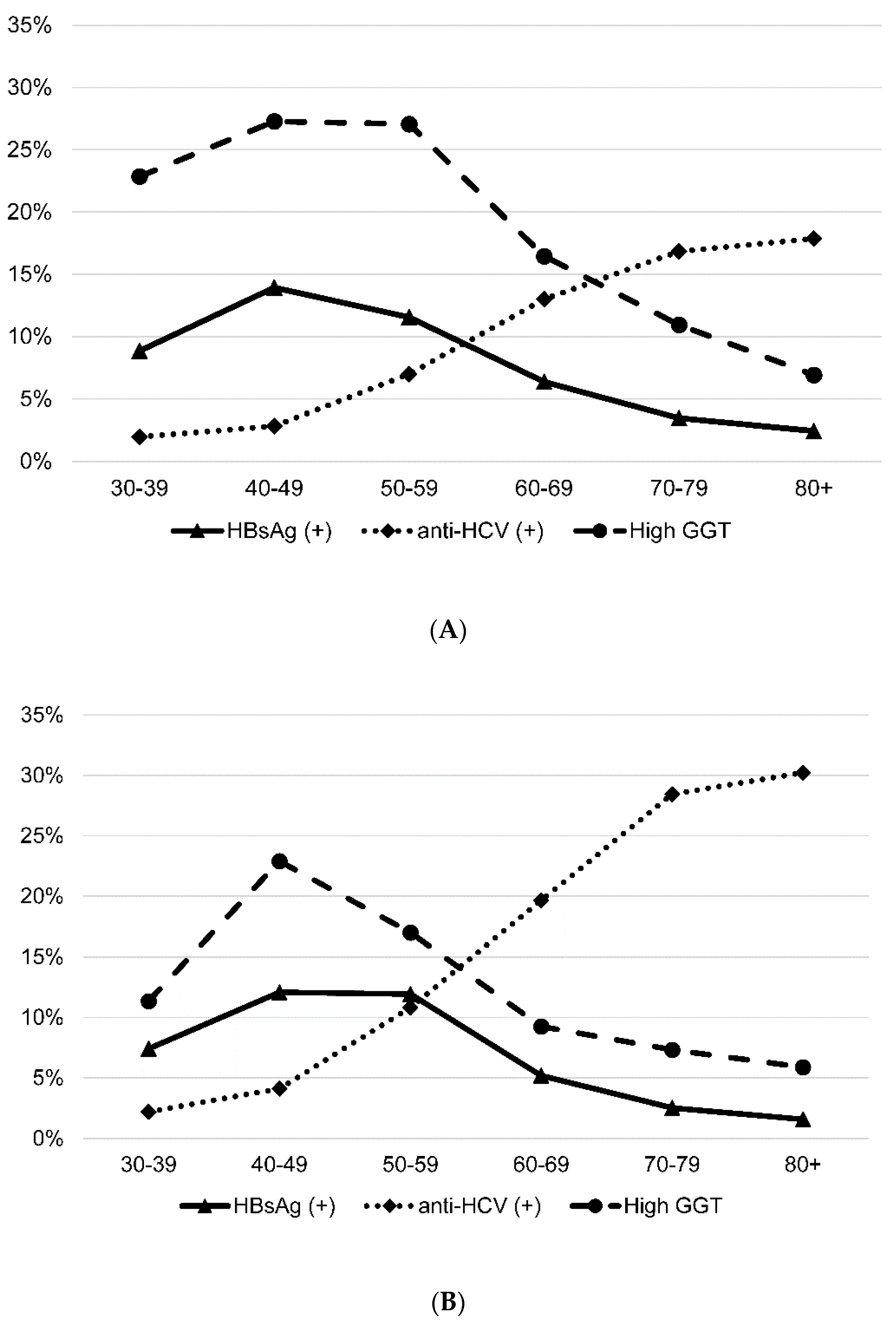
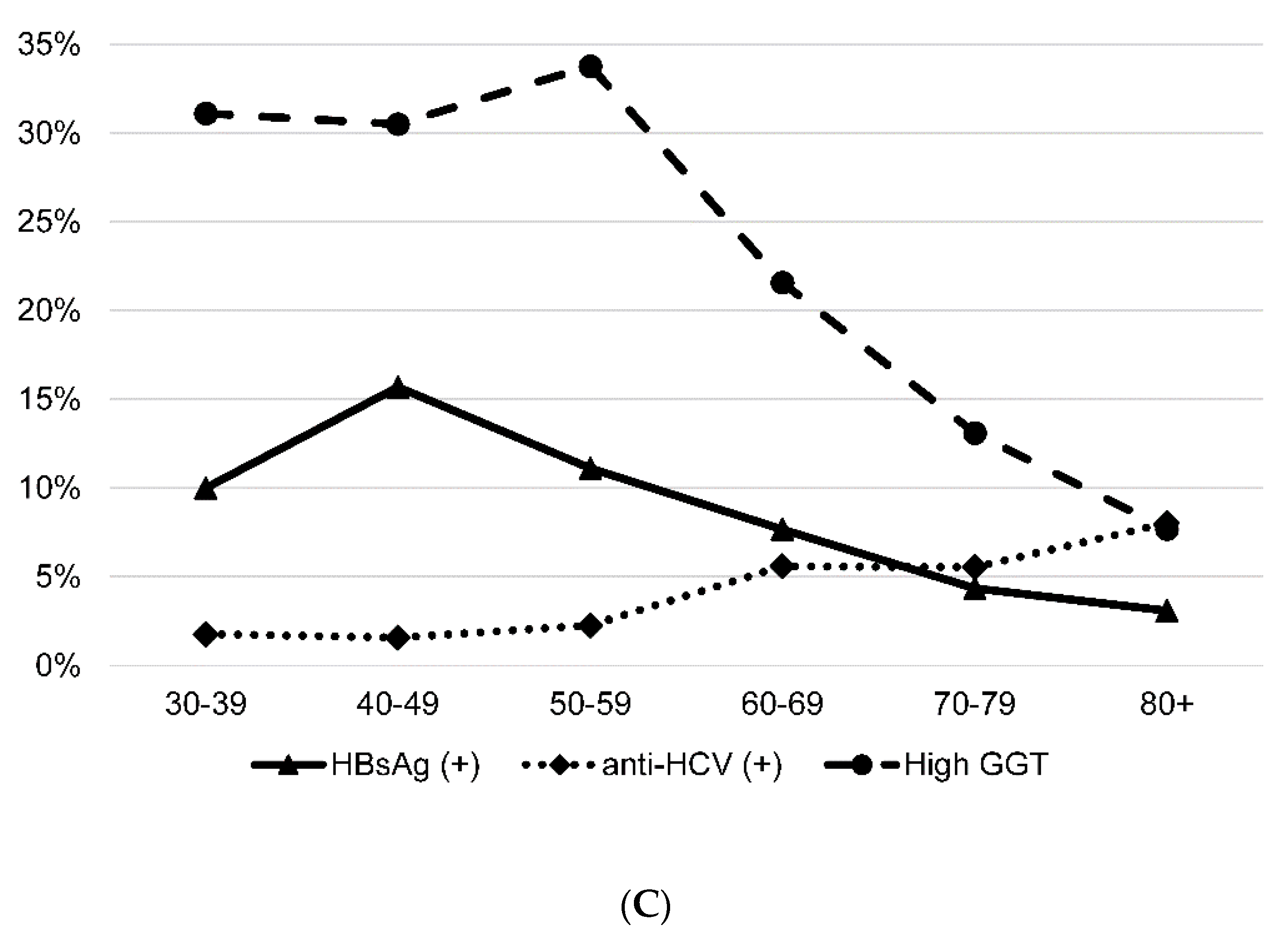
3.2. Prevalence of HBsAg, Anti-HCV and High GGT Level
3.3. HCV Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Yang, H.I.; Lu, S.N.; Jen, C.-L.; You, S.-L.; Wang, L.-Y.; Wang, C.-H.; Chen, W.J.; Chen, C.-J.; for the R.E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: A community-based long-term prospective study. J. Infect. Dis. 2012, 206, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The polaris observatory HCV collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, L.; Peacock, A.; Colledge, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, e1192–e1207. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Dore, G.J.; Matthews, G.; Luhmann, N.; Macdonald, V.; Bajis, S.; Baggaley, R.; Mathers, B.; Verster, A.; Grulich, E.A. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 39–56. [Google Scholar] [CrossRef]
- Jadoul, M.; Bieber, B.A.; Martin, P.; Kiba, T.; Nwankwo, C.; Arduino, J.M.; Goodkin, D.A.; Pisoni, R.L. Prevalence, incidence, and risk factors for hepatitis C virus infection in hemodialysis patients. Kidney Int. 2019, 95, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.L.; Chen, P.J.; Dai, C.Y.; Hu, T.-H.; Huang, C.-F.; Huang, Y.-H.; Hung, C.-H.; Liu, C.-H.; Liu, C.-J.; Peng, C.-Y.; et al. 2020 Taiwan consensus statement on the management of hepatitis C: Part (I) general population. J. Formos. Med. Assoc. 2020, 119, 1019–1040. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, C.L.; Chen, J.W.; Hsu, N.; Wei, S.; Hou, S.; Lu, S.; Chen, P. Secular trends and geographic maps of hepatitis C virus infection among 4 million blood donors in Taiwan from 1999 to 2017. Hepatol. Commun. 2020, 4, 1193–1205. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sectors Strategy on Viral Hepatitis 2016–2021: Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Wu, G.H.; Pwu, R.F.; Chen, S.C. Achieving hepatitis C elimination in Taiwan-Overcoming barriers by setting feasible strategies. J. Formos. Med. Assoc. 2018, 117, 1044–1045. [Google Scholar] [CrossRef]
- Taiwan Hepatitis C Policy Guideline 2018–2025. Available online: https://www.mohw.gov.tw/cp-4464-52812-1.html (accessed on 1 February 2022).
- Cheng, A.T.; Chen, W.J. Alcoholism among for aboriginal groups in Taiwan: High prevalences and their implications. Alcohol Clin. Exp. Res. 1995, 19, 81–91. [Google Scholar] [CrossRef]
- Juan, S.C.; Awerbuch-Friedlander, T.; Levins, R. Ethnic density and mortality: Aboriginal population health in Taiwan. Public Health Rev. 2016, 37, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Yang, P.M.; Huang, G.T.; Lee, H.S.; Sung, J.L.; Sheu, J.C. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J. Formos. Med. Assoc. 2007, 106, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.C.; Ko, Y.C.; Chen, C.J.; Wu, C.-C.; Chen, E.-R.; Liaw, Y.-F.; Hwang, S.-J. Seroepidemiological studies on hepatitis B and D viruses infection among five ethnic groups in southern Taiwan. J. Med. Virol. 1988, 26, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Nien, H.C.; Sheu, J.C.; Kao, J.H.; Chou, H.C.; Su, C.W.; Chen, C.H. Aboriginal Taiwanese hepatitis B carriers have more favorable viral factors than Han Chinese carriers. J. Med. Virol. 2011, 83, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Kao, J.H. Global perspective on the natural history of chronic hepatitis B: Role of hepatitis B virus genotypes A to J. Semin. Liver Dis. 2013, 33, 97–102. [Google Scholar] [CrossRef]
- European association for the study of the liver. EASL Recommendations on treatment of hepatitis C, 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.F.; Kee, K.M.; Chen, Y.D.; Tsai, L.S.; Shih, L.Y.; Lin, L.C.; Chen, T.H.H.; Lu, S.N. Village distribution and geographic variations of the prevalence of chronic hepatitis B, C and hypertransaminemia: An analysis of adult health examinations in 520 villages of Tainan County, Taiwan. J. Intern. Med. Taiwan 2006, 17, 276–290. [Google Scholar]
- Wu, J.S.; Lu, C.F.; Chou, W.H.; Chen, H.-Y.; Lee, H.-F.; Wu, Y.-C.; Lin, S.-Y. High prevalence of hepatitis C virus infection in aborigines in Taiwan. Jpn. J. Med. Sci. Biol. 1992, 45, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.J.; Chen, H.C.; Ying, J.; Lu, C.F.; Ko, Y.C. Risk factors of hepatitis C virus infection in a Taiwanese aboriginal community. Kaohsiung J. Med. Sci. 1996, 12, 241–247. [Google Scholar]
- Lin, H.H.; Li, Y.H.; Yu, J.H.; Wang, Y.W.; Lua, A.C.; Huang, L.C.; Huang, S.C.; Lee, M.L. Ethnic and geographic variations in the prevalence of hepatitis A, B and C among aboriginal villages in Hualien, Taiwan. Infection 2000, 28, 205–208. [Google Scholar] [CrossRef]
- Wang, J.H.; Chen, C.H.; Chang, C.M.; Feng, W.C.; Lee, C.Y.; Lu, S.N. Hepatitis C virus core antigen is cost-effective in community-based screening of active hepatitis C infection in Taiwan. J. Formos. Med. Assoc. 2020, 119, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Chang, K.C.; Chen, W.M.; Hsu, N.-T.; Lee, C.-Y.; Lin, Y.-C.; Huang, W.-C.; Chiu, W.-N.; Hu, J.-H.; Huang, T.-J.; et al. Village-to-village screening for hepatitis B and C using quantitative HBsAg and anti-HCV testing with reflex HCV core antigen tests in the remote communities of a resource-rich setting: A population-based prospective cohort study. BMJ Open 2021, 11, e046115. [Google Scholar] [CrossRef] [PubMed]
- Morales-Arraez, D.; Hernandez-Guerra, M.; Diaz-Flores, F.; Nieto-Bujalance, Y.; Garcia-Dopico, J.; Jimenez, A.; Quintero, E. Hepatitis C virus media coverage favorably impacts on antibody testing in the non-interferon era. J. Public Health 2021, 43, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Amoako, A.; Ortiz-Paredes, D.; Engler, K.; Lebouché, B.; Klein, M.B. Patient and provider perceived barriers and facilitators to direct acting antiviral hepatitis C treatment among priority populations in high income countries: A knowledge synthesis. Int. J. Drug Policy 2021, 96, 103247. [Google Scholar] [CrossRef]
- Radley, A.; Robinson, E.; Aspinall, E.J.; Angus, K.; Tan, L.; Dillon, J.F. A systematic review and meta-analysis of community and primary-care-based hepatitis C testing and treatment services that employ direct acting antiviral drug treatments. BMC Health Serv. Res. 2019, 19, 765. [Google Scholar] [CrossRef] [Green Version]
- Syed, T.A.; Bashir, M.H.; Farooqui, S.M.; Chen, A.; Chen, S.; Nusrat, S.; Fazili, J. Treatment outcomes of hepatitis C-infected patients in specialty clinic vs. primary care physician clinic: A comparative analysis. Gastroenterol. Res. Pract. 2019, 2019, 8434602. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Wu, C.H.; Chen, H.L.; Lin, I.-T.; Chen, M.-J.; Wang, T.-E.; Wang, H.-Y.; Shih, S.-C.; Bair, M.-J. Hepatitis C treatment outcome in relation to alcohol consumption and racial differences in southeastern Taiwan. J. Formos. Med. Assoc. 2015, 114, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- King, M.; Smith, A.; Gracey, M. Indigenous health part 2: The underlying causes of the health gap. Lancet 2009, 374, 76–85. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lee, H.S.; Huang, L.C.; Tsai, K.S.; Chen, D.S.; Cheng, A.T. Alcoholism, hepatitis B and C viral infections, and impaired liver function among Taiwanese aboriginal groups. Am. J. Epidemiol. 1996, 143, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.H.; Wang, L.Y.; Shaw, C.K.; Cheng, M.-L.; Chung, W.-K.; Chiang, H.-J.; Lin, T.-Y.; Chen, C.-J. Combined effects of chronic hepatitis virus infections and substance-use habits on chronic liver diseases in Taiwanese aborigines. J. Formos. Med. Assoc. 2002, 101, 826–834. [Google Scholar]
- Conigrave, K.M.; Davies, P.; Haber, P.; Whitfield, J.B. Traditional markers of excessive alcohol use. Addiction 2003, 98 (Suppl. S2), 31–43. [Google Scholar] [CrossRef] [PubMed]
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and treatment of alcohol-associated liver diseases—2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total (n = 3503) | Laiyi (n = 1784) | Mudan (n = 1719) | |
|---|---|---|---|
| Age (year, mean ± SD) | 59.7 ± 13.7 | 59.6 ± 13.1 | 59.9 ± 14.3 |
| Sex (%) | |||
| Male | 1445 (41.3) | 719 (40.3) | 726 (42.2) |
| Female | 2058 (58.7) | 1065 (59.7) | 993 (57.8) |
| Screen coverage rate (%) | 41.8 | 35.9 | 50.6 |
| Adjusted screen coverage rate (%) | 73.5 | 69.0 # | 78.9 ## |
| Positive HBsAg (%) | 8.2 | 131/1739 (7.5) * | 154/1719 (9.0) * |
| Positive Anti-HCV (%) | 10.0 | 281/1784 (15.8) ** | 70/1717 (4.1) ** |
| Hight GGT (%) | 19.5 | 114/900 (12.7) *** | 316/1302 (24.3) *** |
| Coverage Rate | HBsAg (+) | Anti-HCV(+) | High GGT | |
|---|---|---|---|---|
| Laiyi township | ||||
| Gender-specific (%) | ||||
| Male | 719/2467 (29.1) | 70/697 (10.0) | 92/719 (12.8) | 92/393 (23.4) |
| Female | 1065/2507 (42.5) | 61/1042 (5.9) | 189/1065 (17.7) | 22/507 (4.3) |
| p-value | <0.001 | 0.001 | 0.005 | <0.001 |
| Village-specific (%) | ||||
| Danlin | 234/577 (40.6) | 17/222 (7.7) | 68/234 (29.1) | 17/123 (13.8) |
| Wunle | 228/677 (33.7) | 12/224 (5.4) | 15/228 (6.6) | 8/103 (7.8) |
| Gulou | 298/885 (33.7) | 26/279 (9.3) | 83/298 (27.9) | 24/168 (14.3) |
| Laiyi | 271/737 (36.8) | 22/268 (8.2) | 62/271 (22.9) | 15/131 (11.5) |
| Nanhe | 364/945 (38.5) | 40/363 (11.0) | 12/364 (3.3) | 25/152 (16.4) |
| Wangjia | 200/689 (29.0) | 5/199 (2.5) | 7/200 (3.5) | 11/117 (9.4) |
| Yilin | 189/464 (40.7) | 9/184 (4.9) | 34/189 (18.0) | 14/106 (13.2) |
| p-value | <0.001 | 0.005 | <0.001 | 0.415 |
| Mudan township | ||||
| Gender-specific (%) | ||||
| Male | 726/1687 (43.0) | 80/726 (11.0) | 31/726 (4.3) | 176/546 (32.2) |
| Female | 993/1711 (58.0) | 74/993 (7.5) | 39/991 (3.9) | 140/756 (18.5) |
| p-value | <0.001 | 0.011 | 0.729 | <0.001 |
| Village-specific (%) | ||||
| Sihlin | 214/463 (46.2) | 12/214 (5.6) | 12/214 (5.6) | 55/175 (31.4) |
| Shihmen | 665/1279 (52.0) | 66/665 (9.9) | 23/664 (3.5) | 93/445 (20.9) |
| Syuhai | 168/317 (53.0) | 17/168 (10.1) | 7/168 (4.2) | 39/141 (27.7) |
| Mudan | 290/543 (53.4) | 29/290 (10.0) | 7/289 (2.4) | 61/245 (24.9) |
| Dongyuan | 174/355 (49.0) | 22/174 (12.6) | 17/174 (9.8) | 41/157 (26.1) |
| Gaoshih | 208/441 (47.2) | 8/208 (3.8) | 4/208 (1.9) | 27/139 (19.4) |
| p-value | 0.093 | 0.016 | 0.001 | 0.059 |
| Total (n = 121) | Laiyi (n = 95) | Mudan (n = 26) | |
|---|---|---|---|
| Age (year, mean ± SD) | 69.1 ± 11.6 | 69.6 ± 11.3 | 67.2 ± 12.6 |
| Sex (%) | |||
| Male | 44 (36.4) | 31 (32.6) | 13 (50) |
| Female | 77 (63.6) | 64 (67.4) | 13 (50) |
| DAA # (%) | 116 (95.9) | 93 (97.9) | 23 (88.5) |
| SVR ## (%) | |||
| IT | 111 (91.7) | 88 (92.6) | 23 (88.5) |
| PP | 111 (95.7) | 88 (94.6) | 23 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tien, H.-M.; Cheng, T.-C.; Lien, H.-C.; Yang, K.-F.; Shy, C.-G.; Chen, Y.-L.; Hsu, N.-T.; Lu, S.-N.; Wang, J.-H. Liver Disease Screening and Hepatitis C Virus Elimination in Taiwan Rural Indigenous Townships: Village-By-Village Screening and Linking to Outreach Hepatology Care. Int. J. Environ. Res. Public Health 2022, 19, 3269. https://doi.org/10.3390/ijerph19063269
Tien H-M, Cheng T-C, Lien H-C, Yang K-F, Shy C-G, Chen Y-L, Hsu N-T, Lu S-N, Wang J-H. Liver Disease Screening and Hepatitis C Virus Elimination in Taiwan Rural Indigenous Townships: Village-By-Village Screening and Linking to Outreach Hepatology Care. International Journal of Environmental Research and Public Health. 2022; 19(6):3269. https://doi.org/10.3390/ijerph19063269
Chicago/Turabian StyleTien, Hui-Min, Tai-Chung Cheng, Hsiao-Chu Lien, Kuei-Fei Yang, Cherng-Gueih Shy, Yu-Ling Chen, Nien-Tzu Hsu, Sheng-Nan Lu, and Jing-Houng Wang. 2022. "Liver Disease Screening and Hepatitis C Virus Elimination in Taiwan Rural Indigenous Townships: Village-By-Village Screening and Linking to Outreach Hepatology Care" International Journal of Environmental Research and Public Health 19, no. 6: 3269. https://doi.org/10.3390/ijerph19063269
APA StyleTien, H.-M., Cheng, T.-C., Lien, H.-C., Yang, K.-F., Shy, C.-G., Chen, Y.-L., Hsu, N.-T., Lu, S.-N., & Wang, J.-H. (2022). Liver Disease Screening and Hepatitis C Virus Elimination in Taiwan Rural Indigenous Townships: Village-By-Village Screening and Linking to Outreach Hepatology Care. International Journal of Environmental Research and Public Health, 19(6), 3269. https://doi.org/10.3390/ijerph19063269







