The Effects of Action Observation Therapy as a Rehabilitation Tool in Parkinson’s Disease Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Data Items and Collection Process
2.5. Synthesis Methods
2.6. Risk of Bias Assessment
3. Results
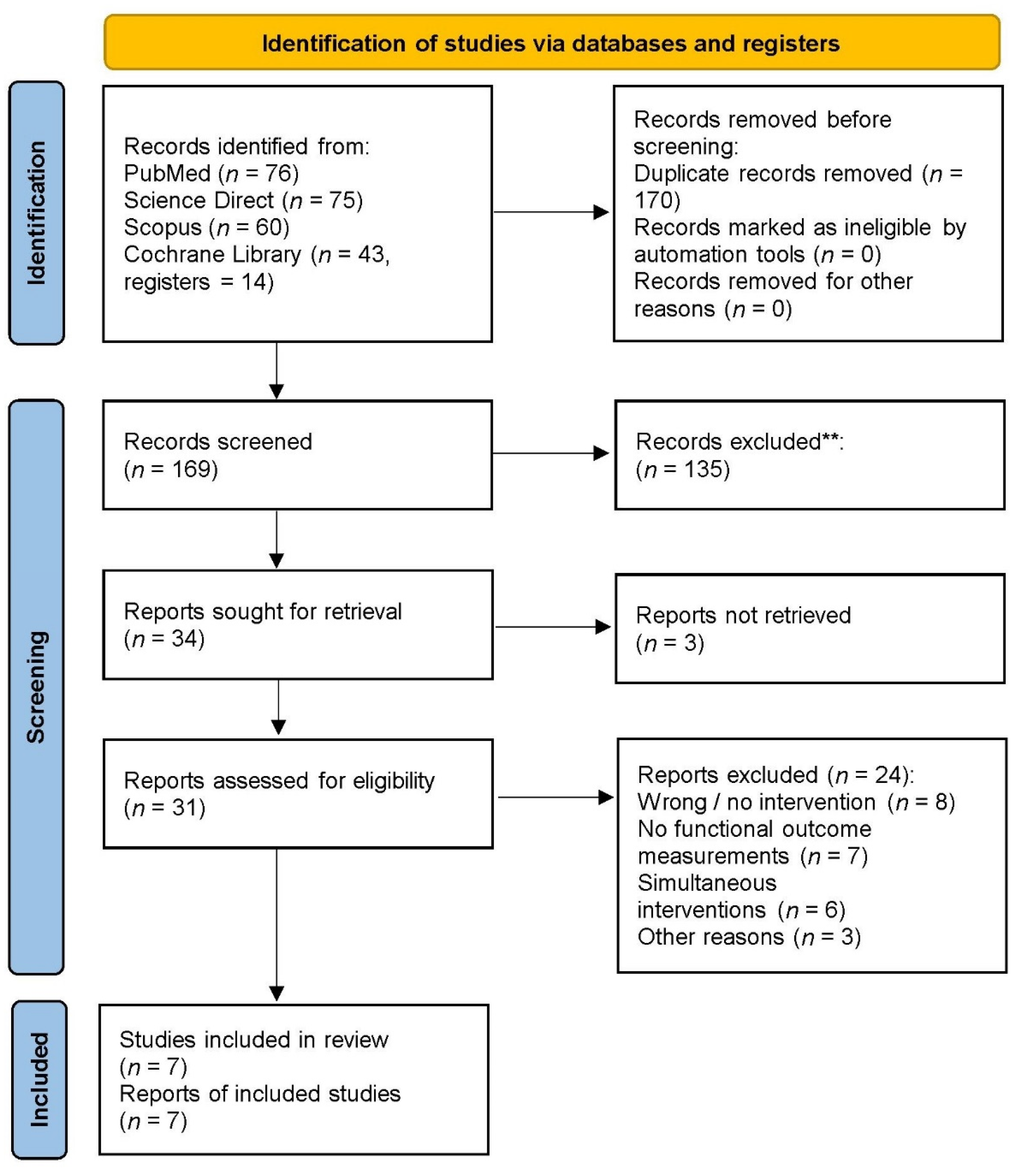
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Participants
| Study | Participants’ Characteristics | AO | Control | Design/Dose | Task/Stimulus | Outcome Measures |
|---|---|---|---|---|---|---|
| Pelosin et al., 2010 [42] | PD patients: (n = 18), FOG-Q item 3 ≥ 2 and item 4 ≥ 1, MMSE > 24 AO group: (n = 9), 68.8 ± 4.1 years, F:M 7/6 Control group: (n = 9), 70.2 ± 6.8 years, F:M 6/4 | Watched videos of movements and strategies to circumvent FoG episodes and then practiced the observed actions | Watched sequences of static pictures of landscapes and then practiced the same actions as the experimental group | Sessions: 3 per week Session duration: 1 h Protocol duration: 4 weeks Total sessions: 12 | Movements: weight shifting, step, turn around chair, step over obstacle, walk straight-through doorway Perspectives: 3rd person—frontal | FOG-Q, FoG-diary, TUG, 10M-WT, BBS, Tinetti scale and PDQ-39 Time points: baseline; 2 days after; 1, 2, 3, and 4 weeks after |
| Pelosin et al., 2013 [30] | PD patients: (n =20), H&Y: 1–3, MMSE ≥ 24 Healthy patients: (n = 14) AO group: n =10 (PD), 68.8 ± 7.4 years, F:M 3/7, DD: 9.1 ± 3.7, UPDRS: 18.9 ± 4.2 n = 7 (H), 64.3 ± 8.6 years, F:M 3/4 Control group: n = 10 (PD), 66.4 ± 8.9 years, F:M 6/4, DD: 8.9 ± 3.1, UPDRS: 19.2 ± 5.4 n = 7 (H), 69.2 ± 9.6 years, F:M 4/3 | Watched videos of repetitive finger movements | Listened acoustic cues | Sessions: 1 Duration: 6 min | Movements: opposition of right thumb to all other fingers at 3 HZ pace Perspectives: 3rd person | Primary: SMR of self-paced finger movements Secondary: Inter-tapping interval and touch duration (kinematic parameters) Time points: Baseline, after, 45′ after, 2 days after |
| Jaywant et al., 2016 [43] | PD patients: (n = 23), H&Y 1–3, UPDRS gait item ≥ 1 AO group: (n = 12), 63.7 ± 6.2 years, DD: 11.6 ± 4.9 years Control group: (n = 10), 70.2 ± 6.8 years, DD: 9.5 ± 3.7 years | Watched videos of walking trials and judged whether the observed action was a PD or healthy pattern | Watched videos of water moving roughly and calmly and judged whether the motion of the water was rough or calm | Sessions: 1 per day Session duration: not specified Protocol duration: 1 week Total sessions: 7 | Movements: walking in hallway Perspectives: 3rd person—frontal, lateral, and posterior views | PDQ-39 and stride frequency, number-duration of walking periods during straight walking, walking with turns, and dual task walking Time points: baseline, 1 day after |
| Agosta et al., 2017 [38] | PD patients:(n =25), H&Y < 4, MMSE > 24, FOG-Q item 3 ≥ 2, DD ≥ 5 years Healthy patients: (n = 19), 66 ± 8 years, F:M 10/9 AO group: n =12 (PD), 69 ± 8 years, F:M 2/10 Control group: n = 13 (PD), 64 ± 7 years, F:M 5/8 | Watched videos of movements with the help of auditory cues and then imitated them at the same beats | Watched videos of static landscape images and then executed the same movements as the experimental group | Sessions: 3 per week Session duration: 1 h (24 min observation—36 min action) Protocol duration: 4 weeks Total sessions: 12 | Movements: weight shifting, stepping forward-backward-side, turn around chair, step over obstacle, walk straight-through doorway Perspectives: 3rd person—frontal view | UPDRS III (on/off), H&Y (on/off), FOG-Q, UPDRS II-FoG (on/off), PDQ-39, BBS, 10M-WT Time points: baseline, after (week 4), after 1 month (week 8) |
| Mezzarobba et al., 2017 [40] | PD patients: (n = 22), FoG, H&Y 1–3, BDI ≤ 16, MMSE > 24 AO group: (n = 12), 74.6 ± 5.9 years, F:M 5/7, DD: 10.7 ± 3.44 years Control group: (n = 10), 72 ± 5.8 years, F:M 3/7, DD: 9.4 ± 4.8 years | Watched videos of gait-related gestures and after video clip practiced the same observed action for the same amount of time (x2) | The same motor gestures performed in the same order and time by means of visual (floor) or auditory (metronome) cues | Sessions: 2 per week Session duration: 1 h Protocol duration: 8 weeks Total sessions: 16 | Movements: weight shifting + step, gait initiation, turn around, step over obstacle, STW, walk straight- through doorway Perspectives: 3rd person—frontal-lateral views | Primary: NFOGQ (duration & severity) Secondary: UPDRS II, III, H&Y, PDQ-39, 6M-WT, BBS, TUG, improvement index Time points: baseline, after, 1 month after, 3 months after |
| Pelosin et al., 2018 [39] | PD patients: (n = 64), FOG-Q: item 2 ≥ 1 & item 4 ≥ 2, H&Y 2–3, MMSE > 24, unassisted walk AO group: (n = 33), 70.4 ± 4.5 years, F:M 17/16, DD: 10.7 ± 3.9 years Control group: (n = 31), 72.8 ± 3.1 years, F:M 16/15, DD: 9.5 ± 4.2 years | Watched videos of functional movements and then practiced the observed actions with the help of physiotherapist | Watched videos of static landscape images and then practiced the same actions as the experimental group | Sessions: 2 per week Session duration: 45 min Protocol duration: 5 weeks Total sessions: 10 | Movements: weight shifting, weight shifting + step, turn around chair, step over obstacle, walk straight-through doorway Perspective: 3rd person—frontal view | Primary: FOG-Q Secondary: TUG, 10M-WT, BBS Time points: baseline, 1 week after training, 4 weeks after training |
| Mezzarobba et al., 2020 [41] | PD patients: (n = 22), FoG, H&Y 1–3, BDI ≤ 16, MMSE > 24 AO group: (n = 12), 74.6 ± 5.9 years, F:M 5/7, DD: 10.7 ± 3.44 years Control group: (n = 10), 72 ± 5.8 years, F:M 3/7, DD: 9.4 ± 4.8 years | Watched videos of gait-related gestures and after video clip practiced the same observed action for the same amount of time (x2) | The same motor gestures performed in the same order and time by means of visual (floor) or auditory (metronome) cues | Sessions: 2 per week Session duration: 1 h Protocol duration: 8 weeks Total sessions: 16 | Movements: weight shifting + step, gait initiation, turn around, step over obstacle, STW, walk straight—through doorway Perspectives: 3rd person—frontal—lateral views | STW time, COM’s & COP’s time—position, Task: STW Time events: initiation, flexion phase, extension phase, unloading phase, and stance phase Time points: baseline, after, 1 month after, 3 months after |
3.2.2. Action Observation Interventions
3.2.3. Control Interventions
3.2.4. Outcome Measures and Time Points
3.3. Risk of Bias in Studies
3.4. Results of Included Studies
4. Discussion
4.1. Dose/Design of the Interventions
4.2. Characteristics of the Stimuli/Task
4.3. Outcomes Measures
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallese, V.; Fadiga, L.; Fogassi, L.; Rizzolatti, G. Action recognition in the premotor cortex. Brain 1996, 119, 593–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Fadiga, L.; Gallese, V.; Fogassi, L. Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res. 1996, 3, 131–141. [Google Scholar] [CrossRef]
- Hari, R.; Forss, N.; Avikainen, S.; Kirveskari, E.; Salenius, S.; Rizzolatti, G. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc. Natl. Acad. Sci. USA 1998, 95, 15061–15065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri-Destro, M.; Rizzolatti, G. Mirror neurons and mirror systems in monkeys and humans. Physiology 2008, 23, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Fadiga, L.; Fogassi, L.; Pavesi, G.; Rizzolatti, G. Motor facilitation during action observation: A magnetic stimulation study. J. Neurophysiol. 1995, 73, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- Buccino, G.; Binkofski, F.; Fink, G.R.; Fadiga, L.; Fogassi, L.; Gallese, V.; Seitz, R.J.; Zilles, Z.; Rizzolatti, G.; Freund, H.-J. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur. J. Neurosci. 2001, 13, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage 2007, 36, T164–T173. [Google Scholar] [CrossRef] [PubMed]
- Buccino, G. Action observation treatment: A novel tool in neurorehabilitation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130185. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fogassi, L.; Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001, 2, 661–670. [Google Scholar] [CrossRef]
- Mattar, A.A.; Gribble, P.L. Motor learning by observing. Neuron 2005, 46, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porro, C.A.; Facchin, P.; Fusi, S.; Dri, G.; Fadiga, L. Enhancement of force after action observation: Behavioural and neurophysiological studies. Neuropsychologia 2007, 45, 3114–3121. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Thomaschke, R. From visuo-motor interactions to imitation learning: Behavioural and brain imaging studies. J. Sports Sci. 2007, 25, 497–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Helden, J.; Van Schie, H.T.; Rombouts, C. Observational Learning of New Movement Sequences Is Reflected in Fronto-Parietal Coherence. PLoS ONE 2011, 5, e14482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, S.; Holle, H.; Roberts, N.; Eickhoff, S.B.; Vogt, S. Imitation and observational learning of hand actions: Prefrontal involvement and connectivity. NeuroImage 2012, 59, 1668–1683. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malouin, F.; Jackson, P.L.; Richards, C.L. Towards the integration of mental practice in rehabilitation programs. A critical review. Front. Hum. Neurosci. 2013, 7, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yutaka, O. Applications of Observational Learning in Neurorehabilitation. Int. J. Phys. Med. Rehabil. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Celnik, P.; Webster, B.; Glasser, D.M.; Cohen, L.G. Effects of action observation on physical training after stroke. Stroke 2008, 39, 1814–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschini, M.; Agosti, M.; Cantagallo, A.; Sale, P.; Mancuso, M.; Buccino, G. Mirror neurons: Action observation treatment as a tool in stroke rehabilitation. Eur. J. Phys. Rehabil. 2010, 46, 517–523. [Google Scholar]
- Franceschini, M.; Ceravolo, M.G.; Agosti, M.; Cavallini, P.; Bonassi, S.; Dall’Armi, V.; Massucci, M.; Schifini, F.; Sale, P. Clinical relevance of action observation in upper-limb stroke rehabilitation: A possible role in recovery of functional dexterity. A randomized clinical trial. Neurorehabilit. Neural Repair 2012, 26, 456–462. [Google Scholar] [CrossRef]
- Sampson, M.; Shau, Y.W.; King, M.J. Bilateral upper limb trainer with virtual reality for post-stroke rehabilitation: Case series report. Disabil. Rehabil. Assist. Technol. 2012, 7, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sugg, K.; Müller, S.; Winstein, C.; Hathorn, D.; Dempsey, A. Does Action Observation Training With Immediate Physical Practice Improve Hemiparetic Upper-Limb Function in Chronic Stroke? Neurorehabil. Neural Repair 2015, 29, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Action observation for upper limb function after stroke: Evidence-based review of randomized controlled trials. J. Phys. Ther. Sci. 2015, 27, 3315–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.J.; Fong, K.N.; Welage, N.; Liu, K.P. The Activation of the Mirror Neuron System during Action Observation and Action Execution with Mirror Visual Feedback in Stroke: A Systematic Review. Neural Plast. 2018, 2321045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kan, L.; Dong, A.; Zhang, J.; Bai, Z.; Xie, Y.; Liu, Q.; Peng, Y. The effects of action observation training on improving upper limb motor functions in people with stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.-H.; Zhu, J.-D.; Chen, C.-C.; Tai, R.-Y.; Lee, C.-Y.; Hsieh, Y.-W. Action observation therapy for improving arm function, walking ability, and daily activity performance after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1277–1285. [Google Scholar] [CrossRef]
- Sánchez Silverio, V.; Abuín Porras, V.; Rodríguez Costa, I.; Cleland, J.A.; Villafañe, J.H. Effects of action observation training on the walking ability of patients post stroke: A systematic review. Disabil. Rehabil. 2021, 1–10. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Avanzino, L.; Marchese, R.; Pelosin, E. Action Observation and Motor Imagery: Innovative Cognitive Tools in the Rehabilitation of Parkinson’s Disease. Parkinsons Dis. 2015, 2015, 124214. [Google Scholar] [CrossRef] [Green Version]
- Castiello, U.; Ansuini, C.; Bulgheroni, M.; Scaravilli, T.; Nicoletti, R. Visuomotor priming effects in Parkinson’s disease patients depend on the match between the observed and the executed action. Neuropsychologia 2009, 47, 835–842. [Google Scholar] [CrossRef]
- Pelosin, E.; Bove, M.; Ruggeri, P.; Avanzino, L.; Abbruzzese, G. Reduction of bradykinesia of finger movements by a single session of action observation in Parkinson disease. Neurorehabil. Neural Repair 2013, 27, 552–560. [Google Scholar] [CrossRef]
- Di Iorio, W.; Ciarimboli, A.; Ferriero, G.; Feleppa, M.; Baratto, L.; Matarazzo, G.; Gentile, G.; Masiero, S.; Sale, P. Action Observation in People with Parkinson’s Disease. A Motor(-)Cognitive Combined Approach for Motor Rehabilitation. A Preliminary Report. Diseases 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plata Bello, J.; Modroño, C.; Marcano, F.; González-Mora, J.L. The mirror neuron system and motor dexterity: What happens? Neuroscience 2014, 275, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. Open 2021, 88, 105906. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.Z.; Moseley, A.M.; Maher, C.G.; Nascimento, D.P.; Costa, L.; Costa, L.O. Methodologic Quality and Statistical Reporting of Physical Therapy Randomized Controlled Trials Relevant to Musculoskeletal Conditions. Arch. Phys. Med. Rehabil. 2018, 99, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Gatti, R.; Sarasso, E.; Antonietta Volonté, M.; Canu, E.; Meani, A.; Sarro, L.; Copetti, M.; Cattrysse, E.; Kerckhofs, E. Brain plasticity in Parkinson’s disease with freezing of gait induced by action observation training. J. Neurol. 2017, 264, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Pelosin, E.; Barella, R.; Bet, C.; Magioncalda, E.; Putzolu, M.; Di Biasio, F.; Cerulli, C.; Casaleggio, M.; Abbruzzese, G.; Avanzino, L. Effect of Group-Based Rehabilitation Combining Action Observation with Physiotherapy on Freezing of Gait in Parkinson’s Disease. Neural Plast. 2018, 2018, 4897276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzarobba, S.; Grassi, M.; Pellegrini, L.; Catalan, M.; Kruger, B.; Furlanis, G.; Manganotti, P.; Bernardis, P. Action Observation Plus Sonification. A Novel Therapeutic Protocol for Parkinson’s Patient with Freezing of Gait. Front. Neurol. 2017, 8, 723. [Google Scholar] [CrossRef] [Green Version]
- Mezzarobba, S.; Grassi, M.; Pellegrini, L.; Catalan, M.; Krüger, B.; Stragapede, L.; Manganotti, P.; Bernardis, P. Action observation improves sit-to-walk in patients with Parkinson’s disease and freezing of gait. Biomechanical analysis of performance. Parkinsonism Relat. Disord. 2020, 80, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Pelosin, E.; Avanzino, L.; Bove, M.; Stramesi, P.; Nieuwboer, A.; Abbruzzese, G. Action Observation Improves Freezing of Gait in Patients With Parkinson’s Disease. Neurorehabil. Neural Repair 2010, 24, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Jaywant, A.; Ellis, T.D.; Roy, S.; Lin, C.-C.; Neargarder, S.; Cronin-Golomb, A. Randomized Controlled Trial of a Home-Based Action Observation Intervention to Improve Walking in Parkinson Disease. Arch. Phys. Med. Rehabil. 2016, 97, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. 2004, 19, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.M.; McGinley, J.L.; Danoudis, M.E.; Iansek, R.; Morris, M.E. Freezing of Gait and Activity Limitations in People with Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2011, 92, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H.; Takakusaki, K.; Debu, B.; Lozano, A.M.; Krishna, V.; Fasano, A.; Aziz, T.Z.; Papa, S.M.; Factor, S.A.; Hallett, M. Physiology of freezing of gait. Ann. Neurol. 2016, 80, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Temporiti, F.; Adamo, P.; Cavalli, E.; Gatti, R. Efficacy and Characteristics of the Stimuli of Action Observation Therapy in Subjects With Parkinson’s Disease: A Systematic Review. Syst. Rev. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Rodríguez-Oroz, M.C.; Benitez-Temino, B.; Blesa, F.J.; Guridi, J.; Marin, C.; Rodriguez, M. Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov. Disord. 2008, 23, S548–S559. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Marchese, R.; Avanzino, L.; Pelosin, E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism Relat. Disord. 2016, 22, S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, E.; Gemma, M.; Agosta, F.; Filippi, M.; Gatti, R. Action observation training to improve motor function recovery: A systematic review. Arch. Physiother. 2015, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Merino, B.; Glaser, D.E.; Grèzes, J.; Passingham, R.E.; Haggard, P. Action Observation and Acquired Motor Skills: An fMRI Study with Expert Dancers. Cereb. Cortex 2004, 15, 1243–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Merino, B.; Grèzes, J.; Glaser, D.E.; Passingham, R.E.; Haggard, P. Seeing or Doing? Influence of Visual and Motor Familiarity in Action Observation. Curr. Biol. 2006, 16, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Filimon, F.; Nelson, J.D.; Hagler, D.J.; Sereno, M.I. Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. NeuroImage 2007, 37, 1315–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, M.; Siebner, H.R.; Biermann-Ruben, K.; Kessler, K.; Bäumer, T.; Büchel, C.; Schnitzler, A.; Münchau, A. Do simple intransitive finger movements consistently activate frontoparietal mirror neuron areas in humans? Neuroimage 2007, 36, T44–T53. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, V.; Fogassi, L.; Rizzolatti, G.; Pomper, J.K.; Thier, P.; Giese, M.A.; Casile, A. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Curr. Biol. 2011, 21, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Fogassi, L. The mirror mechanism: Recent findings and perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caggiano, V.; Giese, M.; Their, P.; Casile, A. Encoding of point of view during action observation in the local field potentials of macaque area F5. Eur. J. Neurosci. 2015, 41, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Watanabe, S.; Kuruma, H.; Murakami, Y.; Seno, A.; Matsuda, T. Neural activation during imitation of movements presented from four different perspectives: A functional magnetic resonance imaging study. Neurosci. Lett. 2011, 503, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Angelini, M.; Fabbri-Destro, M.; Lopomo, N.F.; Gobbo, M.; Rizzolatti, G.; Avanzini, P. Perspective-dependent reactivity of sensorimotor mu rhythm in alpha and beta ranges during action observation: An EEG study. Sci. Rep. 2018, 8, 12429. [Google Scholar] [CrossRef]
- Jackson, P.L.; Meltzoff, A.N.; Decety, J. Neural circuits involved in imitation and perspective-taking. NeuroImage 2006, 31, 429–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villiger, M.; Estévez, N.; Hepp-Reymond, M.-C.; Kiper, D.; Kollias, S.S.; Eng, K.; Hotz-Boendermaker, S. Enhanced Activation of Motor Execution Networks Using Action Observation Combined with Imagination of Lower Limb Movements. PLoS ONE 2013, 8, e72403. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Mohammadi, B.; Hammer, A.; Heldmann, M.; Samii, A.; Münte, T.F.; Effenberg, A.O. Observation of sonified movements engages a basal ganglia frontocortical network. BMC Neurosci. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Eligibility Criteria & Source | Random Allocation | Concealed Allocation | Baseline Comparability | Blinding of Participants | Blinding of Therapists | Blinding of Assessors | Adequate Follow-Up (>85%) | Intention-to-Treat Analysis | Between-Group Statistical Comparisons | Reporting of Point Measures & Variability | Total Score (0–10) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pelosin et al., 2013 [30] | yes | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 5 |
| Agosta et al., 2017 [38] | yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Pelosin et al., 2018 [39] | yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Mezzarobba et al., 2017 [40] | yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Mezzarobba et al., 2020 [41] | yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Pelosin et al., 2010 [42] | yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Jaywant at al., 2016 [43] | yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Outcome Measures | Time Points | Experimental Group | Control Group | Mean Difference [95% CI] | |
|---|---|---|---|---|---|
| Pelosin et al., 2010 [42] | |||||
| Action Observation Training Group (Experimental Group) vs. Landscape Observation Training Group (Control Group) | |||||
| FoG-Q | Post 2 Days | 12.8 (2.0) | 14.4 (1.9) | −1.6 [−3.40. 0.20] | |
| Post 4 Weeks | 14.1 (2.8) | 16.4 (2.5) | −2.3 [−4.75, 0.15] | ||
| TUG, 10M-WT, Tinetti Scale, BBS and PDQ-39 | Post 2 Days | Not significant | |||
| Post 1 Week | Not significant | ||||
| Post 2 Weeks | Not significant | ||||
| Post 3 Weeks | Not significant | ||||
| Post 4 Weeks | Not significant | ||||
| FoG-diary (total number of episodes) | Post 2 Days | Not significant | |||
| Post 1 Week | Not significant | ||||
| Post 2 Weeks | p < 0.05 | ||||
| Post 3 Weeks | p < 0.05 | ||||
| Post 4 Weeks | p < 0.05 | ||||
| Outcome Measures | Time Points | BetweenGroups Difference | |||
| Pelosin et al., 2013 [30] | |||||
| Action Observation Training Group (Experimental Group) vs. Acoustic Training Group (Control Group) | |||||
| Self-paced Movement Rate | Post | Not significant | |||
| Post 45′ | p = 0.007 | ||||
| Post 2 Days | p = 0.004 | ||||
| Inter-tapping Interval | Post | p = 0.019 | |||
| Post 45′ | p < 0.001 | ||||
| Post 2 Days | p < 0.001 | ||||
| Touch Duration | Post | Not significant | |||
| Post 45′ | Not significant | ||||
| Post 2 Days | Not significant | ||||
| Outcome Measures | Time Points | Experimental Group | Control Group | Mean Difference [95% CI] | |
| Jaywant et al., 2016 [43] | |||||
| Action Observation Training Group (Experimental Group) vs. Landscape Observation Training Group (Control Group) | |||||
| PDQ-39 | Follow-up (1 week) | n/a | n/a | 3.08 [−2.97, 9.12] | |
| Straight Line Walking | Walking Speed (m/s) | Follow-up (1 week) | 1.19 (0.15) | 1.18 (0.08) | 0.01 [−0.32, 0.34] |
| Stride Length (m) | Follow-up (1 week) | 1.35 (0.21) | 1.34 (0.12) | 0.01 [−0.46, 0.48] | |
| Stride Frequency (strides/s) | Follow-up (1 week) | 0.89 (0.06) | 0.89 (0.06) | 0.00 [−0.17, 0.17] | |
| Swing Time (% of stride) | Follow-up (1 week) | 45.6 (1.6) | 44.8 (1.7) | 0.80 [−3.78, 5.38] | |
| Gait Asymmetry | Follow-up (1 week) | 0.03 (0.02) | 0.02 (0.01) | 0.01 [−0.03, 0.05] | |
| Walking with Turns | Walking Speed (m/s) | Follow-up (1 week) | 1.19 (0.13) | 1.19 (0.08) | 0.00 [−0.30, 0.30] |
| Stride Length (m) | Follow-up (1 week) | 1.36 (0.20) | 1.35 (0.11) | 0.01 [−0.44, 0.46] | |
| Stride Frequency (strides/s) | Follow-up (1 week) | 0.89 (0.07) | 0.88 (0.06) | 0.01 [−0.17, 0.19] | |
| Swing Time (% of stride) | Follow-up (1 week) | 45.3 (1.3) | 44.7 (1.6) | 0.60 [−3.44, 4.64] | |
| Gait Asymmetry | Follow-up (1 week) | 0.03 (0.01) | 0.03 (0.01) | 0.00 [−0.03, 0.03] | |
| Dual Task Walking | Walking Speed (m/s) | Follow-up (1 week) | 1.17 (0.18) | 1.17 (0.15) | 0.00 [−0.46, 0.46] |
| Stride Length (m) | Follow-up (1 week) | 1.34 (0.23) | 1.34 (0.14) | 0.00 [−0.53, 0.53] | |
| Stride Frequency (strides/s) | Follow-up (1 week) | 0.88 (0.07) | 0.88 (0.08) | 0.00 [−0.21, 0.21] | |
| Swing Time (% of stride) | Follow-up (1 week) | 45.3 (1.7) | 44.6 (1.9) | 0.70 [−4.30, 5.70] | |
| Gait Asymmetry | Follow-up (1 week) | 0.03 (0.03) | 0.03 (0.02) | 0.00 [−0.07, 0.07] | |
| Outcome Measures | Time Points | Experimental Group | Control Group | Mean Difference [95% CI] | |
| Agosta et al., 2017 [38] | |||||
| Action Observation Training Group (Experimental Group) vs. Landscape Observation Training Group (Control Group) | |||||
| H&Y-off | Post (W4) | 2.5 (0.5) | 2.3 ± 0.4 | 0.20 [−0.17, 0.57] | |
| H&Y-on | Post (W4) | 2.4 (0.4) | 2.2 ± 0.3 | 0.20 [−0.09, 0.491] | |
| Post (W8) | 2.2 (0.4) | 2.2 ± 0.4 | 0.00 [−0.33, 0.33] | ||
| UPDRS-III-off | Post (W4) | 35.0 (10.9) | 33.8 ± 9.0 | 1.20 [−6.89, 9.29] | |
| UPDRS-III-on | Post (W4) | 23.3 (7.8) | 24.2 ± 8.3 | −1.10 [−7.55, 5.35] | |
| Post (W8) | 23.3 (10.1) | 22.1 ± 8.4 | 1.20 [−6.55, 8.95] | ||
| FoG-Q | Post (W4) | 9.7 (3.4) | 10.9 ± 3.0 | −1.20 [−3.79, 1.39] | |
| Post (W8) | 10.2 (2.4) | 11.3 ± 3.0 | −1.10 [−3.31, 1.11] | ||
| UPDRS-II-FoG-off | Post (W4) | 1.64 (0.94) | 1.92 ± 0.79 | −0.28 [−0.98, 0.42] | |
| Post (W8) | 2.13 (0.99) | 2.0 ± 1.1 | 0.13 [−0.73, 0.99] | ||
| UPDRS-II-FoG-on | Post (W4) | 1.18 (0.87) | 1.25 ± 0.75 | −0.07 [−0.73, 0.59] | |
| Post (W8) | 0.89 (0.93) | 0.92 ± 0.95 | −0.03 [−0.80, 0.74] | ||
| PDQ-39 | Post (W4) | 19.0 (9.2) | 14.0 ± 8.9 | −0.07 [−0.73, 0.59] | |
| Post (W8) | 17.0 (7.0) | 16.7 ± 10.5 | −0.03 [−0.80, 0.74] | ||
| BBS | Post (W4) | 53.6 (2.6) | 54.4 ± 2.4 | −0.80 [−2.82, 1.22] | |
| Post (W8) | 53.4 (2.7) | 54.4 ± 2.2 | −1.00 [−3.06, 1.06] | ||
| 10 M-WT-normal (s) | Post (W4) | 8.2 (1.1) | 7.2 ± 1.2 | 1.00 [0.08, 1.92] | |
| Post (W8) | 8.2 (1.4) | 7.68 ± 1.7 | 0.52 [−0.75, 1.79] | ||
| 10 M-WT-fast (s) | Post (W4) | 6.0 (1.4) | 5.6 ± 1.0 | 0.40 [−0.59, 1.39] | |
| Post (W8) | 6.1 (2.0) | 6.0 ± 1.6 | 0.00 [−1.51, 1.51] | ||
| Outcome Measures | Time Points | Between Groups Difference | |||
| Mezzarobba et al., 2017 [40] | |||||
| Action Observation plus Sonification Training Group (Experimental Group) vs. Motor Gesture with Visual & Auditory Cues Training Group (Control Group) | |||||
| NFoG-Q | Post | p ≤ 0.001 | |||
| Post 1 Month | p ≤ 0.001 | ||||
| Post 3 Months | p ≤ 0.001 | ||||
| PDQ-39 mobility | Post | p ≤ 0.05 | |||
| Post 1 Month | p ≤ 0.001 | ||||
| Post 3 Months | p ≤ 0.001 | ||||
| UPDRS-III | Post | p ≤ 0.001 | |||
| Post 1 Month | p ≤ 0.05 | ||||
| Post 3 Months | p ≤ 0.05 | ||||
| PDQ-39-bodily discomfort | Post | p ≤ 0.001 | |||
| Post 1 Month | p ≤ 0.05 | ||||
| Post 3 Months | p ≤ 0.05 | ||||
| PDQ-39-Total | Post | Not significant | |||
| Post 1 Month | p ≤ 0.01 | ||||
| Post 3 Months | p ≤ 0.01 | ||||
| UPDRS-II | Post | Not significant | |||
| Post 1 Month Post 3 Months | p ≤ 0.05 | ||||
| p ≤ 0.01 | |||||
| BBS | Post | Not significant | |||
| Post 1 Month | p ≤ 0.05 | ||||
| Post 3 Months | Not significant | ||||
| 6MWT | Post | Not significant | |||
| Post 1 Month | Not significant | ||||
| Post 3 Months | p ≤ 0.05 | ||||
| TUG | Post | Not significant | |||
| Post 1 Month | Not significant | ||||
| Post 3 Months | Not significant | ||||
| MPAS | Post | Not significant | |||
| Post 1 Month | Not significant | ||||
| Post 3 Months | Not significant | ||||
| PDQ-39 cognitions | Post | Not significant | |||
| Post 1 Month | Not significant | ||||
| Post 3 Months | Not significant | ||||
| Outcome Measures | Time Points | Experimental Group | Control Group | Mean Difference [95% CI] | |
| Pelosin et al., 2018 [39] | |||||
| Action Observation Training Group (Experimental Group) vs. Landscape Observation Training Group (Control Group) | |||||
| FoG-Q | Post 1 Week Post 4 Weeks | 9.7 (5.8) 9.4 (5.7) | 10.5 (4.8) 12.0 (5.7) | −0.8 [−3.47, 1.87] −2.6 [−5.46, 0.26] | |
| TUG | Post 1 Week Post 4 Weeks | 12.2 (4.9) 12.9 (4.1) | 13.4 (6.1) 15.5 (6.8) | −1.2 [−3.98, 1.58] −2.6 [−5.43, 0.23] | |
| BBS | Post 1 Week Post 4 Weeks | 51.3 (5.7) 51.5 (5.5) | 52.4 (4.5) 49.6 (5.7) | −1.1 [−3.67, 1.47] 1.9 [−0.91, 4.71] | |
| 10M-WT | Post 1 Week Post 4 Weeks | 10.7 (3.9) 12.3 (4.3) | 12.9 (4.3) 13.9 (5.4) | −2.2 [−4.26, −0.14] −1.6 [−4.05, 0.85] | |
| Outcome Measures | Time Points | Between Groups Difference | |||
| Mezzarobba et al., 2020 [41] | |||||
| Action Observation plus Sonification Training Group (Experimental Group) vs. Motor Gesture with Visual & Auditory Cues Training Group (Control Group) | |||||
| Sit-to-walk times (s) | Post Post 1 Month Post 3 Months | Not significant Not significant Not significant | |||
| COP Profiles | Post Post 1 Month Post 3 Months | Significant difference (30–50% range) Significant difference (40–50% range) Significant difference (14–50% range) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulos, I.; Karanika, P.; Papaxanthis, C.; Tsaklis, P. The Effects of Action Observation Therapy as a Rehabilitation Tool in Parkinson’s Disease Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 3311. https://doi.org/10.3390/ijerph19063311
Giannakopoulos I, Karanika P, Papaxanthis C, Tsaklis P. The Effects of Action Observation Therapy as a Rehabilitation Tool in Parkinson’s Disease Patients: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(6):3311. https://doi.org/10.3390/ijerph19063311
Chicago/Turabian StyleGiannakopoulos, Ioannis, Panagiota Karanika, Charalambos Papaxanthis, and Panagiotis Tsaklis. 2022. "The Effects of Action Observation Therapy as a Rehabilitation Tool in Parkinson’s Disease Patients: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 6: 3311. https://doi.org/10.3390/ijerph19063311
APA StyleGiannakopoulos, I., Karanika, P., Papaxanthis, C., & Tsaklis, P. (2022). The Effects of Action Observation Therapy as a Rehabilitation Tool in Parkinson’s Disease Patients: A Systematic Review. International Journal of Environmental Research and Public Health, 19(6), 3311. https://doi.org/10.3390/ijerph19063311







