Daily Associations of Air Pollution and Pediatric Asthma Risk Using the Biomedical REAI-Time Health Evaluation (BREATHE) Kit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Asthma Outcomes
2.2.1. Lung Function
2.2.2. Inhaler Use
2.2.3. Asthma Symptoms
2.3. Environmental Exposure Assessment
2.4. Covariate Information
2.5. Statistical Analysis
3. Results
3.1. Descriptive Summaries
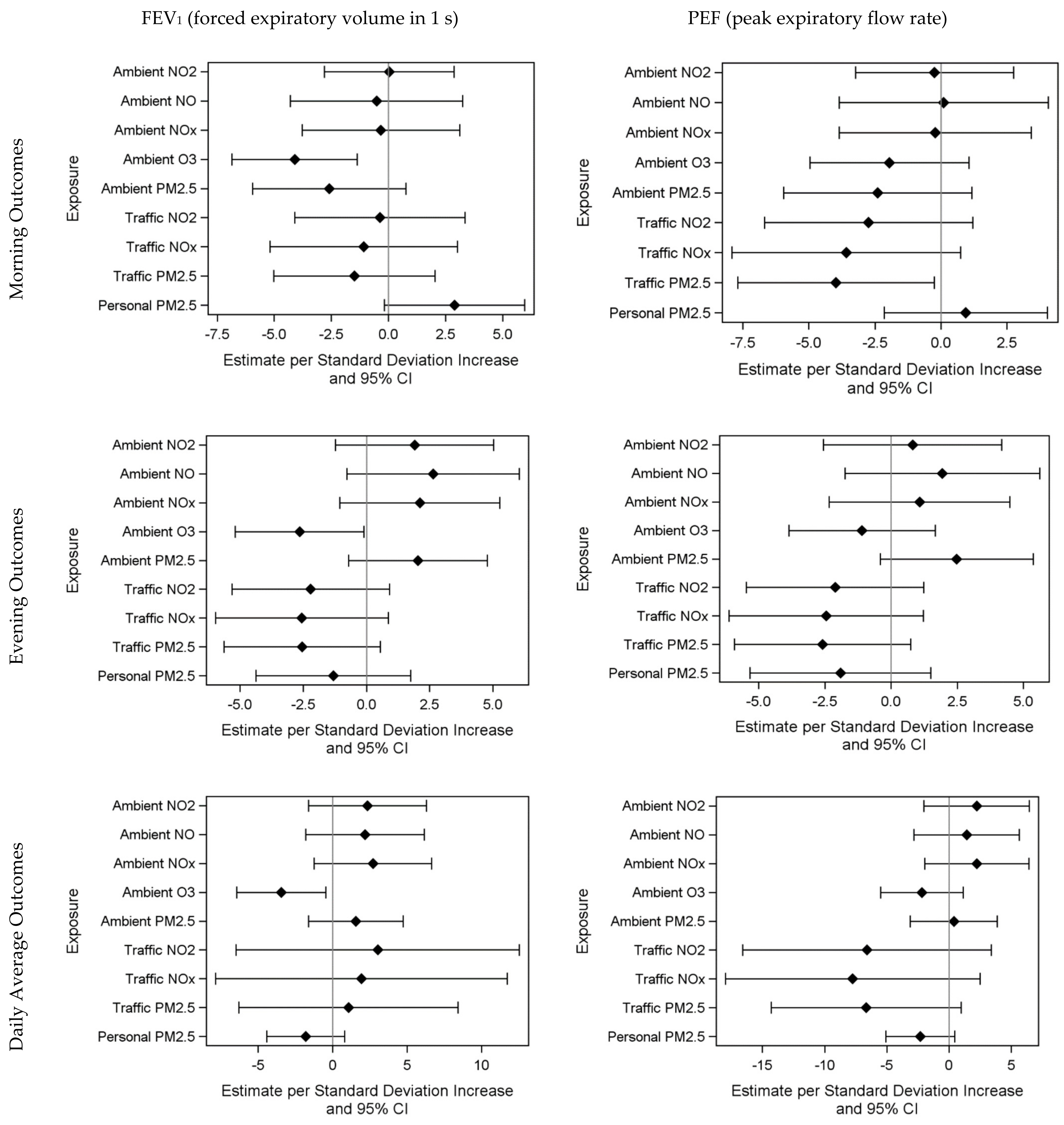
3.2. Air Pollution and Health Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- CDC National Asthma Data. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 13 September 2021).
- Kuschner, W.G. The asthma epidemic. N. Engl. J. Med. 2007, 356, 1073. [Google Scholar] [CrossRef] [PubMed]
- Van Dellen, Q.M.; Stronks, K.; Bindels, P.J.; Ory, F.G.; Bruil, J.; van Aalderen, W.M.; Group, P.S. Predictors of asthma control in children from different ethnic origins living in Amsterdam. Respir. Med. 2007, 101, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinbami, L. Centers for Disease Control and Prevention; National Center for Health Statistics. The state of childhood asthma, United States, 1980–2005. Adv. Data 2006, 381, 1–24. [Google Scholar]
- Bartter, T.; Pratter, M.R. Asthma: Better outcome at lower cost? The role of the expert in the care system. Chest 1996, 110, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Guyatt, G.H.; Feeny, D.H.; Ferrie, P.J.; Griffith, L.E.; Townsend, M. Measuring quality of life in the parents of children with asthma. Qual. Life Res. 1996, 5, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.M.; Teach, S.J.; Lemanske, R.F., Jr.; Blake, K.V. The 2020 Focused Updates to the NIH Asthma Management Guidelines: Key Points for Pediatricians. Pediatrics 2021, 147, e2021050286. [Google Scholar] [CrossRef] [PubMed]
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 2007, 120, S94–S138. [Google Scholar] [CrossRef]
- Burke, W.; Fesinmeyer, M.; Reed, K.; Hampson, L.; Carlsten, C. Family history as a predictor of asthma risk. Am. J. Prev. Med. 2003, 24, 160–169. [Google Scholar] [CrossRef]
- Illi, S.; von Mutius, E.; Lau, S.; Niggemann, B.; Gruber, C.; Wahn, U.; Multicentre Allergy Study, g. Perennial allergen sensitisation early in life and chronic asthma in children: A birth cohort study. Lancet 2006, 368, 763–770. [Google Scholar] [CrossRef]
- Holst, G.J.; Pedersen, C.B.; Thygesen, M.; Brandt, J.; Geels, C.; Bonlokke, J.H.; Sigsgaard, T. Air pollution and family related determinants of asthma onset and persistent wheezing in children: Nationwide case-control study. BMJ 2020, 370, m2791. [Google Scholar] [CrossRef]
- Dezateux, C.; Stocks, J.; Dundas, I.; Fletcher, M.E. Impaired airway function and wheezing in infancy: The influence of maternal smoking and a genetic predisposition to asthma. Am. J. Respir. Crit. Care Med. 1999, 159, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, S.L.; Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Li, Z.; Roberg, K.A.; Anderson, E.L.; Carlson-Dakes, K.T.; Adler, K.J.; Gilbertson-White, S.; et al. Viral infections, cytokine dysregulation and the origins of childhood asthma and allergic diseases. Pediatr. Infect. Dis. J. 2005, 24, S170–S176, disscusion S174–S175. [Google Scholar] [CrossRef] [PubMed]
- Etzel, R.A. How environmental exposures influence the development and exacerbation of asthma. Pediatrics 2003, 112, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Spann, K.; Snape, N.; Baturcam, E.; Fantino, E. The Impact of Early-Life Exposure to Air-borne Environmental Insults on the Function of the Airway Epithelium in Asthma. Ann. Glob. Health 2016, 82, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouazza, N.; Foissac, F.; Urien, S.; Guedj, R.; Carbajal, R.; Treluyer, J.M.; Chappuy, H. Fine particulate pollution and asthma exacerbations. Arch. Dis. Child. 2018, 103, 828–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfino, R.J.; Quintana, P.J.; Floro, J.; Gastanaga, V.M.; Samimi, B.S.; Kleinman, M.T.; Liu, L.J.; Bufalino, C.; Wu, C.F.; McLaren, C.E. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ. Health Perspect. 2004, 112, 932–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.M.; Phaneuf, D.J.; Barrett, M.A.; Su, J.G. Short-term impact of PM2.5 on contemporaneous asthma medication use: Behavior and the value of pollution reductions. Proc. Natl. Acad. Sci. USA 2019, 116, 5246–5253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habre, R.; Moshier, E.; Castro, W.; Nath, A.; Grunin, A.; Rohr, A.; Godbold, J.; Schachter, N.; Kattan, M.; Coull, B.; et al. The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, A.A.T.; Hosseini, A.; Rocchio, R.; Jacobs, N.; Ross, M.K.; Okelo, S.; Lurmann, F.; Eckel, S.; Dzubur, E.; Dunton, G.; et al. Biomedical REAl-Time Health Evaluation (BREATHE): Toward an mHealth informatics platform. JAMIA Open 2020, 3, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Knudson, R.J.; Lebowitz, M.D.; Holberg, C.J.; Burrows, B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am. Rev. Respir. Dis. 1983, 127, 725–734. [Google Scholar] [CrossRef] [PubMed]
- DuRivage, N.; Ross, M.; Mayne, S.L.; Suh, A.; Weng, D.; Grundmeier, R.W.; Fiks, A.G. Asthma Control Test. Clin. Pediatr. 2017, 56, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Alduchov, O.A. Improved Magnus Form Approximation of Saturation Vapor Pressure. J. Appl. Meterology Climatol. 1996, 35, 601–609. [Google Scholar] [CrossRef]
- Snyder, M.G.; Venkatram, A.; Heist, D.K.; Perry, S.G.; Petersen, W.B.; Isakov, V. RLINE: A line source dispersion model for near-surface releases. Atmos. Environ. 2013, 77, 748–756. [Google Scholar] [CrossRef]
- Samoli, E.; Dimakopoulou, K.; Evangelopoulos, D.; Rodopoulou, S.; Karakatsani, A.; Veneti, L.; Sionidou, M.; Tsolakoglou, I.; Krasanaki, I.; Grivas, G.; et al. Is daily exposure to ozone associated with respiratory morbidity and lung function in a representative sample of schoolchildren? Results from a panel study in Greece. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Angelis, N.; Spyratos, D.; Domvri, K.; Dimakopoulou, K.; Samoli, E.; Kalamaras, G.; Karakatsani, A.; Grivas, G.; Katsouyanni, K.; Papakosta, D. Effect of Ambient Ozone Exposure Assessed by Individual Monitors on Nasal Function and Exhaled NO Among School Children in the Area of Thessaloniki, Greece. J Occup. Environ. Med. 2017, 59, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.V.; Bohnet, W.; Frischer, T.; Ulmer, C.; Studnicka, M.; Ihorst, G.; Gardner, C.; Forster, J.; Urbanek, R.; Kuehr, J. Effects of ambient ozone on lung function in children over a two-summer period. Eur. Respir. J. 2000, 16, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.K.; Wu, C.C.; Lee, L.T.; Lin, R.S.; Yu, Y.H.; Chen, Y.C. The short-term effects of air pollution on adolescent lung function in Taiwan. Chemosphere 2012, 87, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Tager, I.B.; Balmes, J.; Lurmann, F.; Ngo, L.; Alcorn, S.; Kunzli, N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology 2005, 16, 751–759. [Google Scholar] [CrossRef]
- Geyh, A.S.; Xue, J.; Ozkaynak, H.; Spengler, J.D. The Harvard Southern California Chronic Ozone Exposure Study: Assessing ozone exposure of grade-school-age children in two Southern California communities. Environ. Health Perspect. 2000, 108, 265–270. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project: Technical Report; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Bell, M.L.; Zanobetti, A.; Dominici, F. Who is more affected by ozone pollution? A systematic review and meta-analysis. Am. J. Epidemiol. 2014, 180, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Arjomandi, M.; Tager, I.B.; Holland, N.; Balmes, J.R. Effects of antioxidant enzyme polymorphisms on ozone-induced lung function changes. Eur. Respir. J. 2007, 30, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, F.J. Dietary antioxidants and environmental stress. Proc. Nutr. Soc. 2004, 63, 579–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudway, I.S.; Kelly, F.J. Ozone and the lung: A sensitive issue. Mol. Asp. Med. 2000, 21, 1–48. [Google Scholar] [CrossRef]
- Steerenberg, P.A.; Nierkens, S.; Fischer, P.H.; van Loveren, H.; Opperhuizen, A.; Vos, J.G.; van Amsterdam, J.G. Traffic-related air pollution affects peak expiratory flow, exhaled nitric oxide, and inflammatory nasal markers. Arch. Environ. Health 2001, 56, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.M.; Balmes, J.R. Systematic Review of Ozone Effects on Human Lung Function, 2013 Through 2020. Chest 2022, 161, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Lurmann, F.; Avol, E.; Gilliland, F. Emissions reduction policies and recent trends in Southern California’s ambient air quality. J. Air Waste Manag. Assoc. 2015, 65, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Williams, G.; Jalaludin, B.; Baker, P. Panel studies of air pollution on children’s lung function and respiratory symptoms: A literature review. J. Asthma 2012, 49, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef]
- See, S.W.; Balasubramanian, R. Chemical characteristics of fine particles emitted from different gas cooking methods. Atmos. Environ. 2008, 42, 8852–8862. [Google Scholar] [CrossRef]
- Schildcrout, J.S.; Sheppard, L.; Lumley, T.; Slaughter, J.C.; Koenig, J.Q.; Shapiro, G.G. Ambient air pollution and asthma exacerbations in children: An eight-city analysis. Am. J. Epidemiol. 2006, 164, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Pepper, J.R.; Barrett, M.A.; Su, J.G.; Merchant, R.; Henderson, K.; Van Sickle, D.; Balmes, J.R. Geospatial-temporal analysis of the impact of ozone on asthma rescue inhaler use. Environ. Int. 2020, 136, 105331. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Mar, T.F.; Larson, T.V.; Stier, R.A.; Claiborn, C.; Koenig, J.Q. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal. Toxicol. 2004, 16, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Galeone, C.; Lelii, M.; Longhi, B.; Ascolese, B.; Senatore, L.; Prada, E.; Montinaro, V.; Malerba, S.; Patria, M.F.; et al. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulm. Med. 2014, 14, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timonen, K.L.; Pekkanen, J. Air pollution and respiratory health among children with asthmatic or cough symptoms. Am. J. Respir. Crit. Care Med. 1997, 156, 546–552. [Google Scholar] [CrossRef]
- Jalaludin, B.B.; O’Toole, B.I.; Leeder, S.R. Acute effects of urban ambient air pollution on respiratory symptoms, asthma medication use, and doctor visits for asthma in a cohort of Australian children. Environ. Res. 2004, 95, 32–42. [Google Scholar] [CrossRef]
- Williams, R.; Duvall, R.; Kilaru, V.; Hagler, G.; Hassinger, L.; Benedict, K.; Rice, J.; Kaufman, A.; Judge, R.; Pierce, G.; et al. Deliberating performance targets workshop: Potential paths for emerging PM2.5 and O3 air sensor progress. Atmos. Environ. X 2019, 2, 100031. [Google Scholar] [CrossRef]
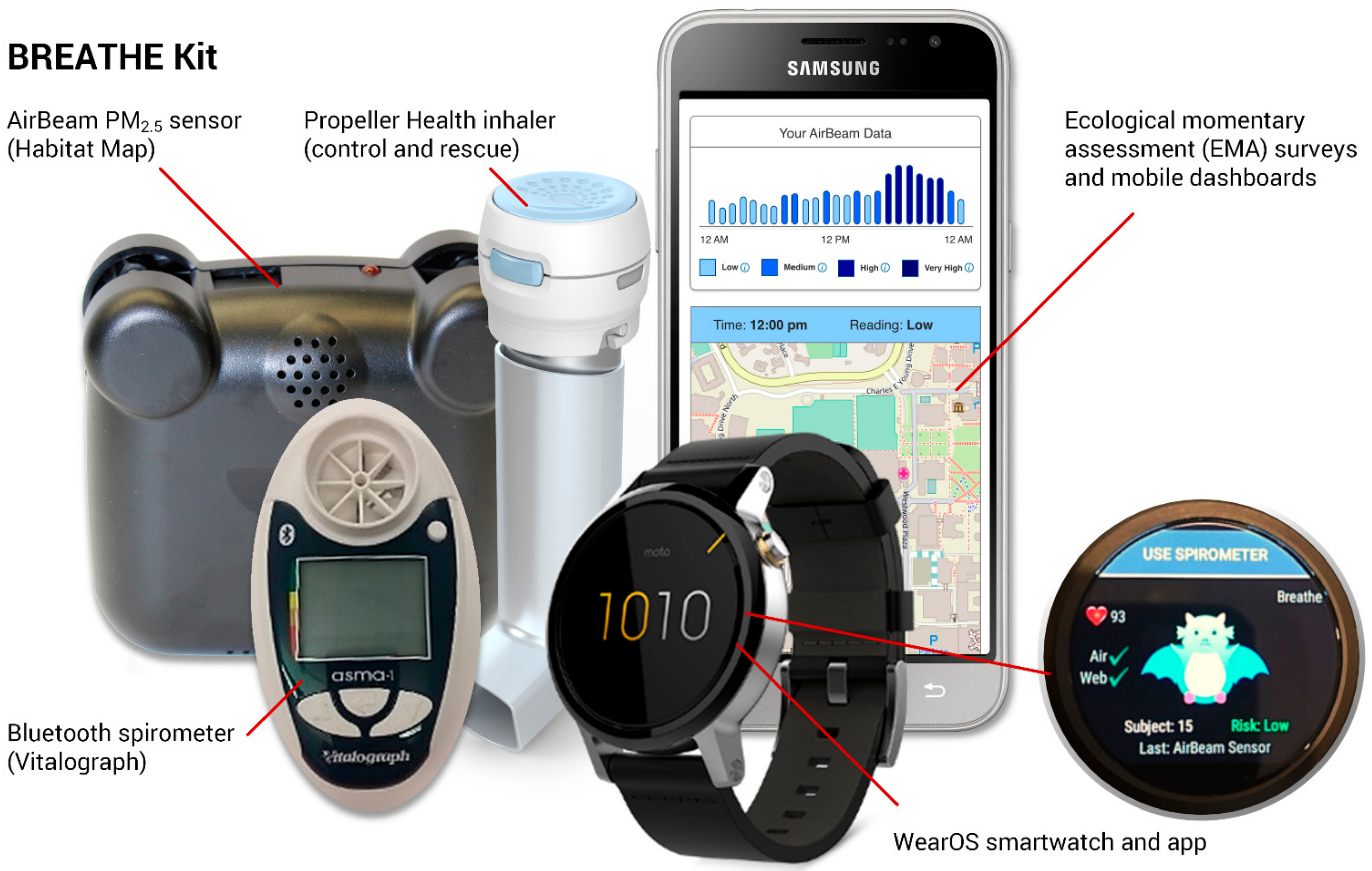
| Characteristics | Statistics |
|---|---|
| Age (years, mean (range)) | 12.0 (8.4–16.8) |
| Sex (n (%)) | |
| Female | 18 (45.0) |
| Male | 22 (55.0) |
| Race (n (%)) | |
| White | 15 (37.5) |
| Black/African American | 2 (5.0) |
| Black/Not African American | 1 (2.5) |
| Asian | 4 (10.0) |
| Other | 15 (37.5) |
| Missing | 3 (7.5) |
| Hispanic Ethnicity (n (%)) | |
| No | 19 (47.5) |
| Yes | 17 (42.5) |
| Missing | 4 (10.0) |
| Caretaker’s highest completed educational grade (n (%)) | |
| High school or GED | 3 (7.5) |
| Some college or trade school | 9 (22.5) |
| College | 9 (22.5) |
| Graduate school | 15 (37.5) |
| Missing | 4 (10.0) |
| Total household income per year (n (%)) | |
| Prefer not to say | 12 (30) |
| USD 30,000–40,000 | 2 (5.0) |
| Over USD 50,000 | 23 (57.5) |
| Missing | 3 (7.5) |
| Type of Health Insurance (n (%)) | |
| HMO | 18 (45.0) |
| PPO or POS | 20 (50.0) |
| Missing | 2 (5.0) |
| Lung Function | Mean ± SD |
|---|---|
| Percent-predicted FEV1 (%) | |
| Morning (n = 175) | 67.9 ± 17.3 |
| Evening (n = 147) | 70.9 ± 17.7 |
| Daily Average (n = 96) | 68.7 ± 15.7 |
| Percent-predicted PEF (%) | |
| Morning (n = 175) | 69.1 ± 18.4 |
| Evening (n = 147) | 73.8 ± 18.3 |
| Daily Average (n = 96) | 69.3 ± 15.8 |
| Inhaler Medication | Mean ± SD |
| Number of rescue inhaler puffs per day (n = 324) | 1.4 ± 3.5 |
| Number of control inhaler puffs per day (n = 312) | 1.5 ± 1.9 |
| Asthma Symptoms | n (%) |
| Did you wake up last night because of your asthma? | |
| No | 123 (93.2) |
| Yes | 9 (6.8) |
| How many times did you use your inhaler during the night? | |
| Never | 111 (84.1) |
| One or more times | 21 (15.9) |
| How much of the time did your asthma keep you from getting as much done at school or at home today? | |
| Not at all | 94 (86.2) |
| A little/Quite a bit/Very much so | 15 (13.8) |
| Did your chest feel tight because of asthma today? | |
| Not at all | 138 (63.6) |
| A little/Quite a bit/Very much so | 79 (26.4) |
| Did you feel wheezy because of your asthma today? | |
| Not at all | 179 (82.5) |
| A little/Quite a bit/Very much so | 38 (17.5) |
| Did you have trouble breathing because of your asthma today? | |
| Not at all | 147 (67.7) |
| A little/Quite a bit/Very much so | 70 (32.3) |
| Did you cough because of your asthma today? | |
| Not at all | 124 (57.1) |
| A little/Quite a bit/Very much so | 93 (42.9) |
| How much of a problem was your asthma when you ran, exercised, or played sports today? | |
| Not at all | 79 (71.8) |
| A little/Quite a bit/Very much so | 31 (28.2) |
| In the past hour, did you feel scared that you might have trouble breathing because of your asthma? | |
| Not at all | 152 (81.7) |
| A little/Quite a bit/Very much so | 34 (18.3) |
| In the past hour, have you avoided strenuous activities, or had to slow down or stop exercising because of your asthma? | |
| Not at all | 155 (83.3) |
| A little/Quite a bit/Very much so | 31 (16.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Eckel, S.P.; Hosseini, A.; Van Vliet, E.D.S.; Dzubur, E.; Dunton, G.; Chang, S.Y.; Craig, K.; Rocchio, R.; Bastain, T.; et al. Daily Associations of Air Pollution and Pediatric Asthma Risk Using the Biomedical REAI-Time Health Evaluation (BREATHE) Kit. Int. J. Environ. Res. Public Health 2022, 19, 3578. https://doi.org/10.3390/ijerph19063578
Hao H, Eckel SP, Hosseini A, Van Vliet EDS, Dzubur E, Dunton G, Chang SY, Craig K, Rocchio R, Bastain T, et al. Daily Associations of Air Pollution and Pediatric Asthma Risk Using the Biomedical REAI-Time Health Evaluation (BREATHE) Kit. International Journal of Environmental Research and Public Health. 2022; 19(6):3578. https://doi.org/10.3390/ijerph19063578
Chicago/Turabian StyleHao, Hua, Sandrah P. Eckel, Anahita Hosseini, Eleanne D. S. Van Vliet, Eldin Dzubur, Genevieve Dunton, Shih Ying Chang, Kenneth Craig, Rose Rocchio, Theresa Bastain, and et al. 2022. "Daily Associations of Air Pollution and Pediatric Asthma Risk Using the Biomedical REAI-Time Health Evaluation (BREATHE) Kit" International Journal of Environmental Research and Public Health 19, no. 6: 3578. https://doi.org/10.3390/ijerph19063578
APA StyleHao, H., Eckel, S. P., Hosseini, A., Van Vliet, E. D. S., Dzubur, E., Dunton, G., Chang, S. Y., Craig, K., Rocchio, R., Bastain, T., Gilliland, F., Okelo, S., Ross, M. K., Sarrafzadeh, M., Bui, A. A. T., & Habre, R. (2022). Daily Associations of Air Pollution and Pediatric Asthma Risk Using the Biomedical REAI-Time Health Evaluation (BREATHE) Kit. International Journal of Environmental Research and Public Health, 19(6), 3578. https://doi.org/10.3390/ijerph19063578






