An Assessment of the Metal Removal Capability of Endemic Chilean Species
Abstract
:1. Introduction
2. Materials and Methods
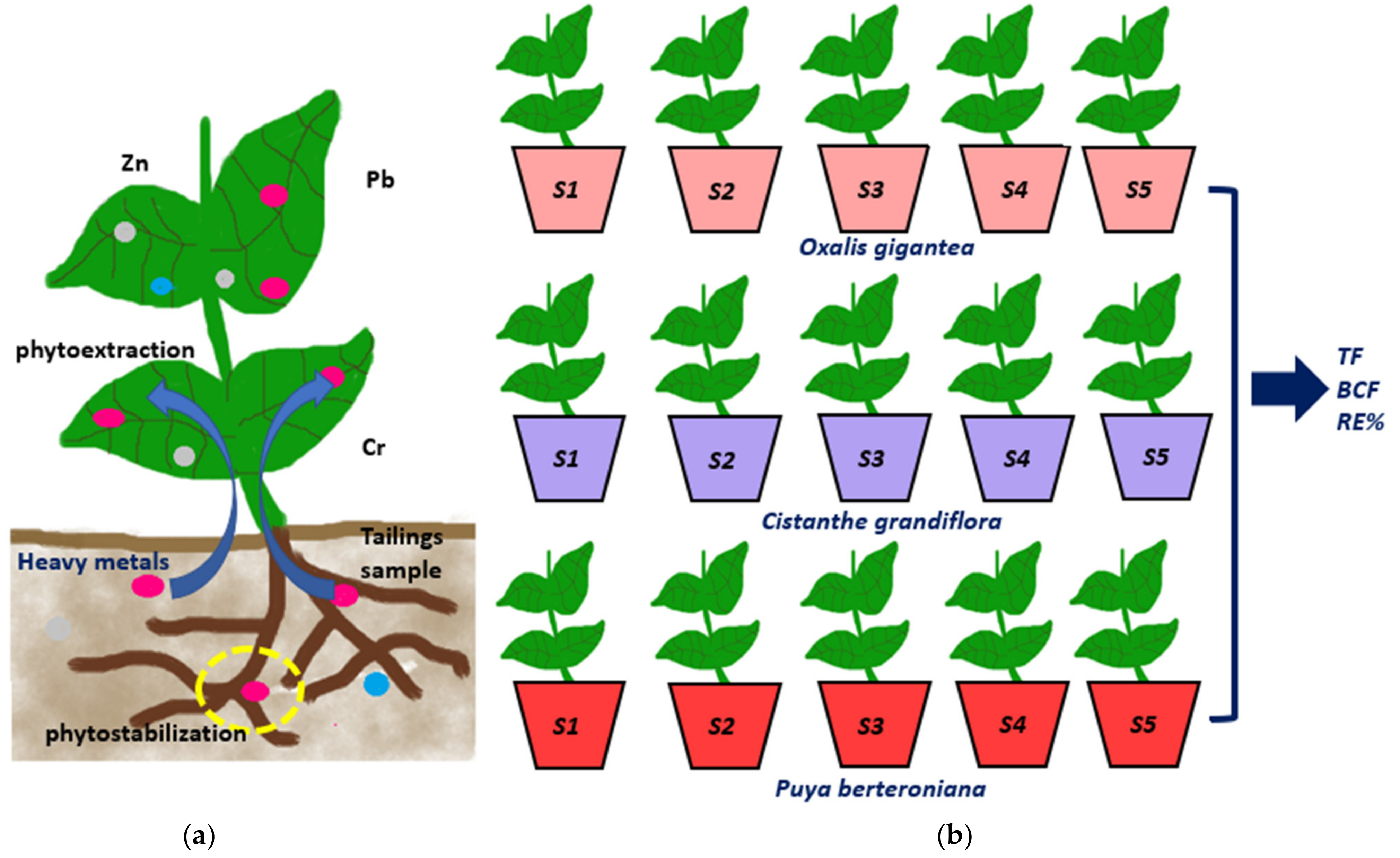
2.1. Plant Species
- Oxalis gigantea Barnéoud, commonly called Churqui or Churco. It is a very common endemic Chilean shrub belonging to the Oxalidaceae family. This species can be found between the regions of Antofagasta and Coquimbo.
- Cistanthe grandiflora, a succulent plant, commonly called Pata de guanaco or Doquilla, is a perennial species endemic to Chile. It can be found frequently between the regions of Antofagasta and Ñuble, and it belongs to the Portulacaceae family.
- Puya berteroniana is an endemic Chilean shrub from the Bromeliaceae family. This species grows between the regions of Coquimbo and Maule. It is commonly called Chagual or Magüey, and it has excellent ornamental value.
2.2. Tailing Samples
2.3. Sample Preparation and ICP-OES Measurements
2.4. Theory/Calculations
3. Results
3.1. Concentration of Metals in Tailings
3.2. Concentration of Metals in Plants and Control Samples
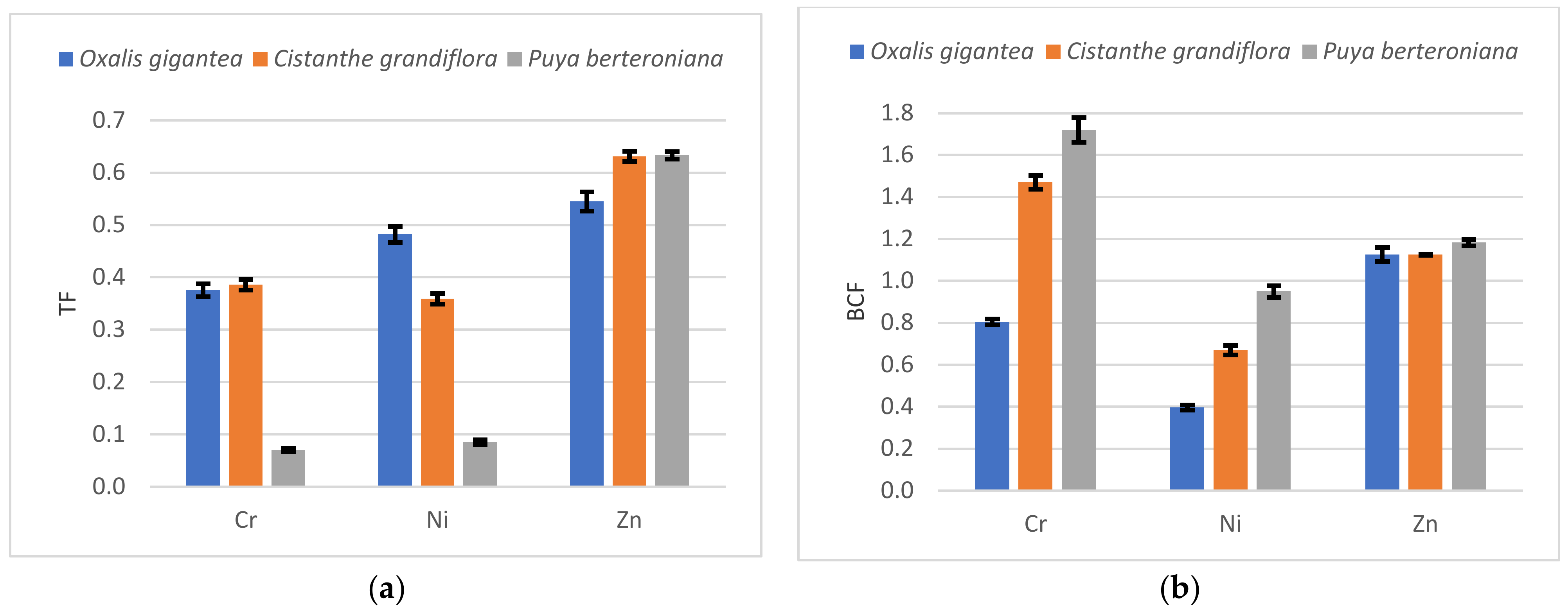
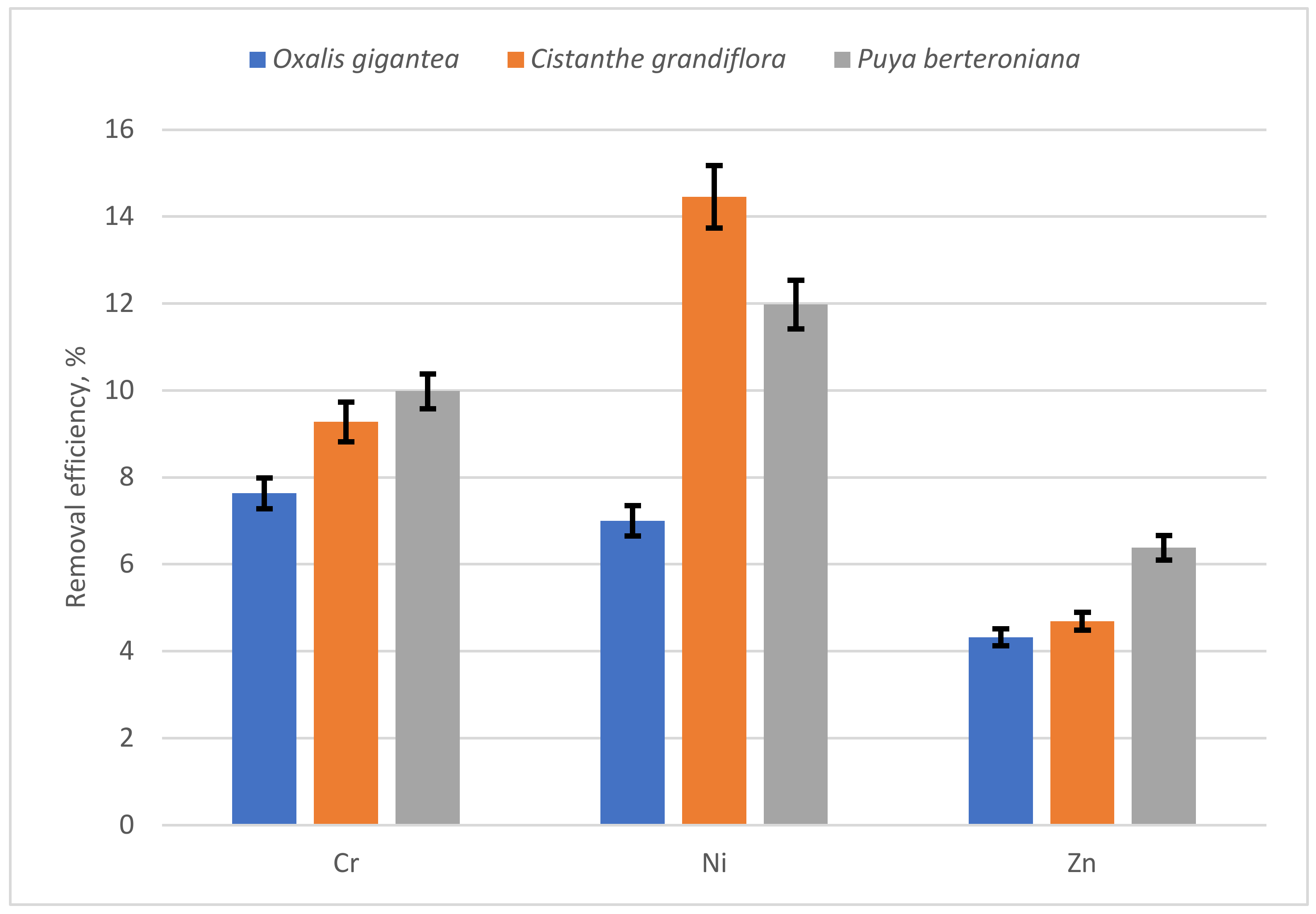
3.3. Phytoremediation Factors and Removal Efficiency
4. Discussion
4.1. Presence of Metals in the Original Tailings
4.2. Efficiency of the Phytoremediation Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Rev. Environ. Sci. Biotechnol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Lam, E.J.; Zetola, V.; Ramírez, Y.; Montofré, Í.L.; Pereira, F. Making Paving Stones from Copper Mine Tailings as Aggregates. Int. J. Environ. Res. Public Health 2020, 17, 2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sernageomin. Servicio Nacional de Geología y Minería (Chile). Available online: https://www.sernageomin.cl/datos-publicos-deposito-de-relaves/ (accessed on 21 February 2022).
- Uugwanga, M.; Kgabi, N. Assessment of Metals Pollution in Sediments and Tailings of Klein Aub and Oamites Mine Sites, Namibia. Environ. Adv. 2020, 2, 100006. [Google Scholar] [CrossRef]
- Radziemska, M.; Vaverková, M.D.; Baryla, A. Phytostabilization—Management Strategy for Stabilizing Trace Elements in Contaminated Soils. Int. J. Environ. Res. Public Health 2017, 14, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourret, O.; Bollinger, J.-C.; Hursthouse, A. Heavy metal: A misused term. Acta Geochim. 2021, 40, 466–471. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, J.; Wang, T.; Moryani, H.T.; Kang, W.; Zheng, J.; Zhan, C.; Xiao, W. Hazardous Heavy Metals Accumulation and Health Risk Assessment of Different Vegetable Species in Contaminated Soils from a Typical Mining City, Central China. Int. J. Environ. Res. Public Health 2021, 18, 2617. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, X.; Wang, P.; Hu, Y.; Cheng, H. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef]
- Luo, C.; Routh, J.; Dario, M.; Sarkar, S.; Wei, L.; Luo, D.; Liu, Y. Distribution and mobilization of heavy metals at an acid mine drainage affected region in South China, a post-remediation study. Sci. Total Environ. 2020, 724, 138122. [Google Scholar] [CrossRef]
- Akoto, R.; Anning, A.K. Heavy metal enrichment and potential ecological risks from different solid mine wastes at a mine site in Ghana. Environ. Adv. 2021, 3, 100028. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Yan, X.; Liang, T.; Yang, X.; El-Naggar, A.; Liu, J.; Chen, H. Evaluation of potential ecological risks in potential toxic elements contaminated agricultural soils: Correlations between soil contamination and polymetallic mining activity. J. Environ. Manag. 2021, 300, 113679. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, L.; Rajendran, M.; Ramamoorthy, K.; Narayanan, M.; Kandasamy, S. 11-Phytostabilization of metal mine tailings—A green remediation technology. In Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water; Kumar, V., Shah, M.P., Kumar Shahi, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Urrutia, C.; Mansilla-Yañez, R.; Jeison, D. Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res. 2019, 43, 101659. [Google Scholar] [CrossRef]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in an abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2021, 220, 112368. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Tiwari, M.; Dutta, P.; Singh, P.; Chawda, K.; Kumari, M.; Chakrabarty, D. Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation. Sustainability 2021, 13, 4629. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Rijkswaterstaat Ministry of Infrastructure and the Environment. Into Dutch Soils. 2014. Available online: www.rijkswaterstaat.nl/en (accessed on 24 November 2021).
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- WHO. Permissible Limits of Heavy Metals in Soil and Plants; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Yan, A.; Wang, Y.; Ngin, T.S.; Lokman, M.Y.M.; Subhadip, G.; Zhong, C. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Lan. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Futughe, A.E.; Purchase, D.; Jones, H. Phytoremediation Using Native Plants. In Phytoremediation. Concepts and Strategies in Plant Sciences; Shmaefsky, B., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Policarpo, F.C.; Policarpo, F.M.; Severino, M.; De Melo Nunes, N.A. Chapter 3—Mechanisms of phytoremediation. In Phytoremediation Biotechnological Strategies for Promoting Invigorating Environs; Ahmad Bhat, R., Policarpo, M.F., Hamid Dar, G., Hakeem, K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 37–64. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, W.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, S.; Kumar, A.; Singh, A.N. Chapter 15—Phytoremediation: A wonderful cost-effective tool. In Advances in Environmental Pollution Research, Cost Effective Technologies for Solid Waste and Wastewater Treatment; Kathi, S., Devipriya, S., Thamaraiselvi, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 179–208. [Google Scholar] [CrossRef]
- Bilal, T.; Malik, B.; Tahir, I.; Kumar, M.; Varma, A.; Rehman, R. Chapter 5—Phytoremediation: An Eco-Friendly Green Technology for Pollution Prevention, Control and Remediation. In Soil Remediation and Plants, Prospects and Challenges, 1st ed.; Hakeem, K., Sabir, M., Ozturkt, M., Mermut, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 107–129. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Van der Ent, A. A global database for plants that hyper accumulate metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Zeremski, T.; Ranđelović, D.; Jakovljević, K.; Marjanović Jeromela, A.; Milić, S. Brassica Species in Phytoextractions: Real Potentials and Challenges. Plants 2021, 10, 2340. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Kumar, V.; Tripathi, S.; Sharma, P. Heavy metal phytoextraction potential of native weeds and grasses from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng. 2018, 111, 143–156. [Google Scholar] [CrossRef]
- Jeleni, M.N.; Gumbo, J.R.; Muzerengi, C.; Dacosta, F.A. An Assessment of Toxic Metals in Soda Mine Tailing and a Native Grass: A Case Study of an Abandoned Nyala Magnesite Mine, Limpopo, South Africa. WIT Trans. Ecol. Environ. 2012, 164, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Ji, P.; Huang, X.; Jiang, Y.; Zhao, H. Potential of enhancing the phytoremediation efficiency of Solanum nigrum L. by earthworms. Int. J. Phytoremediat. 2020, 22, 529–533. [Google Scholar] [CrossRef]
- Cailian, Y.; Xianlong, P.; Hong, Y.; Xiaoxia, L.; Zhenhua, Z.; Tingliang, Y. Phytoremediation Ability of Solanum nigrum L. to Cd-Contaminated Soils with High Levels of Cu, Zn and Pb. Water Air Soil Pollut. 2015, 226, 157. [Google Scholar] [CrossRef]
- Lam, E.J.; Keith, B.F.; Montofré, Í.L.; Gálvez, M.E. Copper Uptake by Adesmia atacamensis in a Mine Tailing in an Arid Environment. Air Soil Water Res. 2018, 11, 1178622118812462. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.; Cánovas, M.; Gálvez, M.E.; Montofré, I.L.; Keith, B.F.; Faz, Á. Evaluation of the phytoremediation potential of native plants growing on a copper mine tailing in northern Chile. J. Geochem. Explor. 2017, 182, 210–217. [Google Scholar] [CrossRef]
- Chileflora. Available online: http://www.chileflora.com/Florachilena/FloraSpanish/PIC_NORTHERN_PLANTS_0.php (accessed on 29 November 2020).
- Lazo, P.; Lazo, A. Assessment of Native and Endemic Chilean Plants for Removal of Cu, Mo and Pb from Mine Tailings. Minerals 2020, 10, 1020. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. SW-846 Test Method 9045D: Soil and Waste pH. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-9045d-soil-and-waste-ph (accessed on 21 February 2022).
- Mishra, T.; Pandey, V.C. Chapter 16—Phytoremediation of Red Mud Deposits Through Natural Succession. In Phytomanagement of Polluted Sites, Market Opportunities in Sustainable Phytoremediation; Mishra, T., Pandey, V.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–424. [Google Scholar] [CrossRef]
- Sulaiman, F.R.; Hamzah, H.A. Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol. Process. 2018, 7, 28. [Google Scholar] [CrossRef]
- Gajić, G.; Mitrović, M.; Pavlović, P. 6—Feasibility of Festuca rubra L. native grass in phytoremediation. In Phytoremediation Potential of Perennial Grasses; Pandey, C.V., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–164. [Google Scholar] [CrossRef]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Silveira, M.; Camargo, F.A.O.; Andreazza, R. Bioprospection of indigenous flora grown in copper mining tailing area for phytoremediation of metals. J. Environ. Manag. 2020, 256, 109953. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.; Chakravarty, P.; Sharma, P.; Sinha, R.; Kumar, M.; Bauddh, K. Chapter 1—Phytoremediation: A sustainable method for cleaning up the contaminated sites. In Phytorestoration of Abandoned Mining and Oil Drilling Sites; Bauddh, K., Korstad, J., Sharma, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–32. [Google Scholar] [CrossRef]
- Chamba, I.; Gazquez, M.J.; Selvaraj, T.; Calva, J.; Toledo, J.J.; Armijos, C. Selection of a suitable plant for phytoremediation in mining artisanal zones. Int. J. Phytoremediat. 2016, 9, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Soil Rijkswaterstaat Environment. Soil Remediation Circular 2013. Available online: https://rwsenvironment.eu/subjects/soil/legislation-and/soil-remediation/ (accessed on 24 November 2021).
- Rodríguez, F.; Moraga, C.; Castillo, J.; Gálvez, E.; Robles, P.; Toro, N. Submarine Tailings in Chile—A Review. Metals 2021, 11, 780. [Google Scholar] [CrossRef]
- Servicio Nacional de Geología y Minería. Geoquímica de Superficie de Depósitos de Relaves de Chile; Servicio Nacional de Geología y Minería: Santiago, Chile, 2018.
- Manikandan, M.; Kannan, V.; Mahalingam, K.; Vimala, A.; Chun, S. Phytoremediation Potential of Chromium Containing Tannery Effluent Contaminated Soil by Native Indian Timber Yielding Tree Species. Prep. Biochem. Biotechnol. 2015, 46, 100–108. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Vecino-Bueno, I.; Feldman, S. Accumulation and tolerance characteristics of chromium in a cordgrass Cr-hyperaccumulator, Spartina argentinensis. J. Hazard. Mater. 2011, 185, 862–869. [Google Scholar] [CrossRef]

| Parameter | Value ± Standard Deviation |
|---|---|
| Specific gravity | 2.78 ± 0.25 |
| pH | 7.30 ± 0.10 |
| Solid concentration (weight %) | 82.00 ± 1 |
| Granulometry d50 micrometers | 0.046 ± 0.001 |
| Electric conductivity (dS m−1 at 25 °C) | 32.20 ± 0.10 |
| Cu mg kg−1 dry weight | 1582.22 ± 78.31 |
| Mo mg kg−1 dry weight | 3.86 ± 0.17 |
| Pb mg kg−1 dry weight | 228.15 ± 2.79 |
| Zn mg kg−1 dry weight | 869.80 ± 31.54 |
| Ni mg kg−1 dry weight | 94.64 ± 2.57 |
| Cr mg kg−1 dry weight | 154.63 ± 5.41 |
| Cd mg kg−1 dry weight | Under detection limit |
| Calcium percentage as oxide, CaO% w/w | 9.92 ± 0.32 |
| Magnesium percentage as oxide, MgO% w/w | 5.56 ± 0.27 |
| Manganese percentage as oxide, MnO% w/w | 0.26 ± 0.01 |
| Sodium percentage as oxide, Na2O% w/w | 2.63 ± 0.11 |
| Potassium percentage as oxide, K2O% w/w | 2.24 ± 0.12 |
| Phosphorous percentage as oxide, P2O5% w/w | 0.23 ± 0.01 |
| Element | Concentration mg kg−1 Dry Weight ± Standard Deviation | |||
|---|---|---|---|---|
| Initial Tailings | Final Tailings | |||
| Oxalis gigantea | Cistanthe grandiflora | Puya berteroniana | ||
| Zn | 869.80 ± 31.54 | 832.17 ± 32.67 | 828.96 ± 25.67 | 803.88 ± 37.89 |
| Ni | 94.64 ± 2.57 | 87.98 ± 3.32 | 80.93 ± 2.89 | 83.27 ± 4.06 |
| Cr | 154.63 ± 5.41 | 142.79 ± 6.52 | 140.25 ± 5.98 | 141.83 ± 4.87 |
| Element | Concentration mg kg−1 Dry Weight ± Standard Deviation | ||
|---|---|---|---|
| Oxalis gigantea | Cistanthe grandiflora | Puya berteroniana | |
| Cr stems and leaves t = 0 | <d.l | <d.l | <d.l |
| Cr stems and leaves t = tfinal | 0.01 ± 0.00 | 0.01 ± 0.00 | <d.l |
| Cr roots t = 0 | 0.01 ± 0.00 | <d.l | <d.l |
| Cr roots t = tfinal | 0.01 ± 0.00 | <d.l | <d.l |
| Ni stems and leaves t = 0 | 1.85 ± 0.08 | 6.12 ± 0.28 | 5.76 ± 0.08 |
| Ni stems and leaves t = tfinal | 2.01 ± 0.06 | 6.25 ± 0.19 | 6.76 ± 0.12 |
| Ni roots t = 0 | 2.55 ± 0.10 | 4.14 ± 0.52 | 1.97 ± 0.05 |
| Ni roots t = tfinal | 2.85 ± 0.07 | 4.56 ± 0.47 | 1.81 ± 0.05 |
| Zn stems and leaves t = 0 | 13.39 ± 0.45 | 28.79 ± 0.60 | 24.10 ± 0.95 |
| Zn stems and leaves t = tfinal | 13.25 ± 0.55 | 32.55 ± 0.42 | 23.55 ± 0.88 |
| Zn roots t = 0 | 16.82 ± 0.50 | 16.12 ± 0.09 | 17.96 ± 0.88 |
| Zn roots t = tfinal | 17.76 ± 0.61 | 17.65 ± 0.08 | 18.33 ± 0.95 |
| Element | Concentration mg kg−1 Dry Weight ± Standard Deviation | ||
|---|---|---|---|
| Oxalis gigantea | Cistanthe grandiflora | Puya berteroniana | |
| Cr stems and leaves | <d.l | <d.l | <d.l |
| Cr roots | 0.14 ± 0.00 | <d.l | <d.l |
| Ni stems and leaves | 1.98 ± 0.09 | 6.32 ± 0.30 | 5.53 ± 0.06 |
| Ni roots | 2.86 ± 0.09 | 3.80 ± 0.45 | 1.97 ± 0.07 |
| Zn stems and leaves | 13.97 ± 0.50 | 28.24 ± 0.65 | 24.75 ± 1.10 |
| Zn roots | 16.79 ± 0.47 | 16.44 ± 0.09 | 18.25 ± 0.90 |
| Cr Stems and Leaves | Sum of Squares | df | Mean Square | F | p-Value | F. crit | |
|---|---|---|---|---|---|---|---|
| Between groups | 9457.54 | 2 | 4728.77 | 1115.96 | 2.34 × 10−14 | 3.89 | |
| Within groups | 50.87 | 12 | 4.24 | ||||
| Total | 9508.41 | 14 | |||||
| mean | Std. error | q-stat | lower | upper | p-value | ||
| Oxalis gigantea | Puya berteroniana | 34.62 | 0.92 | 37.60 | 31.15 | 38.10 | <0.05 |
| Oxalis gigantea | Cistanthe grandiflora | 26.71 | 0.92 | 29.01 | 23.24 | 30.19 | <0.05 |
| Puya berteroniana | Cistanthe grandiflora | 61.34 | 0.92 | 66.62 | 57.86 | 64.81 | <0.05 |
| Cr roots | Sum of groups | df | Mean square | F | p-value | F. crit | |
| Between groups | 39,951.43 | 2 | 19,975.71 | 338.42 | 1.44 × 10−12 | 3.89 | |
| Within groups | 429.27 | 12 | 35.77 | ||||
| Total | 40,380,70 | ||||||
| mean | Std. error | q-stat | lower | upper | p-value | ||
| Oxalis gigantea | Puya berteroniana | 90.47 | 2.67 | 33.83 | 80.36 | 100.57 | <0.05 |
| Oxalis gigantea | Cistanthe grandiflora | 121.70 | 2.67 | 45.97 | 111.60 | 131.79 | <0.05 |
| Puya berteroniana | Cistanthe grandiflora | 31.22 | 2.67 | 11.67 | 21.13 | 41.31 | <0.05 |
| Ni stems and leaves | Sum of squares | df | Mean square | F | p-value | F. crit | |
| Between groups | 411.61 | 2 | 205.81 | 819.49 | 1.47 × 10−34 | 3.89 | |
| Within groups | 3.01 | 12 | 0.25 | ||||
| Total | 14 | ||||||
| Mean | Std. error | q-stat | Lower | Upper | p-value | ||
| Oxalis gigantea | Puya berteroniana | 2.75 | 0.22 | 12.27 | 1.90 | 3.60 | <0.05 |
| Oxalis gigantea | Cistanthe grandiflora | 9.48 | 0.22 | 42.30 | 8.63 | 10.32 | <0.05 |
| Puya berteroniana | Cistanthe grandiflora | 12.23 | 0.22 | 54.57 | 11.38 | 13.07 | <0.05 |
| Ni roots | Sum of squares | df | Mean square | F | p-value | F. crit | |
| Between groups | 4459.05 | 2 | 3229.53 | 1061.87 | 3.15 × 10−14 | 3.89 | |
| Within groups | 36.50 | 12 | 3.04 | ||||
| Total | 6495.55 | 14 | |||||
| Mean | Std. error | q-stat | Lower | Upper | p-value | ||
| Oxalis gigantea | Puya berteroniana | 18.21 | 0.78 | 23.36 | 15.27 | 21.16 | <0.05 |
| Oxalis gigantea | Cistanthe grandiflora | 50.20 | 0.78 | 64.37 | 47.26 | 53.14 | <0.05 |
| Puya berteroniana | Cistanthe grandiflora | 31.99 | 0.78 | 41.01 | 29.04 | 34.93 | <0.05 |
| Zn stems and leaves | Sum of squares | df | Mean square | F | p-value | F. crit | |
| Between groups | 9457.54 | 2 | 4728.77 | 1115.60 | 2.34 × 10−14 | 3.89 | |
| Within groups | 50.86 | 12 | 4.24 | ||||
| Total | 9508.41 | 14 | |||||
| Mean | Std. error | q-stat | Lower | Upper | p-value | ||
| Oxalis gigantea | Puya berteroniana | 83.87 | 3.16 | 26.52 | 71.94 | 95.79 | <0.05 |
| Oxalis gigantea | Cistanthe grandiflora | 95.45 | 3.16 | 30.19 | 83.52 | 107.238 | <0.05 |
| Puya berteroniana | Cistanthe grandiflora | 11.59 | 3.16 | 3.67 | −0.34 | 23.52 | 0.06 |
| Zn roots | Sum of squares | df | Mean square | F | p-value | F. crit | |
| Between groups | 2118.66 | 2 | 1059.33 | 4.41 | 0.04 | 3.89 | |
| Within groups | 2880.03 | 12 | 240.00 | ||||
| Total | 4998.69 | 14 | |||||
| Mean | Std. error | q-stat | Lower | Upper | p-value | ||
| Oxalis gigantea | Puya berteroniana | 2.31 | 6.93 | 0.33 | −23.83 | 28.44 | 0.97 |
| Oxalis gigantea | Cistanthe grandiflora | 26.28 | 6.93 | 3.79 | 0.14 | 52.43 | 0.05 |
| Puya berteroniana | Cistanthe grandiflora | 23.98 | 6.93 | 3.46 | −2.16 | 50.12 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazo, A.; Lazo, P.; Urtubia, A.; Lobos, M.G.; Hansen, H.K.; Gutiérrez, C. An Assessment of the Metal Removal Capability of Endemic Chilean Species. Int. J. Environ. Res. Public Health 2022, 19, 3583. https://doi.org/10.3390/ijerph19063583
Lazo A, Lazo P, Urtubia A, Lobos MG, Hansen HK, Gutiérrez C. An Assessment of the Metal Removal Capability of Endemic Chilean Species. International Journal of Environmental Research and Public Health. 2022; 19(6):3583. https://doi.org/10.3390/ijerph19063583
Chicago/Turabian StyleLazo, Andrea, Pamela Lazo, Alejandra Urtubia, María Gabriela Lobos, Henrik K. Hansen, and Claudia Gutiérrez. 2022. "An Assessment of the Metal Removal Capability of Endemic Chilean Species" International Journal of Environmental Research and Public Health 19, no. 6: 3583. https://doi.org/10.3390/ijerph19063583
APA StyleLazo, A., Lazo, P., Urtubia, A., Lobos, M. G., Hansen, H. K., & Gutiérrez, C. (2022). An Assessment of the Metal Removal Capability of Endemic Chilean Species. International Journal of Environmental Research and Public Health, 19(6), 3583. https://doi.org/10.3390/ijerph19063583







