Abstract
Breast cancer treatments can trigger respiratory sequelae. Respiratory physiotherapy helps to eliminate or mitigate the sequelae by optimizing respiratory function. This systematic review aims to synthesize the scientific evidence and assess its quality regarding the use of respiratory physiotherapy in the sequelae of breast cancer. The Cochrane Library, Physiotherapy Evidence Database, PubMed, Web of Science, Scientific Electronic Library Online, Cumulative Index of Nursing and Allied Literature Complete, and Scopus were searched. Study quality was determined using the PEDro scale, STROBE Statement, and Single-Case Experimental Design Scale. Ten studies, six clinical trials, one case study, and three observational studies were selected. The mean methodological quality of the clinical trials was 5.6, that of the case study was 7, and that of the observational studies was 56%. Respiratory physiotherapy has been observed to improve respiratory capacity, lung function, respiratory muscle strength, effort tolerance, dyspnea, fatigue, thoracic mobility, upper limb volume, sleep quality and quality of life, as well as sensitivity to adverse physiological reactions, nausea, vomiting, and anxiety. However, it is not effective for vasomotor symptoms. More clinical trials are needed. These studies should homogenize the techniques used, as well as improve their methodological quality.
1. Introduction
Breast cancer (BC) is the most common malignant tumors in women worldwide, with the exception of skin cancer [1].
According to the World Health Organization, more than one million new cases are diagnosed each year, accounting for almost a quarter of all malignant tumors in women and affecting one in 100 men. In the West, it has been shown that one in nine to twelve women will suffer from the disease in her lifetime [2].
The incidence rate is increasing. This is indicative of earlier detection, as the mortality rate has not increased at the same rate. This seems to be due to the fact that, together with early diagnosis, treatment intervention is more appropriate to the pathology and the patient, although it is one of the main causes of death from cancer among women in developed countries [3].
However, with improved survival rates, more patients are facing persistent treatment-related symptoms. These treatments can be surgical, systemic (hormonal therapy and chemotherapy), and radiotherapy which, in turn, can have adverse effects on the respiratory system [4].
With regard to the consequences of surgical treatment, it can be found related to immobilization and postoperative injury, including respiratory disorders, reduced mobility of the thorax due to postoperative pain, circulatory disorders, as well as reduced muscle strength and alteration of the cough reflex [5]. In addition, in the case of radical mastectomy, it can lead to disturbances in body posture, causing winged scapulae, ascended shoulders, and increased curvature of the cervical and thoracic spine [6], reducing thoracic and fascial mobility, disturbing ventilatory mechanics, and impairing the functions of the respiratory system [7]. This leads to a reduction in the mobility of the thorax, resulting in reduced respiratory muscle efficiency and fatigue, as well as a decrease in the range of motion of the diaphragm [5].
There is evidence that chest radiation may affect the cardiorespiratory capacity of women with breast cancer due to reduced maximal oxygen consumption compared to healthy people [7].
In addition, incidental exposure to the heart may occur, increasing the risk of coronary heart disease and cardiovascular mortality [8]. Thoracic radiotherapy also decreases respiratory and exercise capacity, probably due to restricted chest wall mobility [9]. There may also be risks of lung parenchymal damage [10,11], loss of type II pneumocytes, and loss of surfactant and basement membrane edema impacting respiratory function and impairing the ability to perform physical activities [12]. It can progress to pulmonary fibrosis that induces a restrictive pattern.
Finally, hormone therapy with tamoxifen and some chemotherapy drugs can also contribute to the appearance of pulmonary toxicity [13,14], decreasing pulmonary function tests such as forced vital capacity, forced expiratory volume in 1 s, total lung capacity, peak expiratory flow at 50% and 25% of vital capacity, and carbon monoxide diffusion capacity [15]. In addition, there is peripheral muscle weakness [16] and respiratory muscle weakness with increased exercise intolerance [17,18].
These findings highlight the importance of respiratory muscle function, especially with regard to exertional dyspnea and reduced exercise performance reported in breast cancer [19].
Physiotherapy is an integral part of treatment for breast cancer patients. It allows patients to regain physical fitness and reduce the side effects of treatment. Respiratory physiotherapy (RP), which consists of a combination of strategies aimed at preventing, treating, and stabilizing cardiorespiratory disorders in adult and pediatric patients [20], is an accepted method to maintain and improve respiratory capacity, quality of life, and post-treatment sequelae of breast cancer [21].
RP has been shown to be helpful in other types of cancer, such as lung cancer [22].
There are numerous studies related to physiotherapy for lymphoedema [23,24,25,26,27], pain [28,29,30,31,32], restoration of shoulder mobility [33,34], and physical training [35,36,37,38]. However, they do not take into account respiratory and other harmful symptoms associated with treatments used in breast cancer.
Therefore, the aim of our study is to synthesize the scientific evidence and assess its quality regarding the use of intervention strategies in RP in the aftermath of breast cancer. It also aims to know the use of respiratory physiotherapy on the negative effects of the treatments used in breast cancer, as well as to know the techniques used.
2. Materials and Methods
A systematic review and meta-analysis was conducted and recorded in PROSPERO (CRD42021227590) using the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [39]. The PRISMA checklist is detailed in Appendix A.
2.1. Search Strategy
The search was conducted from September to October 2020 in the following databases: The Cochrane Library, Physiotherapy Evidence Database (PEDro), PubMed, Web of Science, Scientific Electronic Library Online (SciELO), Cumulative Index of Nursing and Allied Literature Complete (CINAHL), and Scopus.
“Breast Neoplasms”, “physiotherapy”, “breathing exercises”, “breast cancer”, “physical therapy”, “rehabilitation”, “respiratory muscle training”, were used as keywords. These were combined with AND and OR.
2.2. Eligibility Criteria
Eligibility criteria were based on the PICO framework [40]: (P) Participants, over 18 years of age diagnosed with breast cancer who had received adjuvant therapy, after surgery; (I) Intervention, any patient that had undergone any technique within the field of respiratory physiotherapy defined as combination of strategies aimed at preventing, treating, and stabilizing cardiorespiratory disorders in adult and pediatric patients [21]; (C) Comparison, no treatment, placebo, or other intervention; Outcome, any clinical variable that could be improved following respiratory physiotherapy treatment.
No limitations were made in terms of language. Regarding the design of the study, all types of study designs were considered. The search was limited to the last 10 years.
Studies where the intervention was aerobic or resistance exercise programs, breathing exercises in yoga, qigong, tai chi, and pilates, and those where the patient was receiving palliative treatment, were excluded.
2.3. Study Selection Process and Data Extraction
The papers were independently reviewed and selected by two of the researchers. The final result was agreed with a third investigator.
The information extracted from each study was related to authors, number and characteristics of the sample, specific treatment used for cancer, type of respiratory physiotherapy, duration of treatment, outcome measures, measurement instrument, and results obtained.
2.4. Assessment of Methodological Quality
To assess the methodological quality of the clinical trials, the PEDro scale that is based on the Delphi list developed by Verhagen et al. [41] was used: (item 1) specified choice criteria, (item 2) random allocation, (item 3) covert allocation, (item 4) baseline similarity, (item 5) subject blinding, (item 6) therapist blinding, (item 7) assessor blinding, (item 8) more than 85% follow-up for at least one key outcome, (item 9) intention-to-treat analysis, (item 10) statistical comparison between groups for at least one key outcome, and (item 11) point measures and variability for at least one key outcome. Item 1 is not scored. It is scored 1 when the condition is met and 0 when it is not met.
The PEDro scale categorizes clinical trials as “good” quality (score 6–10), “fair” quality (score 4–5), and “poor” quality (score < 4) [42].
For observational studies, the STROBE Statement was used: it looks at the quality of information from observational studies with a focus on prevalence (cut-off, case-control, cross-sectional). It consists of 22 items on the title of articles, abstract, introduction, methods, results, discussion sections, and other information. A total of 18 items are common to all three designs; the other items are design-specific. For some items, information should be given separately for cases and controls in case-control studies, or exposed and non-exposed groups in the cross-sectional study and cross-sectional studies [43].
For the assessment of case studies we used the Single-Case Experimental Design Scale (SCED) which includes 11 items, of which 10 are used to assess methodological quality and the use of statistical analysis [44]. An additional item (specification of the clinical history) is included which is not scored. The items are (item 1) clinical history, (item 2), target behaviors, (item 3), design, (item 4) baseline, (item 5) sampling behavior during treatment, (item 6) raw data record, (item 7) inter-rater reliability, (item 8) independence of assessors, (item 9) statistical analysis, (item 10) replication, and (item 11) generalization.
A dichotomous response format (present/absent) is used, with 1 point if the criterion has been met. Thus, the score ranges from 0 to 10, with higher scores indicating better methodological quality.
2.5. Risk of Bias of Included Studies
The risk of bias was calculated for each study selected using the Cochrane Collaboration Tool [45]. The following types of bias were assessed: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. Two reviewers (M.J.V.-G. and R.M.-V.) assessed the methodological quality and the risk of bias of the studies. In case of doubt, authors resolved disagreements by consensus and consulting a third author (G.G.-M.) when necessary.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results and their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Selection of Studies
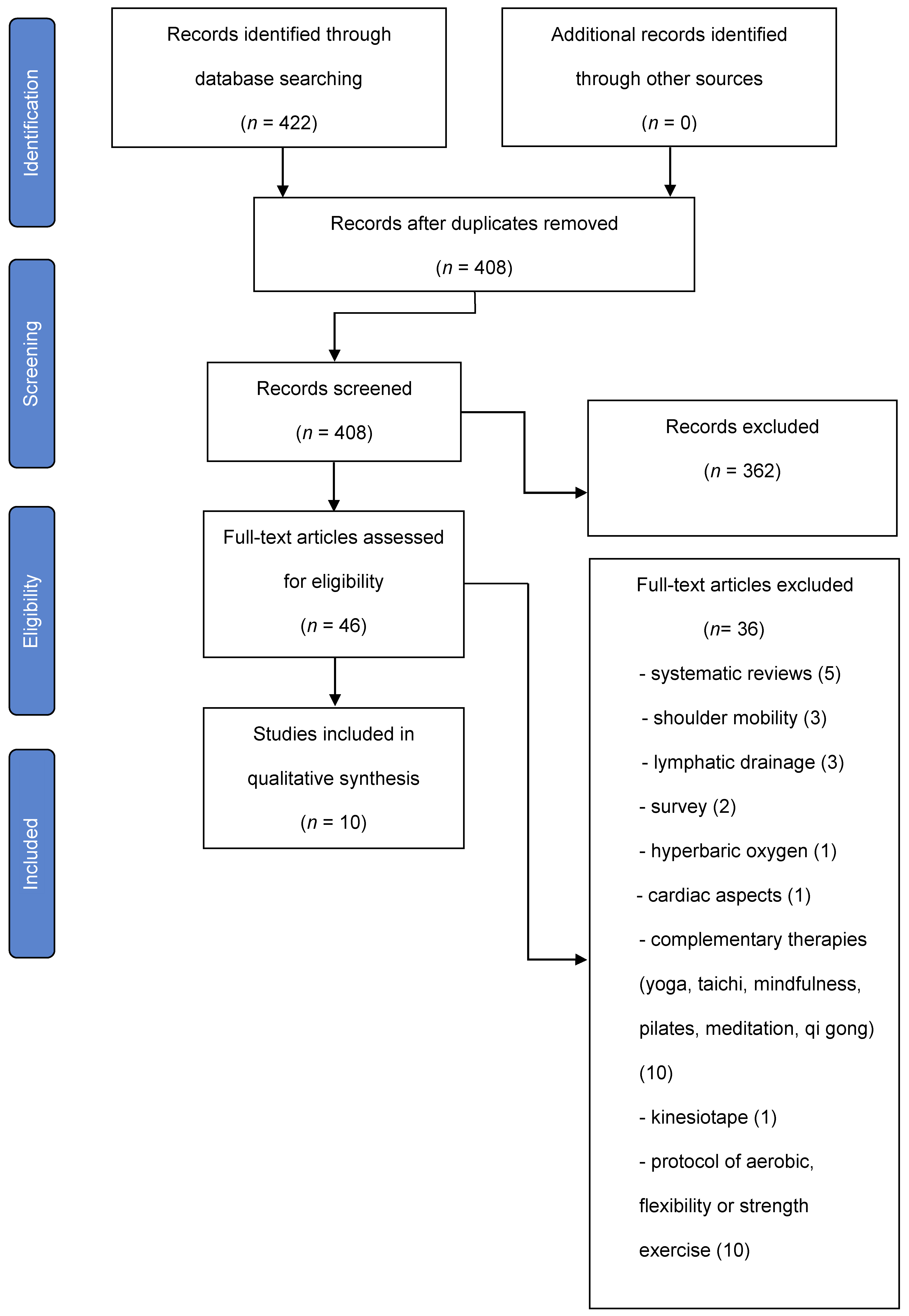
The entire selection process in the different phases is detailed in a PRISMA flow chart (Figure 1).
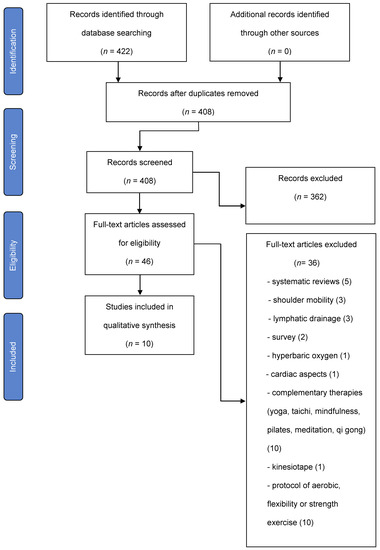
Figure 1.
Flow diagram.
The main characteristics of the studies are shown in Table 1.

Table 1.
Characteristics of the study intervention.
3.2. Data Extraction
The sample consisted of 908 patients, where only 8% were male [50,51]. The number of subjects participating in the studies ranged from 5 [49] to 315 [51] persons and the age of the participants ranged from 54 [46] to 62.5 [55] years, with a mean age of 54. Regarding the type of surgery, radical mastectomy [47,49,51,52,54,55], segmentectomy [49,51,55], and axillary lymphadenectomy [49,51,55] were performed.
In reference to the stage of the cancer, according to the tumor-node-metastasis (TNM) staging system: “stage” I [48,53] and II [47,48,49,52,53] were used. Only one article specified tumor size [52]: 2.5 ± 1.6 (0.1–9) cm3. In the studies in which radiotherapy was used and its doses were specified, it was found that the most commonly used dose was between 40 Gy [47] and 50 Gy [49,52].
All patients underwent surgery, 12% also received radiotherapy and chemotherapy, 42% radiotherapy, 17% chemotherapy, and less than 1% received radiotherapy, chemotherapy, and hormone therapy.
In three of the studies, the sample also consisted of lung cancer patients [50], menopausal women [53], or people with cancer in the abdominal region [51].
Regarding the RP intervention strategies used, there is a lot of variability. RP was used as a sole treatment [50] or was combined with other exercises [47,51,54,55].
Among the RP interventions used were the use of the incentive spirometer and PEP mask [49], respiratory muscle training [56], and the lumbar quadratus lumborum muscle energy technique [47]. In one of the studies the technique or techniques used were not specified [52].
In four of them [47,51,54,55], RP was performed within a broader protocol in combination with other techniques, such as muscle relaxation [51,54], guided imagery and music [51], soft tissue therapy on muscle fascia and postoperative scar [47], and upper limb exercises [47].
Interventions used in the control groups included aerobic exercise [48], nursing care [46,54], or “no training” or rapid shallow breathing exercises [53].
Concerning the number of sessions, frequency and total duration of treatment was very heterogeneous, ranging from 5 times a week, 5 times a day, 30 min [53] to a single session of 45–60 min [51].
The variables studied were inconsistent except for spirometric data [47,48,49,50,52]. Parameters related to respiratory alterations such as fatigue [49,50], effort tolerance [49,50], aerobic capacity [50], dyspnea [50], quality of life [50], and thoracic mobility [47] were evaluated. Vasomotor symptoms [53], nausea [46], vomiting [46], satisfaction [51], pain perception [47], anxiety [54], upper limb volumen [55], mood [53], sleep disturbances [53], and functional status [46] were also assessed.
Regarding the measurement instruments, there is little homogeneity. Spirometric data [47,48,49,50,52] such as FEV1, FVC, and VVM were used to assess lung function; PIM and PEM to assess respiratory muscle strength [48]; Borg scale [49] and FACIT-F [49,50] to measure fatigue; 6 MWT to assess exercise tolerance [49,50]; MRC, BDI, and TDI to measure dyspnea [50]; TUG to assess lower limb mobility and risk of falls [50]; SF-36 [50], QOL37 [50], and MCGill quality of life questionnaire [55], RSCL [54] to measure quality of life; VAS to measure pain [47], STAI to assess anxiety [54], and PANAS and HFRDIS to assess hot flushes [53].
Variables related to upper limb symptomatology [55] were assessed by perimetry, bioimpedance and tonometry, mood [53] with POMS-SF, sleep quality [53] with PSQI and functional status with FLI-C [46].
In terms of outcomes, there were improvements in lung function [47,48,49,50,52], respiratory muscle strength [48,50], exercise tolerance [49], dyspnea [50], fatigue [49], thoracic mobility [47], upper limb volumen [55], sleep quality [53], and quality of life [50,51], as well as a reduction in sensitivity to adverse physiological reactions [54], number of vomiting [46], nausea [46], and anxiety [54].
3.3. Methodological Quality Assessment
The results of the quality assessment of the different studies are shown in Table 2, Table 3 and Table 4. Table 2 presents the methodological quality of the clinical trials. Table 3 and Table 4 show the methodological quality of the observational studies and the case study, respectively.

Table 2.
Quality of Clinical Trials measured with the PEDro Scale.

Table 3.
Quality assessment of observational studies using the STROBE Statement [51].

Table 4.
Quality of the case studies, as measured by the SCED scale.
The mean methodological quality of the clinical trials as measured by the PEDro scale was 4.5, that of the case study as measured by the SCED scale was 7, and in the case of the observational studies, 56% of the recommendations of the STROBE Statement were met.
3.4. Risk of Bias of Included Studies
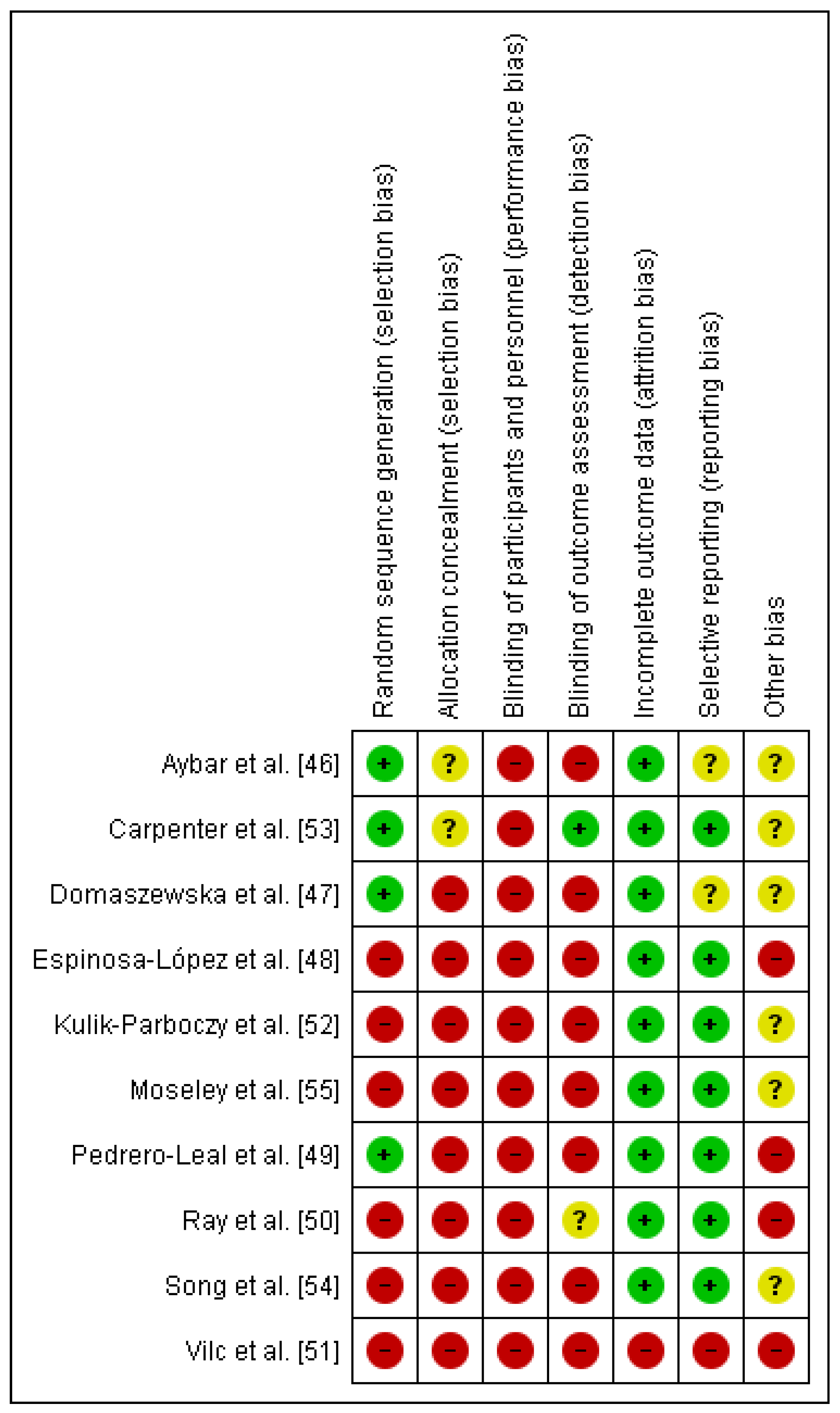
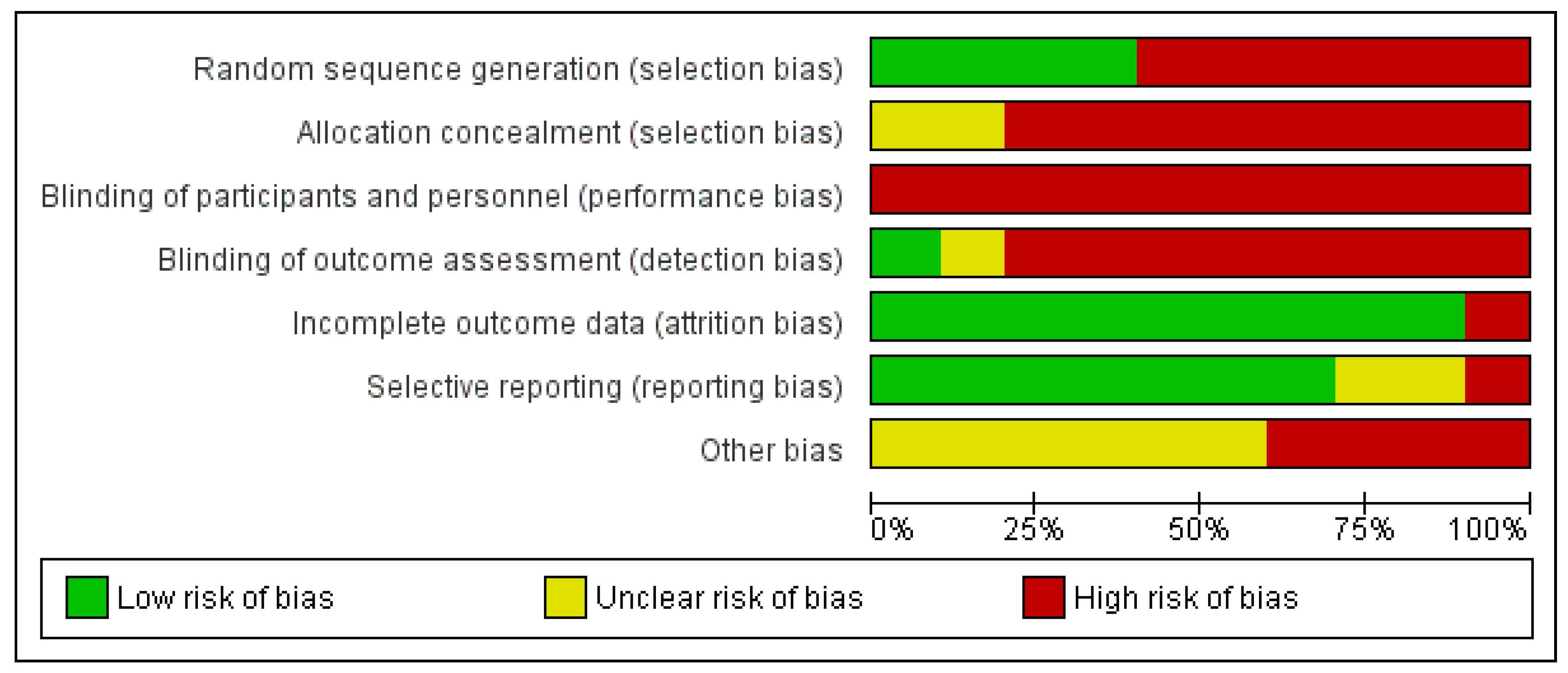
The Cochrane Risk of Bias Assessment Tool was used to assess the risk of bias of the articles included in this review. The results of the risk of bias can be observed in Figure 2. It should be noted that the risk of bias is high in relation to performance bias and detection bias because patients and therapists were not blinded, and in only one article were the evaluators blinded [53]. The risk of bias is also high in relation to selection bias because there was random sequence generation in only four of the trials [46,47,49,53]. With respect to attrition bias, all of the them were low-risk, except one [51] (Figure 3).
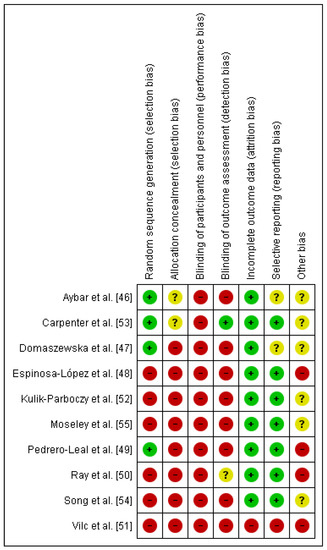
Figure 2.
Risk of bias summary [46,47,48,49,50,51,52,53,54,55].
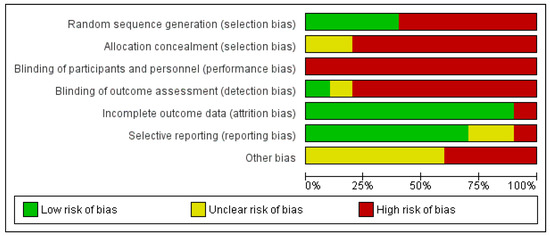
Figure 3.
Risk of bias graph.
4. Discussion
A systematic review was carried out to synthesize the scientific evidence and evaluate its quality regarding the use of RP intervention strategies in the treatment of the sequelae of breast cancer.
4.1. Characteristics of the Sample
With regard to the characteristics of the sample, it was homogeneous in terms of sex, as the number of women was always higher than men. This is due to the fact that breast cancer affects women to a greater extent [56,57]. According to the age of the participants, the sample was heterogeneous, as was the specific treatments previously received by the study participants. Of the 10 studies, all had surgery, 5 had radiotherapy [47,49,51,52,53], 5 had chemotherapy [46,49,52,53,54], and 2 had hormone therapy [47,49]. Adjuvant radiotherapy is the standard treatment following breast conserving surgery in early breast cancer [58].
4.2. Measuring Instruments
Concerning measuring instruments, the most commonly used in our paper was spirometry, which is the main pulmonary function test, fundamental for the evaluation and follow-up of respiratory diseases [59], coinciding with other studies in which pulmonary function in breast cancer was also measured with this test [60,61].
4.3. Intervention Strategies in RP
The most commonly used techniques in RP in general are drainage of secretions, mobilization of the rib cage, and ventilatory techniques [21].
In comparison with our review, we would agree with the ventilatory techniques [49], since, given the type of alteration that occurs in breast cancer, secretion drainage would not be the technique of choice in principle.
Within the ventilatory techniques we can find the thoracic mobility that may increase the vital capacity in patients with chronic respiratory disease [62].
In one of the trials in our review [47], the aim was to improve thoracic mobility, but this was not achieved through specific mobilization of the thorax, but rather with respiratory and circulatory exercises and soft tissue therapy, the latter being used in several of the studies evaluated in this document to treat muscle fascia and postoperative scarring [47,48]. However, among the techniques, the most widely used was deep diaphragmatic breathing exercises [47,51,53,54,55].
In some of the papers in this review, breathing exercises have been combined with other techniques, such as muscle relaxation [51,54], coinciding with other trials carried out in other cancer populations, in which anxiety and emotional distress were reduced [63,64]. Along the same lines, Stoerkel et al., using guided mind-body techniques (breathing, meditation, guided imagery, self-hypnosis suggestions), obtained improvements in pain, nausea, sleep, fatigue, global health, and quality of life after surgery in breast cancer [65].
These latter aspects can also be improved in people with cancer, who are undergoing treatment, through physical training [66], which we know is a mainstay of RP, but is not the focus of this manuscript.
Another study used breathing exercises together with soft tissue techniques, which has also been used as the sole technique, in post-mastectomy patients to eliminate muscle and fascia stiffness in the postoperative scar area [67]. Other therapeutic methods, which are not commonly used, but could be applicable to improve the functions of the respiratory system by restoring the correct mobility of the thorax and improving the work of the respiratory muscles in the operated area, are thoracic rib and joint mobilization, trigger point therapy, and kinesiotaping [68].
Breathing exercises are widely used in breast cancer within broader interventions such as yoga or telerehabilitation platforms.
Considering the effects of therapeutic yoga in breast cancer, yogic breathing (pranayama) has shown numerous beneficial health effects in breast cancer patients undergoing radiotherapy [69] or chemotherapy [70], with an improvement in quality of life and fatigue [71], findings also found in our review. These authors suggested, as a possible cause, that during controlled breathing exercises, the stretching of lung tissue produces inhibitory signals in the vagus nerve, which ultimately shifts the autonomic nervous system towards the parasympathetic domain, resulting in a calm and alert state of mind, coinciding with our review where a reduction in sensitivity to adverse physiological reactions and anxiety [54] was found.
In terms of telerehabilitation, the e-CUIDATE platform provides access to a range of content such as breathing, mobility, strength, and stretching exercises to breast cancer patients during adjuvant treatment, achieving improvements in terms of functional and cognitive performance in breast cancer survivors, as well as decreasing cost and increasing accessibility [72].
4.4. Main Results
The usefulness of RP has been observed in the improvement of pulmonary function [47,48,49,50,52], respiratory muscle strength [48,50], effort tolerance [49], dyspnea [50], fatigue [49], thoracic mobility [47], upper limb volumen [55], sleep quality [53], and quality of life [46,50,51], as well as a reduction in sensitivity to adverse physiological reactions [54], nausea [46], vomiting [46], and anxiety [54]. However, it is not useful in improving vasomotor symptoms [53].
Where it seems to be most effective is in respiratory capacity, as improvements were found in spirometric data such as FEV1, CV, CVF, MVVV, and in the improvement of muscle strength of the respiratory muscles, improving fatigue, dyspnea, and mobility of the rib cage. These results coincide with those obtained in other types of cancer, such as lung cancer, achieving a significant decrease in the severity of dyspnea and fatigue, although they were not significant in respiratory capacity [73], contrasting the latter finding with that obtained in our review.
This variable has been studied through spirometric data from spirometry and in only one of the articles was the Peak Exercise Test with a cycle ergometer applied to the study participants to determine the VO2 peak [50]. In other populations, such as heart patients or athletes, its use is very frequent [74].
Breast cancer patients suffer from impaired respiratory capacity as measured by VO2 peak [75]. Poor VO2 peak is associated with poorer quality of life [76] and increased morbidity and mortality in cancer survivors [77], and may be an independent predictor of survival in metastatic disease [78].
In relation to the above, there is also a decrease in inspiratory and inspiratory muscle function in these patients [19]. In only one study in our review, training of PIM and PEM was performed [48], but their training would be crucial to improve O2 consumption [56].
For all these reasons, it is striking that there is very little research related to respiratory physiotherapy that takes these reflections into account and we recommend that future studies include the measurement of this variable, as well as specific training of the respiratory musculature.
It is worth mentioning the only study in our review that found some important correlations between the results obtained and the type of intervention [52]. Kulik-Parobczy et al. found differences in means of spirometric indicators before and after rehabilitation, especially in patients who underwent mastectomy and lymph node status, radically reducing the level of PEF by as much as 64 units. Other findings included were a positive influence of chemotherapy on the spirometric indicator before and after rehabilitation and a significant impact of the rehabilitation on FEV1
Another variable studied by 30% of the studies in our review was quality of life [46,50,51], obtaining positive results, coinciding with the Cochrane’s review that evaluated the quality of life in breast cancer patients after physical activity. Their trials had as low methodological quality as those reviewed in this manuscript [66].
On the other hand, it is a treatment that does not require large investments in technology [79] and it could easily be implemented in patients with sequelae of breast cancer treatment. Even so, its use is not widespread [21] and it is being underutilized, although it is in demand by the patients themselves [80].
4.5. Strengths and Limitations
The present study has several strengths, including the broad and easily reproducible search strategy applied to seven major medical databases. In addition, studies have been systematically selected by applying well-defined inclusion/exclusion criteria. However, there are several limitations that need to be addressed before drawing conclusions from the results of the present analysis. Heterogeneity among the different studies was so extensive that a meta-analysis could not be performed.
There was little uniformity in study populations (some of them were not unique to breast cancer patients), sample sizes, RP interventions and their duration, measured variables, and different measurement instruments.
Despite a thorough search, the literature found was sparse: there were only five clinical trials with “fair” scores, one case study, and three observational studies that met half of the recommendations of the STROBE Statement. One of the reasons for the low scores of these studies could have been the low baseline similarity and the use of single blinding, due to the inherent nature of the studies in clinical trials, and in the case of the observational and case studies, the low external validity which would make it difficult to generalize the results. For all the above reasons, positive results should therefore be interpreted with caution.
More studies are needed to prove the efficacy of RP so that it can be more widely used in breast cancer, given all the problems associated with its treatment, as well as more in-depth research to broaden the therapeutic options for this type of patient, including determining which type of treatment (radiotherapy, chemotherapy, surgery, hormone therapy) could be more effective. In addition, it could be convenient to investigate whether respiratory physiotherapy can help not only the complications arising from the treatment but also the treatment itself.
5. Conclusions
In conclusion, it is observed that respiratory physiotherapy is not widely used in the sequelae of breast cancer treatment. Respiratory physiotherapy improves lung function, exercise tolerance, dyspnea, fatigue, thoracic mobility, upper limb volume, sleep quality, functional status, and quality of life, as well as reducing sensitivity to adverse physiological reactions, nausea, vomiting, and anxiety. RP is not effective in improving vasomotor symptoms. In terms of RF interventions, diaphragmatic deep breathing exercises were the most commonly used.
This review confirms the limited evidence in favor of the benefits of these RP intervention strategies for breast cancer sequelae.
Future studies with low risk of bias are required to determine the respiratory physiotherapy techniques needed to improve specific outcomes among women who have undergone surgical treatment and adjuvant therapy.
Author Contributions
M.J.V.-G.: conceptualization, resources, data curation, formal analysis, methodology, writing—original draft preparation, writing—review and editing. R.M.-V.: formal analysis, methodology, writing—original draft preparation, writing—review and editing. F.J.M.-V.: resources, investigation, writing—review and editing. V.P.-C.: writing—review and editing. G.G.-M.: writing—original draft preparation. M.R.-H.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Nursing and Physiotherapy (University of Cadiz).
Data Availability Statement
Not applicable.
Acknowledgments
Our thanks to Silvia Viñolo Gil, for her help in translating the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Figure A1.
PRISMA Checklist.
Figure A1.
PRISMA Checklist.
Figure A2.
PRISMA Checklist [39].
Figure A2.
PRISMA Checklist [39].
References
- Rivera Ledesma, E.; Fornaris Hernández, A.; Rosa Mariño Membribes, E.; Alfonso Díaz, K.; María Ledesma Santiago, R.; Cristina Abreu Carter, I.; Habana Policlínico Docente, L.; Fernández Chardiet, A.; Habana, L.; Ledesma, R.E.; et al. Factores de riesgo del cáncer de mama en un consultorio de la Atención Primaria de Salud. Rev. Habanera Cienc. Méd. 2019, 18, 308–322. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1729-519X2019000200308&lng=es&nrm=iso&tlng=es (accessed on 21 July 2020).
- López-Sánchez, I.; Casado-Méndez, P.R.; Santos-Fonseca, R.S.; Méndez-Jiménez, O.; Estrada-Sosa, R.; Guzmán-González, A.J. Prevalencia de factores de riesgo del cáncer de mama en población rural femenina. Rev. Arch. Méd. Camagüey 2019, 23, 563–572. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-02552019000500563&lng=es&nrm=iso&tlng=es (accessed on 21 July 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Gomide, L.B.; Matheus, J.P.C.; Candido Dos Reis, F.J. Morbidity after breast cancer treatment and physiotherapeutic performance. Int. J. Clin. Pract. 2007, 61, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.M.; Maruyama, N.C. Exercise in the rehabilitation of breast cancer survivors. Psychooncology 1999, 8, 191–206. [Google Scholar] [CrossRef]
- McNeely, M.L.; Binkley, J.M.; Pusic, A.L.; Campbell, K.L.; Gabram, S.; Soballe, P.W. A prospective model of care for breast cancer rehabilitation: Postoperative and postreconstructive issues. Cancer 2012, 118, 2226–2236. [Google Scholar] [CrossRef]
- Winick, L.; Robbins, G.F. The post-mastectomy rehabilitation group program. Structure, procedure, and population demography. Am. J. Surg. 1976, 132, 599–602. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Nie, X.Y.; Ji, C.C.; Lin, X.X.; Liu, L.J.; Chen, X.M.; Yao, H.; Wu, S.H. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J. Am. Heart Assoc. 2017, 6, e005633. [Google Scholar] [CrossRef]
- Suesada, M.M.; Carvalho, H.D.A.; de Albuquerque, A.L.P.; Salge, J.M.; Stuart, S.R.; Takagaki, T.Y. Impact of thoracic radiotherapy on respiratory function and exercise capacity in patients with breast cancer. J. Bras. Pneumol. 2018, 44, 469–476. [Google Scholar] [CrossRef]
- Jaén, J.; Vázquez, G.; Alonso, E.; León, A.; Guerrero, R.; Almansa, J.F. Changes in pulmonary function after incidental lung irradiation for breast cancer: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1381–1388. [Google Scholar] [CrossRef]
- Ciesla, S.; Polom, K. The effect of immediate breast reconstruction with Becker-25 prosthesis on the preservation of proper body posture in patients after mastectomy. Eur. J. Surg. Oncol. 2010, 36, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.E.D.; Rett, M.T.; Mendonça, A.C.R.; Bezerra, T.S.; Santana, J.M.D.; Silva Júnior, W.M.D. Efeito da radioterapia na função pulmonar e na fadiga de mulheres em tratamento para o câncer de mama. Fisioter. Pesqui. 2013, 20, 50–55. [Google Scholar] [CrossRef][Green Version]
- Erven, K.; Weltens, C.; Nackaerts, K.; Fieuws, S.; Decramer, M.; Lievens, Y. Changes in pulmonary function up to 10 years after locoregional breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Mayer, E.L.; Burstein, H.J.; Winer, E.P.; Goldhirsch, A. Cardiac toxicity from systemic cancer therapy: A comprehensive review. Prog. Cardiovasc. Dis. 2010, 53, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Krengli, M.; Sacco, M.; Loi, G.; Masini, L.; Ferrante, D.; Gambaro, G.; Ronco, M.; Magnani, C.; Carriero, A. Pulmonary Changes after Radiotherapy for Conservative Treatment of Breast Cancer: A Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1460–1467. [Google Scholar] [CrossRef]
- Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Effects and potential mechanisms of exercise training on cancer progression: A translational perspective. Brain. Behav. Immun. 2013, 30, S75–S87. [Google Scholar] [CrossRef]
- Ci, R.; Degens, W.H. Factors Contributing to Muscle Wasting and Dysfunction in COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 289. [Google Scholar]
- Spyropoulou, D.; Leotsinidis, M.; Tsiamita, M.; Spiropoulos, K.; Kardamakis, D. Pulmonary function testing in women with breast cancer treated with radiotherapy and chemotherapy. In Vivo 2009, 23, 867–871. Available online: https://europepmc.org/article/med/19779125 (accessed on 14 October 2020).
- O’Donnell, D.E.; Webb, K.A.; Langer, D.; Elbehairy, A.F.; Neder, J.A.; Dudgeon, D.J. Respiratory Factors Contributing to Exercise Intolerance in Breast Cancer Survivors: A Case-Control Study. J. Pain Symptom Manag. 2016, 52, 54–63. [Google Scholar] [CrossRef]
- McCool, F.D.; Rosen, M.J. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 250S–259S. [Google Scholar] [CrossRef]
- Martí, J.D.; Muñoz, G.; Gimeno-Santos, E.; Balañá, A.; Vilaró, J. Análisis descriptivo de la fisioterapia respiratoria en España. Rehabilitacion 2016, 50, 160–165. [Google Scholar] [CrossRef]
- Varela, G.; Novoa, N.M.; Agostini, P.; Ballesteros, E. Chest Physiotherapy in Lung Resection Patients: State of the Art. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wilson, C.M. Clinical Outcomes after Physical Therapy Treatment for Secondary Lymphedema after Breast Cancer. Cureus 2019, 11, e4779. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, F.F.; Aykut, M.; Genç, H.; Manslz Kaplan, B.; Soran, A. Is complex decongestive physical therapy safe for median nerve at the level of carpal tunnel in breast cancer related lymphedema? Lymphat. Res. Biol. 2019, 17, 78–86. [Google Scholar] [CrossRef]
- Cho, Y.; Do, J.; Jung, S.; Kwon, O.; Jeon, J.Y. Effects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissection. Support. Care Cancer 2016, 24, 2047–2057. [Google Scholar] [CrossRef]
- Flores, A.M.; Nelson, J.; Sowles, L.; Stephenson, R.; Robinson, K.; Blot, W.J. 1704 Lymphedema signs, symptoms, self-reported diagnosis and referral to physical therapy among African American and low-income breast cancer survivors. Eur. J. Cancer 2015, 51, S249–S250. [Google Scholar] [CrossRef]
- Tambour, M.; Tange, B.; Christensen, R.; Gram, B. Effect of physical therapy on breast cancer related lymphedema: Protocol for a multicenter, randomized, single-blind, equivalence trial. BMC Cancer 2014, 14, 239. [Google Scholar] [CrossRef]
- Fernández-Lao, C.; Cantarero-Villanueva, I.; Fernández-De-Las-Peñas, C.; Del Moral-Ávila, R.; Castro-Sánchez, A.M.; Arroyo-Morales, M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: A randomized controlled clinical trial. Clin. J. Pain 2012, 28, 113–121. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Fernández-de-las-Peñas, C.; López-Barajas, I.B.; Del-Moral-Ávila, R.; de la-Llave-Rincón, A.I.; Arroyo-Morales, M. Effectiveness of Water Physical Therapy on Pain, Pressure Pain Sensitivity, and Myofascial Trigger Points in Breast Cancer Survivors: A Randomized, Controlled Clinical Trial. Pain Med. 2012, 13, 1509–1519. [Google Scholar] [CrossRef]
- De Groef, A.; Van Kampen, M.; Vervloesem, N.; De Geyter, S.; Christiaens, M.R.; Neven, P.; Vos, L.; De Vrieze, T.; Geraerts, I.; Devoogdt, N. Myofascial techniques have no additional beneficial effects to a standard physical therapy programme for upper limb pain after breast cancer surgery: A randomized controlled trial. Clin. Rehabil. 2017, 31, 1625–1635. [Google Scholar] [CrossRef]
- De Groef, A.; Van Kampen, M.; Dieltjens, E.; Christiaens, M.R.; Neven, P.; Geraerts, I.; Devoogdt, N. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: A systematic review. Arch. Phys. Med. Rehabil. 2015, 96, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Feyzioğlu, Ö.; Dinçer, S.; Akan, A.; Algun, Z.C. Is Xbox 360 Kinect-based virtual reality training as effective as standard physiotherapy in patients undergoing breast cancer surgery? Support. Care Cancer 2020, 28, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa Diaz, I.; Torres Lacomba, M.; Cerezo Tellez, E.; del Campo Gomez-Rico, C.D.; Gutierrez Ortega, C. Accessory Joint and Neural Mobilizations for Shoulder Range of Motion Restriction after Breast Cancer Surgery: A Pilot Randomized Clinical Trial. J. Chiropr. Med. 2017, 16, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.A.; Levy, E.; McGarvey, C.; Pfalzer, L.A.; Stout, N.L.; Gerber, L.H.; Soballe, P.W.; Danoff, J. Pre-operative assessment enables early diagnosis and recovery of shoulder function in patients with breast cancer. Breast Cancer Res. Treat. 2010, 120, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lopez-Torres, C.; Rice, C.; Dieli-Conwright, C. Effect of High Intensity Interval Training on Cardiorespiratory Fitness in Breast Cancer Patients Undergoing Anthracycline Chemotherapy. Med. Sci. Sports Exerc. 2019, 51, 242–243. [Google Scholar] [CrossRef]
- Carmichael, A.R.; Daley, A.J.; Rea, D.W.; Bowden, S.J. Physical activity and breast cancer outcome: A brief review of evidence, current practice and future direction. Eur. J. Surg. Oncol. 2010, 36, 1139–1148. [Google Scholar] [CrossRef]
- Sprod, L.K.; Hsieh, C.C.; Hayward, R.; Schneider, C.M. Three versus six months of exercise training in breast cancer survivors. Breast Cancer Res. Treat. 2010, 121, 413–419. [Google Scholar] [CrossRef]
- Lee, H.; Uhm, K.E.; Cheong, I.Y.; Yoo, J.S.; Chung, S.H.; Park, Y.H.; Lee, J.Y.; Hwang, J.H. Patient Satisfaction with Mobile Health (mHealth) Application for Exercise Intervention in Breast Cancer Survivors. J. Med. Syst. 2018, 42, 254. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Verhagen, A.P.; De Vet, H.C.W.; De Bie, R.A.; Boers, M.; Van Den Brandt, P.A. The art of quality assessment of RCTs included in systematic reviews. J. Clin. Epidemiol. 2001, 54, 651–654. [Google Scholar] [CrossRef]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Blettner, M.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- Cascaes da Silva, F.; Valdivia Arancibia, B.A.; da Rosa Iop, R.; Barbosa Gutierres Filho, P.J.; da Silva, R. Escalas y listas de evaluación de la calidad de estudios científicos. Rev. Cuba. Inf. Cienc. Salud 2013, 24, 295–312. Available online: http://www.acimed.sld.cu/index.php/acimed/article/view/438/318 (accessed on 24 March 2021).
- Green, S.; Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions—Version 5.0.2; The Cochrane Collaboration: London, UK, 2011; Available online: www.cochrane-handbook.org (accessed on 4 August 2021).
- Aybar, D.O.; Kılıc, S.P.; Çınkır, H.Y. The effect of breathing exercise on nausea, vomiting and functional status in breast cancer patients undergoing chemotherapy. Complement. Ther. Clin. Pract. 2020, 40, 101213. [Google Scholar] [CrossRef]
- Domaszewska, K.; Pieńkowski, T.; Janiak, A.; Bukowska, D.; Laurentowska, M. The Influence of Soft Tissue Therapy on Respiratory Efficiency and Chest Mobility of Women Suffering from Breast Cancer. Int. J. Environ. Res. Public Health 2019, 16, 5092. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-López, A.M.; Daza-Arana, J.E.; Pinzón-Sanabria, L.M.; Perdomo-Quiroga, Y.; Ruiz-Jiménez, J.P. Effects of muscle energy technique for quadratus lumborum on respiratory muscle strength in patients with breast cancer. Rev. Fac. Med. 2019, 67, 469–475. [Google Scholar] [CrossRef]
- Pedrero Leal, C.; Yuste Sánchez, M.J.; Arranz Martín, B. Effect of incentive spirometry and PEP mask on lung function in women treated for breast cancer with radiotherapy: Case study. Fisioterapia 2019, 41, 337–341. [Google Scholar] [CrossRef]
- Ray, A.D.; Williams, B.T.; Mahoney, M.C. Respiratory Muscle Training Improves Exercise Performance and Quality of Life in Cancer Survivors. Rehabil. Oncol. 2017, 35, 81–89. [Google Scholar] [CrossRef]
- Vilč, B.; Šečić, A.; Kirac, I.; Herman, I.; Kraljević, N.; Brnić, S. Applying deep breathing exercises, relaxation techniques, guided imagery and music in the preoperative period and during radiotherapy in University hospital for tumors, Sestre Milosrdnice University Hospital center in Zagreb, Croatia. Libri Oncol. 2019, 47, 78–83. [Google Scholar] [CrossRef]
- Kulik-Parobczy, I. Evaluation of the effectiveness of physiotherapy in patients after oncological breast cancer treatment based on spirometric indicators. Wspolczesna Onkol. 2019, 23, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.S.; Burns, D.S.; Wu, J.; Otte, J.L.; Schneider, B.; Ryker, K.; Tallman, E.; Yu, M. Paced respiration for vasomotor and other menopausal symptoms: A randomized, controlled trial. J. Gen. Intern. Med. 2013, 28, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-H.; Xu, R.-M.; Zhang, Q.-H.; Ma, M.; Zhao, X.-P. Relaxation training during chemotherapy for breast cancer improves mental health and lessens adverse events. Int. J. Clin. Exp. Med. 2013, 6, 979–984. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00911059/full (accessed on 20 October 2020). [PubMed]
- Moseley, A.L.; Piller, N.B.; Carati, C.J. The effect of gentle arm exercise and deep breathing on secondary arm lymphedema. Lymphology 2005, 38, 136–145. [Google Scholar]
- Perez-Gomez, B.; Lope, V. Situación Epidemiológica del Cáncer de Mama en España SEE PROFILE. 2007. Available online: https://www.researchgate.net/publication/27595448 (accessed on 1 March 2022).
- Gladys Ibáñez, R.; María Elsa Calderón, G.; Domingo Márquez, Z. Male breast cancer: Review of a world and national situation. Rev. Chil. Cir. 2011, 63, 95–101. Available online: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0718-40262011000100018&lng=es&nrm=iso&tlng=e (accessed on 21 April 2021).
- Huang, X.-Z.; Chen, Y.; Chen, W.-J.; Zhang, X.; Wu, C.-C.; Zhang, C.-Y.; Sun, S.-S.; Wu, J. Effect of radiotherapy after breast-conserving surgery in older patients with early breast cancer and breast ductal carcinoma in situ: A meta-analysis. Oncotarget 2017, 8, 28215. [Google Scholar] [CrossRef]
- Garcia-Rio, F.; Calle, M.; Burgos, F.; Casan, P.; del Campo, F.; Galdiz, J.B.; Giner, J.; Gonzalez-Mangado, N.; Ortega, F.; Puente Maestu, L. Espirometria. Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef]
- Verbanck, S.; Hanon, S.; Schuermans, D.; Van Parijs, H.; Vinh-Hung, V.; Miedema, G.; Verellen, D.; Storme, G.; Fontaine, C.; Lamote, J.; et al. Mild Lung Restriction in Breast Cancer Patients after Hypofractionated and Conventional Radiation Therapy: A 3-Year Follow-Up. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 937–945. [Google Scholar] [CrossRef]
- Cortés-Flores, A.O.; Jiménez-Tornero, J.; Morgan-Villela, G.; Delgado-Gómez, M.; Zuloaga-Fernández del Valle, C.J.; García-Rentería, J.; Rendón-Félix, J.; Fuentes-Orozco, C.; Macías-Amezcua, M.D.; Ambriz-González, G.; et al. Effects of preoperative dexamethasone on postoperative pain, nausea, vomiting and respiratory function in women undergoing conservative breast surgery for cancer: Results of a controlled clinical trial. Eur. J. Cancer Care 2018, 27, e12686. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; Zu Wallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Fawzy, N.W. A psychoeducational nursing intervention to enhance coping and affective state in newly diagnosed malignant melanoma patients. Cancer Nurs. 1995, 18, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.H.; Richardson, A.; Richardson, J. Managing symptoms in patients with advanced lung cancer during radiotherapy: Results of a psychoeducational randomized controlled trial. J. Pain Symptom Manag. 2011, 41, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Stoerkel, E.; Bellanti, D.; Paat, C.; Peacock, K.; Aden, J.; Setlik, R.; Walter, J.; Inman, A. Effectiveness of a Self-Care Toolkit for Surgical Breast Cancer Patients in a Military Treatment Facility. J. Altern. Complement. Med. 2018, 24, 916–925. [Google Scholar] [CrossRef]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst. Rev. 2018, 2018, CD011292. [Google Scholar] [CrossRef] [PubMed]
- Field, T. Massage therapy research review. Complement. Ther. Clin. Pract. 2016, 24, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Kaplan, C.; Dandin, O. Kinesiotaping for breast cancer related lymphedema. J. Breast Health 2012, 8, 166–168. [Google Scholar]
- Cramer, H. Yoga in the supportive therapy for breast cancer: Scientific evidence. Dtsch. Z. Onkol. 2014, 46, 152–156. [Google Scholar] [CrossRef]
- Dhruva, A.; Miaskowski, C.; Abrams, D.; Acree, M.; Cooper, B.; Goodman, S.; Hecht, F.M. Yoga breathing for cancer chemotherapy-associated symptoms and quality of life: Results of a pilot randomized controlled trial. J. Altern. Complement. Med. 2012, 18, 473–479. [Google Scholar] [CrossRef]
- Jayawardena, R.; Ranasinghe, P.; Ranawaka, H.; Gamage, N.; Dissanayake, D.; Misra, A. Exploring the therapeutic benefits of “Pranayama” (yogic breathing): A systematic review. Int. J. Yoga 2020, 13, 99–110. [Google Scholar] [CrossRef]
- Galiano-Castillo, N.; Arroyo-Morales, M.; Lozano-Lozano, M.; Fernández-Lao, C.; Martín-Martín, L.; Del-Moral-Ávila, R.; Cantarero-Villanueva, I. Effect of an Internet-based telehealth system on functional capacity and cognition in breast cancer survivors: A secondary analysis of a randomized controlled trial. Support. Care Cancer 2017, 25, 3551–3559. [Google Scholar] [CrossRef]
- Ozalevli, S.; Ilgin, D.; Kul Karaali, H.; Bulac, S.; Akkoclu, A. The effect of in-patient chest physiotherapy in lung cancer patients. Support. Care Cancer 2010, 18, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Allison, T.; Burdiat, G. Pruebas de esfuerzo cardiopulmonar en la práctica clínica. Rev. Uruguaya Cardiol. 2010, 25, 17–27. Available online: http://www.scielo.edu.uy/scielo.php?script=sci_arttext&pid=S1688-04202010000100004&nrm=iso (accessed on 1 March 2022).
- Maginador, G.; Lixandrão, M.E.; Bortolozo, H.I.; Vechin, F.C.; Sarian, L.O.; Derchain, S.; Telles, G.D.; Zopf, E.; Ugrinowitsch, C.; Conceição, M.S. Aerobic exercise-induced changes in cardiorespiratory fitness in breast cancer patients receiving chemotherapy: A systematic review and meta-analysis. Cancers 2020, 12, 2240. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.F.; Flynn, J.R.; Moskowitz, C.S.; Scott, J.M.; Oeffinger, K.C.; Dang, C.T.; Liu, J.E.; Jones, L.W.; Steingart, R.M. Long-term Cardiopulmonary Consequences of Treatment-Induced Cardiotoxicity in Survivors of ERBB2-Positive Breast Cancer. JAMA Cardiol. 2020, 5, 309–317. [Google Scholar] [CrossRef]
- Jones, L.W.; Courneya, K.S.; Mackey, J.R.; Muss, H.B.; Pituskin, E.N.; Scott, J.M.; Hornsby, W.E.; Coan, A.D.; Herndon, J.E.; Douglas, P.S.; et al. Cardiopulmonary function and age-related decline across the breast cancer: Survivorship continuum. J. Clin. Oncol. 2012, 30, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Zehr, K.R. Diagnosis and treatment of breast cancer in men. Radiol. Technol. 2019, 91, 51M–63M. Available online: https://pubmed.ncbi.nlm.nih.gov/31471487/ (accessed on 17 March 2021).
- Sarmiento González-Nieto, V.; Tirado Reyes, M.; Villegas Portero, R.; Márquez Calderón, S.; Briones Pérez De La Blanca, E. Respiratory rehabilitation: Situation in Spain. Rehabilitacion 2005, 39, 128–133. [Google Scholar] [CrossRef]
- ten Tusscher, M.R.; Groen, W.G.; Geleijn, E.; Sonke, G.S.; Konings, I.R.; Van der Vorst, M.J.; van Zweeden, A.; Aaronson, N.K.; Stuiver, M.M. Physical problems, functional limitations, and preferences for physical therapist-guided exercise programs among Dutch patients with metastatic breast cancer: A mixed methods study. Support. Care Cancer 2019, 27, 3061–3070. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).