Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
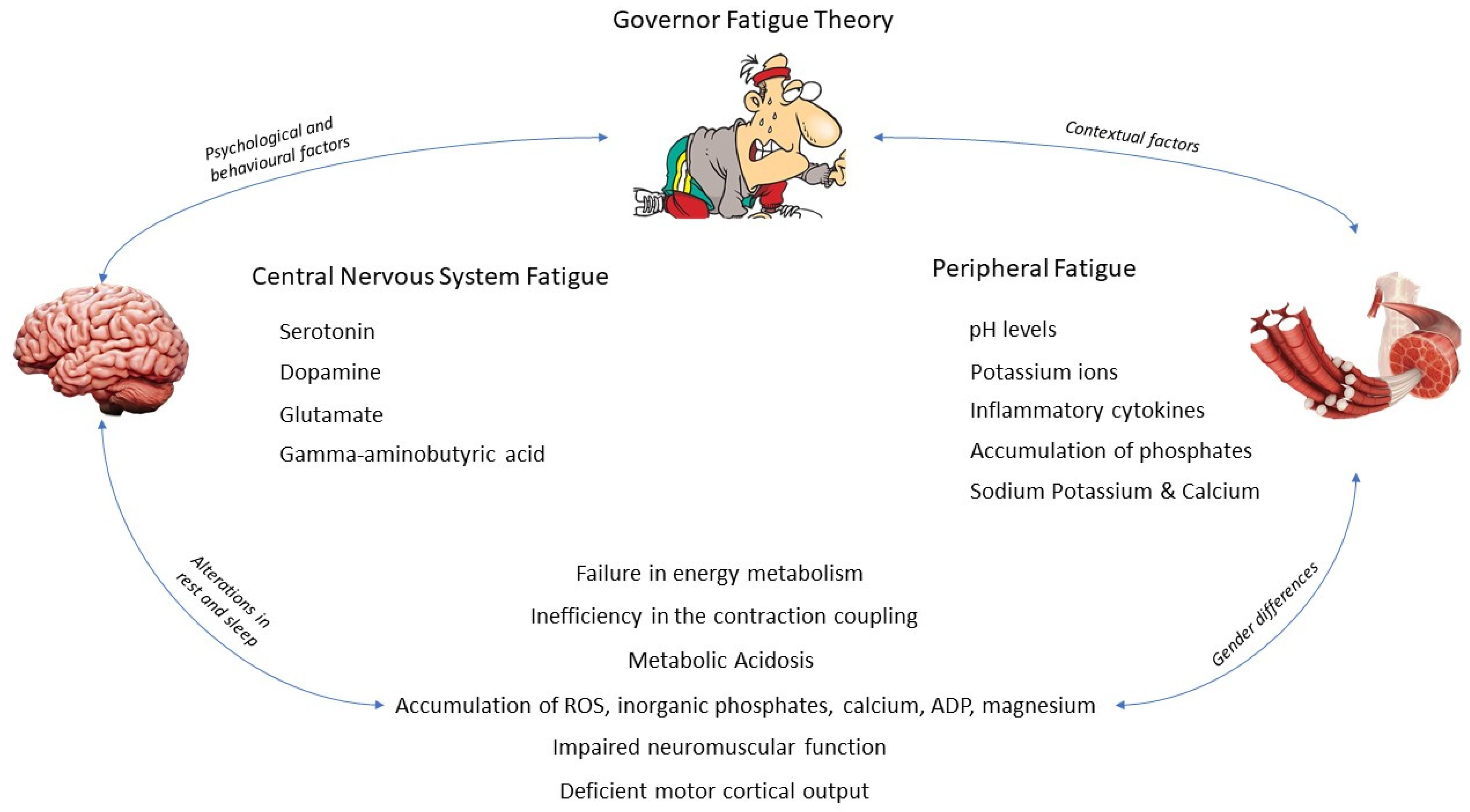
3. Etiology of Central Nervous System and Peripheral Fatigue
3.1. Central Nervous System Fatigue
3.1.1. Serotonin
3.1.2. Dopamine
3.1.3. Glutamate
3.1.4. Gamma-Aminobutyric Acid
3.2. Peripheral Fatigue
3.2.1. What Happens inside the Muscle?
- (i)
- Failure in energy metabolism as the myocyte cannot continue resynthesizing ATP.
- (ii)
- Inefficiency in the contraction coupling mechanism due to an impairment in the number or functionality of the actin and myosin cross-bridges.
- (iii)
- Metabolic acidosis produced by the intramuscular accumulation of Pi and hydrogen ions.
3.2.2. Thus, Who Is the Bad Guy?
4. Psychological and Behavioral Modifications and Conditioning Factors
4.1. The Fight-or-Flight Response
4.2. Alterations in Rest and Sleep
4.3. Gender Differences
5. What Happens during Exercise?
- (i)
- Reactive oxygen species produced during a prolonged eccentric activity produce anions, which damage phospholipids in the muscle cell membrane.
- (ii)
- Hydroxyl radicals generated during muscle stress processes also damage other biomolecules, DNA, and lipids [95].
- (iii)
- A low bioavailability of muscle glycogen and the involvement of glycolysis in ATP hydrolysis for energy during long-lasting physical activity activate nucleotide purine metabolism, leading to an accumulation of inosine monophosphate [96].
- (iv)
- Once glycogen is depleted, brain chain amino acids (BCAA) are oxidized to be used in ATP resynthesis; BCAAs follow the same mechanism as free fatty acids to overcome the brain barrier, where they compete with free tryptophan in the bloodstream. When the BCAA/free tryptophan ratio decreases, serotonin may be accumulated in the brain [97,98], producing a feeling of lethargy and a loss of the neural drive [2]; thus, CNS fatigue.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abd-Elfattah, H.M.; Abdelazeim, F.H.; Elshennawy, S. Physical and Cognitive Consequences of Fatigue: A Review. J. Adv. Res. 2015, 6, 351–358. [Google Scholar] [CrossRef]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central Fatigue: The Serotonin Hypothesis and Beyond. Sports Med. 2006, 36, 881–909. [Google Scholar] [CrossRef]
- Cairns, S.P.; Knicker, A.J.; Thompson, M.W.; Sjøgaard, G. Evaluation of Models Used to Study Neuromuscular Fatigue. Exerc. Sport Sci. Rev. 2005, 33, 9–16. [Google Scholar]
- McKenna, M.J.; Hargreaves, M. Resolving Fatigue Mechanisms Determining Exercise Performance: Integrative Physiology at Its Finest! J. Appl. Physiol. 2008, 104, 286–287. [Google Scholar] [CrossRef] [Green Version]
- Zając, A.; Chalimoniuk, M.; Maszczyk, A.; Gołaś, A.; Lngfort, J. Central and Peripheral Fatigue During Resistance Exercise—A Critical Review. J. Hum. Kinet. 2015, 49, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Tarnopolsky, M.A.; Saris, W.H. Evaluation of gender differences in physiology: An introduction. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Bensing, J.M.; Hulsman, R.L.; Schreurs, K.M. Gender Differences in Fatigue: Biopsychosocial Factors Relating to Fatigue in Men and Women. Med. Care 1999, 37, 1078–1083. [Google Scholar] [CrossRef]
- Hunter, S.K. Sex Differences in Human Fatigability: Mechanisms and Insight to Physiological Responses. Acta Physiol. (Oxf.) 2014, 210, 768–789. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The Effects of Mental Fatigue on Physical Performance: A Systematic Review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef] [Green Version]
- Amann, M. Central and Peripheral Fatigue: Interaction during Cycling Exercise in Humans. Med. Sci. Sports Exerc. 2011, 43, 2039–2045. [Google Scholar] [CrossRef]
- Monster, A.W.; Chan, H. Isometric Force Production by Motor Units of Extensor Digitorum Communis Muscle in Man. J. Neurophysiol. 1977, 40, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P.; Beck, T.W.; Cramer, J.T.; Housh, T.J. Is Fatigue All in Your Head? A Critical Review of the Central Governor Model. Br. J. Sports Med. 2006, 40, 573–586; discussion 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Boord, P. A Controlled Investigation into the Psychological Determinants of Fatigue. Biol. Psychol. 2006, 72, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Desmond, P.A.; Hancock, P.A. Active and Passive Fatigue States. In Stress, Workload, and Fatigue; CRC Press: Boca Raton, FL, USA, 2000; pp. 455–465. [Google Scholar]
- Lock, A.M.; Bonetti, D.L.; Campbell, A.D.K. The Psychological and Physiological Health Effects of Fatigue. Occup. Med. (Lond.) 2018, 68, 502–511. [Google Scholar] [CrossRef]
- Leonard, C.; Fanning, N.; Attwood, J.; Buckley, M. The Effect of Fatigue, Sleep Deprivation and Onerous Working Hours on the Physical and Mental Wellbeing of Pre-Registration House Officers. Ir. J. Med. Sci. 1998, 167, 22–25. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J. The Application of Cortical Arousal Assessment to Control Neuromuscular Fatigue During Strength Training. J. Mot. Behav. 2017, 49, 429–434. [Google Scholar] [CrossRef]
- Ament, W.; Verkerke, G.J. Exercise and Fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Delp, M.D.; Armstrong, R.B.; Godfrey, D.A.; Laughlin, M.H.; Ross, C.D.; Wilkerson, M.K. Exercise Increases Blood Flow to Locomotor, Vestibular, Cardiorespiratory and Visual Regions of the Brain in Miniature Swine. J. Physiol. 2001, 533 Pt 3, 849–859. [Google Scholar] [CrossRef]
- Tomporowski, P.D. Effects of Acute Bouts of Exercise on Cognition. Acta Psychol. (Amst.) 2003, 112, 297–324. [Google Scholar] [CrossRef]
- Williamson, J.W.; McColl, R.; Mathews, D.; Ginsburg, M.; Mitchell, J.H. Activation of the Insular Cortex Is Affected by the Intensity of Exercise. J. Appl. Physiol. 1999, 87, 1213–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leavitt, V.M.; DeLuca, J. Central Fatigue: Issues Related to Cognition, Mood and Behavior, and Psychiatric Diagnoses. PM R 2010, 2, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Behan, P.O. Fatigue and Basal Ganglia. J. Neurol. Sci. 2000, 179, 34–42. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Diaz-Manzano, M. Evaluation of Central Fatigue by the Critical Flicker Fusion Threshold in Cyclists. J. Med. Syst. 2019, 43, 61. [Google Scholar] [CrossRef] [PubMed]
- Clemente Suárez, V.J.; Robles Pérez, J.J. Respuesta Orgánica En Una Simulación de Combate. Sanid. Mil. 2012, 68, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.-H.; Hwang, H.-M.; Chen, J.-J.; Wu, T.; Li, A.H.; Wang, H.-L. Glutamate Transporter Function of Rat Hippocampal Astrocytes Is Impaired Following the Global Ischemia. Neurobiol. Dis. 2005, 18, 476–483. [Google Scholar] [CrossRef]
- Thorstensen, J.R.; Taylor, J.L.; Tucker, M.G.; Kavanagh, J.J. Enhanced Serotonin Availability Amplifies Fatigue Perception and Modulates the TMS-Induced Silent Period during Sustained Low-Intensity Elbow Flexions. J. Physiol. 2020, 598, 2685–2701. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a Prolactin (PRL) Inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical Exercise-Induced Fatigue: The Role of Serotonergic and Dopaminergic Systems. Braz. J. Med. Biol. Res. 2017, 50, e6432. [Google Scholar] [CrossRef]
- Foley, T.E.; Fleshner, M. Neuroplasticity of Dopamine Circuits after Exercise: Implications for Central Fatigue. Neuromolecular Med. 2008, 10, 67–80. [Google Scholar] [CrossRef]
- Meeusen, R.; Van Cutsem, J.; Roelands, B. Endurance Exercise-Induced and Mental Fatigue and the Brain. Exp. Physiol. 2021, 106, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otis, T.S.; Kavanaugh, M.P. Isolation of Current Components and Partial Reaction Cycles in the Glial Glutamate Transporter EAAT2. J. Neurosci. 2000, 20, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain Lactate Metabolism: The Discoveries and the Controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, X. GLT-1 Mediates Exercise-Induced Fatigue through Modulation of Glutamate and Lactate in Rats. Neuropathology 2018, 38, 237–246. [Google Scholar] [CrossRef]
- Maddock, R.J.; Casazza, G.A.; Buonocore, M.H.; Tanase, C. Vigorous Exercise Increases Brain Lactate and Glx (Glutamate+glutamine): A Dynamic 1H-MRS Study. Neuroimage 2011, 57, 1324–1330. [Google Scholar] [CrossRef]
- Coxon, J.P.; Cash, R.F.H.; Hendrikse, J.J.; Rogasch, N.C.; Stavrinos, E.; Suo, C.; Yücel, M. GABA Concentration in Sensorimotor Cortex Following High-Intensity Exercise and Relationship to Lactate Levels. J. Physiol. 2018, 596, 691–702. [Google Scholar] [CrossRef]
- Korzeniewski, B. Pi-Induced Muscle Fatigue Leads to near-Hyperbolic Power—Duration Dependence. Eur. J. Appl. Physiol. 2019, 119, 2201–2213. [Google Scholar] [CrossRef]
- Myburgh, K.H. Can Any Metabolites Partially Alleviate Fatigue Manifestations at the Cross-Bridge? Med. Sci. Sports Exerc. 2004, 36, 20–27. [Google Scholar] [CrossRef]
- Woodward, M.; Debold, E.P. Acidosis and Phosphate Directly Reduce Myosin’s Force-Generating Capacity Through Distinct Molecular Mechanisms. Front. Physiol. 2018, 9, 862. [Google Scholar] [CrossRef] [Green Version]
- Whitten, J.H.D.; Hodgson, D.D.; Drinkwater, E.J.; Prieske, O.; Aboodarda, S.J.; Behm, D.G. Unilateral Quadriceps Fatigue Induces Greater Impairments of Ipsilateral versus Contralateral Elbow Flexors and Plantar Flexors Performance in Physically Active Young Adults. J. Sports Sci. Med. 2021, 20, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Moreno, R.; Robles-Pérez, J.J.; Aznar, S.; Clemente-Suarez, V.J. Inalambric Biofeedback Devices to Analyze Strength Manifestation in Military Population. J. Med. Syst. 2018, 42, 60. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Burnett, A.F.; Newton, M.J. The Effect of Strength Training on Three-Kilometer Performance in Recreational Women Endurance Runners. J. Strength Cond. Res. 2008, 22, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Chycki, J.; Golas, A.; Halz, M.; Maszczyk, A.; Toborek, M.; Zajac, A. Chronic Ingestion of Sodium and Potassium Bicarbonate, with Potassium, Magnesium and Calcium Citrate Improves Anaerobic Performance in Elite Soccer Players. Nutrients 2018, 10, 1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proschinger, S.; Freese, J. Neuroimmunological and Neuroenergetic Aspects in Exercise-Induced Fatigue. Exerc. Immunol. Rev. 2019, 25, 8–19. [Google Scholar]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-Induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529 Pt 1, 237–242. [Google Scholar] [CrossRef]
- Trinh, B.; Peletier, M.; Simonsen, C.; Plomgaard, P.; Karstoft, K.; Klarlund Pedersen, B.; van Hall, G.; Ellingsgaard, H. Blocking Endogenous IL-6 Impairs Mobilization of Free Fatty Acids during Rest and Exercise in Lean and Obese Men. Cell Rep. Med. 2021, 2, 100396. [Google Scholar] [CrossRef]
- Davis, M.P.; Walsh, D. Mechanisms of Fatigue. J. Support. Oncol. 2010, 8, 164–174. [Google Scholar]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal Muscle Fatigue: Cellular Mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [Green Version]
- Corona, B.T.; Balog, E.M.; Doyle, J.A.; Rupp, J.C.; Luke, R.C.; Ingalls, C.P. Junctophilin Damage Contributes to Early Strength Deficits and EC Coupling Failure after Eccentric Contractions. Am. J. Physiol. Cell Physiol. 2010, 298, C365–C376. [Google Scholar] [CrossRef] [Green Version]
- Debold, E.P.; Fitts, R.H.; Sundberg, C.W.; Nosek, T.M. Muscle Fatigue from the Perspective of a Single Crossbridge. Med. Sci. Sports Exerc. 2016, 48, 2270–2280. [Google Scholar] [CrossRef]
- McCarty, R. The Fight-or-Flight Response: A Cornerstone of Stress Research. In Stress: Concepts, Cognition, Emotion, and Behavior; Elsevier: Amsterdam, The Netherlands, 2016; pp. 33–37. [Google Scholar]
- Romero, L.M. Fight or Flight Responses; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tank, A.W.; Lee Wong, D. Peripheral and Central Effects of Circulating Catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.J.; Reaburn, P. Monitoring Changes in Rugby League Players’ Perceived Stress and Recovery during Intensified Training. Percept. Mot. Skills 2008, 106, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Shimo, S.; Sakamoto, Y.; Amari, T.; Chino, M.; Sakamoto, R.; Nagai, M. Differences between the Sexes in the Relationship between Chronic Pain, Fatigue, and QuickDASH among Community-Dwelling Elderly People in Japan. Healthcare 2021, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- LaSorda, K.R.; Gmelin, T.; Kuipers, A.L.; Boudreau, R.M.; Santanasto, A.J.; Christensen, K.; Renner, S.W.; Wojczynski, M.K.; Andersen, S.L.; Cosentino, S.; et al. Epidemiology of Perceived Physical Fatigability in Older Adults: The Long Life Family Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e81–e88. [Google Scholar] [CrossRef]
- Porter, M.M.; Stuart, S.; Boij, M.; Lexell, J. Capillary Supply of the Tibialis Anterior Muscle in Young, Healthy, and Moderately Active Men and Women. J. Appl. Physiol. 2002, 92, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Roepstorff, C.; Steffensen, C.H.; Madsen, M.; Stallknecht, B.; Kanstrup, I.-L.; Richter, E.A.; Kiens, B. Gender Differences in Substrate Utilization during Submaximal Exercise in Endurance-Trained Subjects. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E435–E447. [Google Scholar] [CrossRef] [Green Version]
- Maher, A.C.; Fu, M.H.; Isfort, R.J.; Varbanov, A.R.; Qu, X.A.; Tarnopolsky, M.A. Sex Differences in Global MRNA Content of Human Skeletal Muscle. PLoS ONE 2009, 4, e6335. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.M.; Ferrell, R.E.; Peters, D.G.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Influence of Age, Sex, and Strength Training on Human Muscle Gene Expression Determined by Microarray. Physiol. Genom. 2002, 10, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Qin, Z.; Wang, P.; Sun, Y.; Liu, X. Muscle Fatigue: General Understanding and Treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef]
- Welle, S.; Tawil, R.; Thornton, C.A. Sex-Related Differences in Gene Expression in Human Skeletal Muscle. PLoS ONE 2008, 3, e1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmer, A.R.; Ruell, P.A.; Hunter, S.K.; McKenna, M.J.; Thom, J.M.; Chisholm, D.J.; Flack, J.R. Effects of Type 1 Diabetes, Sprint Training and Sex on Skeletal Muscle Sarcoplasmic Reticulum Ca2+ Uptake and Ca2+-ATPase Activity. J. Physiol. 2014, 592, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Thom, J.M.; Thompson, M.W.; Ruell, P.A.; Bryant, G.J.; Fonda, J.S.; Harmer, A.R.; Janse de Jonge, X.A.; Hunter, S.K. Effect of 10-Day Cast Immobilization on Sarcoplasmic Reticulum Calcium Regulation in Humans. Acta Physiol. Scand. 2001, 172, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M.; et al. Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef]
- Hodes, G.E. Sex, Stress, and Epigenetics: Regulation of Behavior in Animal Models of Mood Disorders. Biol. Sex Differ. 2013, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Koolschijn, P.C.M.P.; Crone, E.A. Sex Differences and Structural Brain Maturation from Childhood to Early Adulthood. Dev. Cogn. Neurosci. 2013, 5, 106–118. [Google Scholar] [CrossRef]
- Gorbet, D.J.; Mader, L.B.; Staines, W.R. Sex-Related Differences in the Hemispheric Laterality of Slow Cortical Potentials during the Preparation of Visually Guided Movements. Exp. Brain Res. 2010, 202, 633–646. [Google Scholar] [CrossRef]
- Lissek, S.; Hausmann, M.; Knossalla, F.; Peters, S.; Nicolas, V.; Güntürkün, O.; Tegenthoff, M. Sex Differences in Cortical and Subcortical Recruitment during Simple and Complex Motor Control: An FMRI Study. Neuroimage 2007, 37, 912–926. [Google Scholar] [CrossRef]
- Hunter, S.K.; Griffith, E.E.; Schlachter, K.M.; Kufahl, T.D. Sex Differences in Time to Task Failure and Blood Flow for an Intermittent Isometric Fatiguing Contraction. Muscle Nerve 2009, 39, 42–53. [Google Scholar] [CrossRef]
- Guenette, J.A.; Romer, L.M.; Querido, J.S.; Chua, R.; Eves, N.D.; Road, J.D.; McKenzie, D.C.; Sheel, A.W. Sex Differences in Exercise-Induced Diaphragmatic Fatigue in Endurance-Trained Athletes. J. Appl. Physiol. 2010, 109, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.K.; Enoka, R.M. Sex Differences in the Fatigability of Arm Muscles Depends on Absolute Force during Isometric Contractions. J. Appl. Physiol. 2001, 91, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Russ, D.W.; Kent-Braun, J.A. Sex Differences in Human Skeletal Muscle Fatigue Are Eliminated under Ischemic Conditions. J. Appl. Physiol. 2003, 94, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M.; Coelho, A.J.; Campy, R.M. Gender Differences in Perceived Exertion during Fatiguing Knee Extensions. Med. Sci. Sports Exerc. 2004, 36, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Fitts, R.H.; Christie, A. Skeletal Muscle Fatigue. Compr. Physiol. 2012, 2, 997–1044. [Google Scholar] [CrossRef] [PubMed]
- Spurway, N.C.; Watson, H.; McMillan, K.; Connolly, G. The Effect of Strength Training on the Apparent Inhibition of Eccentric Force Production in Voluntarily Activated Human Quadriceps. Eur. J. Appl. Physiol. 2000, 82, 374–380. [Google Scholar] [CrossRef]
- Clark, B.C.; Collier, S.R.; Manini, T.M.; Ploutz-Snyder, L.L. Sex Differences in Muscle Fatigability and Activation Patterns of the Human Quadriceps Femoris. Eur. J. Appl. Physiol. 2005, 94, 196–206. [Google Scholar] [CrossRef]
- Hunter, S.K.; Critchlow, A.; Shin, I.-S.; Enoka, R.M. Fatigability of the Elbow Flexor Muscles for a Sustained Submaximal Contraction Is Similar in Men and Women Matched for Strength. J. Appl. Physiol. 2004, 96, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.A.; Smithmyer, S.L.; Pelberg, J.A.; Mishkin, A.D.; Herr, M.D.; Proctor, D.N. Sex Differences in Leg Vasodilation during Graded Knee Extensor Exercise in Young Adults. J. Appl. Physiol. 2007, 103, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Iemitsu, M.; Otsuki, T.; Maeda, S.; Ajisaka, R. Gender Differences in Brachial Blood Flow during Fatiguing Intermittent Handgrip. Med. Sci. Sports Exerc. 2008, 40, 684–690. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fadia, T.; Pincivero, D.M.; Scheuermann, B.W. Forearm Blood Flow Responses to Fatiguing Isometric Contractions in Women and Men. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H805–H812. [Google Scholar] [CrossRef]
- Hunter, S.K.; Schletty, J.M.; Schlachter, K.M.; Griffith, E.E.; Polichnowski, A.J.; Ng, A.V. Active Hyperemia and Vascular Conductance Differ between Men and Women for an Isometric Fatiguing Contraction. J. Appl. Physiol. 2006, 101, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.P.; Hayes, S.G. The Exercise Pressor Reflex. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2002, 12, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Keller, M.L.; De-Lap, B.S.; Harkins, A.; Lepers, R.; Hunter, S.K. Sex Differences in Response to Cognitive Stress during a Fatiguing Contraction. J. Appl. Physiol. 2009, 107, 1486–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russ, D.W.; Lanza, I.R.; Rothman, D.; Kent-Braun, J.A. Sex Differences in Glycolysis during Brief, Intense Isometric Contractions. Muscle Nerve 2005, 32, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Binnert, C.; Koistinen, H.A.; Martin, G.; Andreelli, F.; Ebeling, P.; Koivisto, V.A.; Laville, M.; Auwerx, J.; Vidal, H. Fatty Acid Transport Protein-1 MRNA Expression in Skeletal Muscle and in Adipose Tissue in Humans. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1072–E1079. [Google Scholar] [CrossRef] [Green Version]
- Kiens, B.; Roepstorff, C.; Glatz, J.F.C.; Bonen, A.; Schjerling, P.; Knudsen, J.; Nielsen, J.N. Lipid-Binding Proteins and Lipoprotein Lipase Activity in Human Skeletal Muscle: Influence of Physical Activity and Gender. J. Appl. Physiol. 2004, 97, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Esbjörnsson, M.; Bülow, J.; Norman, B.; Simonsen, L.; Nowak, J.; Rooyackers, O.; Kaijser, L.; Jansson, E. Adipose Tissue Extracts Plasma Ammonia after Sprint Exercise in Women and Men. J. Appl. Physiol. 2006, 101, 1576–1580. [Google Scholar] [CrossRef] [Green Version]
- Maher, A.C.; Akhtar, M.; Tarnopolsky, M.A. Men Supplemented with 17beta-Estradiol Have Increased Beta-Oxidation Capacity in Skeletal Muscle. Physiol. Genom. 2010, 42, 342–347. [Google Scholar] [CrossRef]
- Kacem, M.; Borji, R.; Sahli, S.; Rebai, H. The Disturbing Effect of Neuromuscular Fatigue on Postural Control Is Accentuated in the Premenstrual Phase in Female Athletes. Front. Physiol. 2021, 12, 736211. [Google Scholar] [CrossRef]
- Lee, E.; Vera, K.; Asirvatham-Jeyaraj, N.; Chantigian, D.; Larson, M.; Keller-Ross, M. Menstrual Phase Does Not Influence Ventilatory Responses to Group III/IV Afferent Signaling in Eumenorrheic Young Females. Respir. Physiol. Neurobiol. 2021, 292, 103712. [Google Scholar] [CrossRef]
- Janse DE Jonge, X.A.K.; Thompson, M.W.; Chuter, V.H.; Silk, L.N.; Thom, J.M. Exercise Performance over the Menstrual Cycle in Temperate and Hot, Humid Conditions. Med. Sci. Sports Exerc. 2012, 44, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Pageaux, B.; Marcora, S.M.; Rozand, V.; Lepers, R. Mental Fatigue Induced by Prolonged Self-Regulation Does Not Exacerbate Central Fatigue during Subsequent Whole-Body Endurance Exercise. Front. Hum. Neurosci. 2015, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petibois, C.; Cazorla, G.; Poortmans, J.-R.; Déléris, G. Biochemical Aspects of Overtraining in Endurance Sports: A Review. Sports Med. 2002, 32, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Cazorla, G.; Poortmans, J.-R.; Déléris, G. Biochemical Aspects of Overtraining in Endurance Sports: The Metabolism Alteration Process Syndrome. Sports Med. 2003, 33, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-Chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-Body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Bhat, S.; Patibandla, R. Metal Fatigue and Basic Theoretical Models: A Review. In Alloy Steel—Properties and Use; Valencia Morales, E., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Martin, K.; Meeusen, R.; Thompson, K.G.; Keegan, R.; Rattray, B. Mental Fatigue Impairs Endurance Performance: A Physiological Explanation. Sports Med. 2018, 48, 2041–2051. [Google Scholar] [CrossRef]
- Schiphof-Godart, L.; Roelands, B.; Hettinga, F.J. Drive in Sports: How Mental Fatigue Affects Endurance Performance. Front. Psychol. 2018, 9, 1383. [Google Scholar] [CrossRef] [Green Version]
- Gaschler, R.; Schwager, S.; Umbach, V.J.; Frensch, P.A.; Schubert, T. Expectation Mismatch: Differences between Self-Generated and Cue-Induced Expectations. Neurosci. Biobehav. Rev. 2014, 46, 139–157. [Google Scholar] [CrossRef]
- Amann, M.; Venturelli, M.; Ives, S.J.; McDaniel, J.; Layec, G.; Rossman, M.J.; Richardson, R.S. Peripheral Fatigue Limits Endurance Exercise via a Sensory Feedback-Mediated Reduction in Spinal Motoneuronal Output. J. Appl. Physiol. 2013, 115, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, J.A.; Amann, M.; Romer, L.M.; Miller, J.D. Respiratory System Determinants of Peripheral Fatigue and Endurance Performance. Med. Sci. Sports Exerc. 2008, 40, 457–461. [Google Scholar] [CrossRef]
- Nybo, L. Hyperthermia and Fatigue. J. Appl. Physiol. 2008, 104, 871–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. https://doi.org/10.3390/ijerph19073909
Tornero-Aguilera JF, Jimenez-Morcillo J, Rubio-Zarapuz A, Clemente-Suárez VJ. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(7):3909. https://doi.org/10.3390/ijerph19073909
Chicago/Turabian StyleTornero-Aguilera, José Francisco, Jorge Jimenez-Morcillo, Alejandro Rubio-Zarapuz, and Vicente J. Clemente-Suárez. 2022. "Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 7: 3909. https://doi.org/10.3390/ijerph19073909
APA StyleTornero-Aguilera, J. F., Jimenez-Morcillo, J., Rubio-Zarapuz, A., & Clemente-Suárez, V. J. (2022). Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. International Journal of Environmental Research and Public Health, 19(7), 3909. https://doi.org/10.3390/ijerph19073909







