Assessment of the Impact of Scientific Reports Published by EFSA and GIS on Functional Foods Newly Placed on the Market in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. EFSA
EFSA’s Scientific Opinions
- Scientific opinion on bovine lactoferrin (published 24 May 2012)In its summary of the opinion, EFSA’s Panel on Nutrition, Novel Foods and Food Allergens concluded that lactoferrin does not raise concerns in the area of safety, provided that it is consumed in the amount indicated in the opinion [43];
- Scientific opinion on the evaluation of the safety in use of yohimbe (Pausinystalia yohimbe (K. Schum.) Pierre ex Beille) (published 24 July 2013)The opinion states that the Panel was unable to determine the daily intake norms of Pausinystalia yohimbe bark and its preparations, which did not raise concerns as to their adverse impact on health. An assessment of exposure to Pausinystalia yohimbe from dietary supplements was made that showed that the theoretical maximum daily intake may exceed the maximum approved daily dose of Pausinystalia yohimbe used as a medicinal product [44];
- Statement on the post-marketing monitoring of the use of lycopene (published 9 January 2015)The opinion states that on the basis of previous assessments of intake norms performed by EFSA, the data concerning sale, and the data concerning the placement of products on the market provided for the period from July 2009 to June 2012, the Panel concluded that consumption of naturally occurring lycopene and its use as food colour or a new food ingredient within the accepted use levels does not lead to ADI exceeding 0.5 mg/kg bw/day [45];
- Safety of cranberry extract powder as a novel food ingredient pursuant to Regulation (EC) No 258/97 (published 12 May 2017)The opinion indicates that considering the composition, the process of production, supply, and the history of use of cranberry fruit, the Panel considers that the consumption of its derivatives does not give cause for safety concerns. The Panel concludes that cranberry extract powder is a safe food ingredient for the proposed uses and use levels [46];
- Safety of dried aerial parts of Hoodia parviflora as a novel food pursuant to Regulation (EC) No 258/97 (published 19 October 2017)The opinion is a response to the application submitted by the entrepreneur, who proposed a dose of 15 mg/portion, while the available data shows the safe dose to be 9.4 mg/day. For this reason, a negative decision concerning the application was issued [47];
- Scientific opinion on the safety of green tea catechins (published 14 March 2018)On the basis of the available data on the potentially negative impact of green tea catechins on the liver, the EFSA Panel concluded that evidence from interventional clinical trials shows that consuming doses equal to or exceeding 800 mg of EGCG/day as a dietary supplement induces a statistically significant increase in transaminases in the serum of persons undergoing treatment compared to the control group [48];
- Safety of synthetic trans-resveratrol as a novel food pursuant to Regulation (EC) No 258/97 (published 12 January 2016)The opinion presents EFSA’s position, which indicates that synthetic trans-resveratrol is safe provided that the consumed doses do not exceed 150 mg/day, and in adult persons only [49];
- Scientific opinion on the safety of monacolins in red yeast rice (published 3 August 2018)On the basis of the available information, the opinion emphasises that while the Panel was not able to determine the level of consumption of monacolin sourced from RYR in diet, this does not raise concerns as regards adverse health effects in the general population and, in applicable cases, in sensitive subsets of the population [50];
- Safety of astaxanthin for its use as a novel food in food supplements (published 5 February 2020)The above opinion updates the recommendations concerning astaxanthin intake norms. Considering the updated assessment of exposure to astaxanthin in the basic diet (fish and crustaceans) coupled with the 8 mg from dietary supplements, the NDA panel concluded the following: (i) such total exposure to astaxanthin is safe for adults, (ii) adolescents from 14 to <18 years reach the ADI, and (iii) the ADI exceeds 28% in children aged 10 to <14 years and up to 524% in infants aged 4–6 months [51].
2.2. GIS
2.2.1. The Sanitary and Epidemiological Council’s Team for Dietary Supplements
Resolutions of the Team for Dietary Supplements
2.3. Data Sourced from GIS Register
2.4. Data Analysis
3. Results
3.1. No Joinpoints Identified
3.1.1. Upward Trend: Ashwagandha, Astaxanthin, Lactoferrin, Lycopene, Trans-Resveratrol, White Mulberry
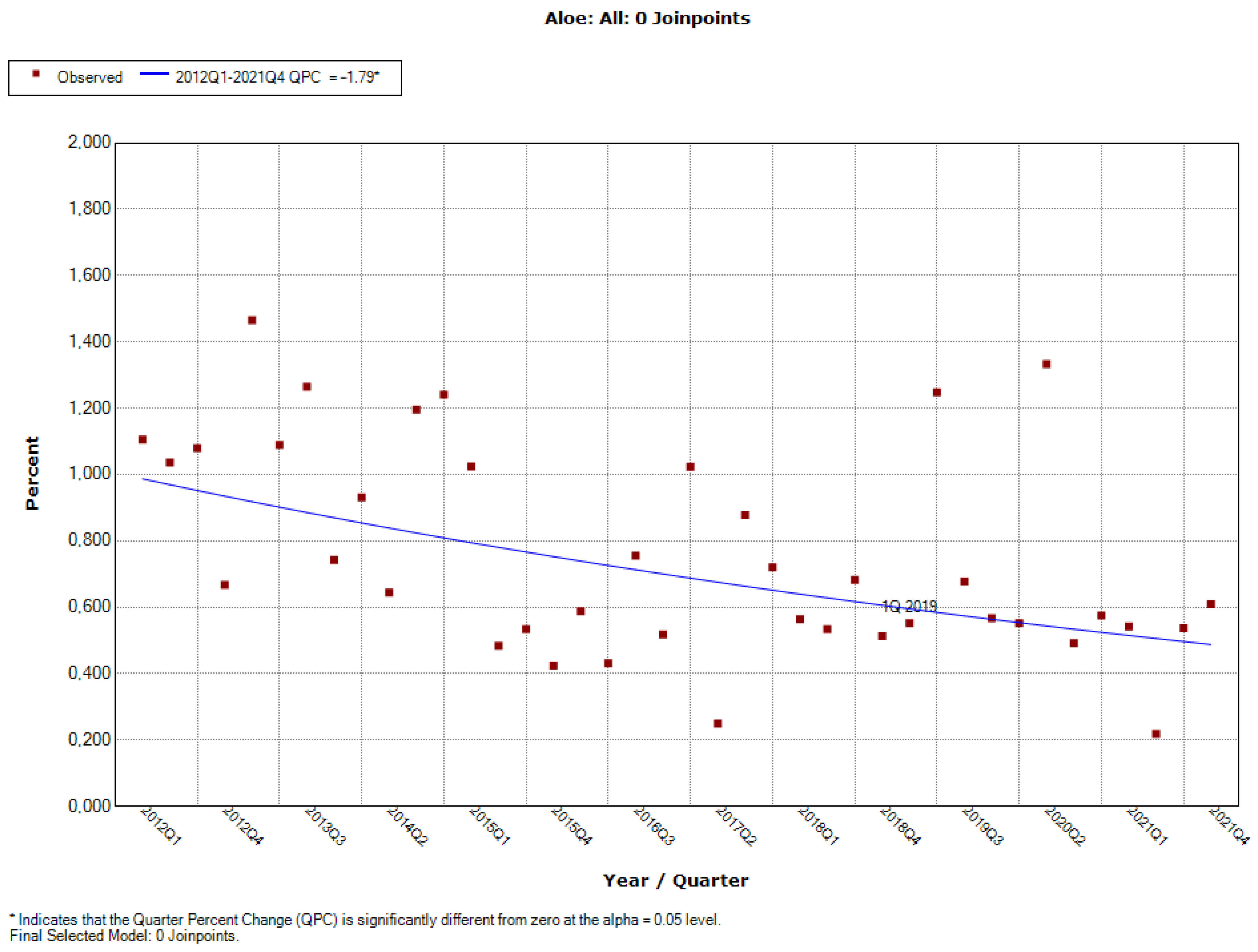
3.1.2. Downward Trend: Aloe, Caffeine, Chromium, ECGC, Hoodia, Niacin, Phosphorus, Red Yeast Rice, Vitamin B12
3.2. One Joinpoint Identified
3.2.1. Upward-Downward Trend: Boron
3.2.2. Downward-Upward Trend: Copper, Fluorine, Iodine, Iron, Isoflavones, Magnesium, Vitamin A, Vitamin B1, Vitamin B2, Vitamin B6, Vitamin C, Vitamin E
3.2.3. Downward-Downward Trend: Pantothenic Acid
3.3. Two Joinpoints Identified
3.3.1. Downward-Upward-Downward Trend: Folic Acid, Manganese, Zinc
3.3.2. Downward-Downward Trend: Cranberry Derivatives
3.3.3. Downward-Upward-Upward Trend: Vitamin K
3.4. Three or More Joinpoints Identified
3.4.1. Increase-Decrease-Increase-Decrease Trend: Yohimbine, Beta-Alanine
3.4.2. Downward-Upward-Upward Trend: Vitamin D
4. Discussion
5. Conclusions
- In most cases, publications of EFSA’s scientific opinions and GIS resolutions do not significantly change the trends of popularity or use of the ingredients that are the subject of the recommendations in newly registered products;
- Changes in trends, understood as joinpoints, appearing chronologically after the publication of a scientific opinion/resolution, seem too removed in time to conclude, on the basis of the collected data, which they are the direct result of GIS and EFSA actions. It can be assumed that those recommendations that determine the maximum permitted levels of ingredients have potentially the strongest impact on the behaviour of producers;
- Further studies are required to assess changes in the levels of particular ingredients. In addition, they may identify potential practises aimed at bypassing a set of norms established by opinion-forming bodies. In order to determine the safest/highest levels of vitamins and minerals in dietary products, this aspect is particularly important as it allows for the creation of proper food regulations;
- A public register of dietary supplements may be an effective tool to support the non-official control of the market known as its self-control. However, any register of supplements can perform its function if and only if there is research, including data on doses of active ingredients in dietary products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Ingredient | Maximum Levels and Other Requirements | Resolution Number (Date) |
|---|---|---|
| Vitamin A | 800 g of retinol equivalent (retinol and retinyl esters); 7 mg of β-carotene. | 6/2019 (11 June 2019) |
| Vitamin D | 2000 IU (50 g) for dietary supplements intended for a healthy adult population up to 75 years of age; 4000 IU (100 g) for dietary supplements intended only for healthy people over 75 years of age. | 1/2021 (19 February2021) |
| Vitamin E | 250 mg | 1/2020 (7 February 2020) |
| Vitamin K | 200 g | 2/2020 (7 February 2020) |
| Vitamin C | 1000 mg | 5/2019 (11 June 2019) |
| Vitamin B1 (thiamine) | 100 mg | 12/2019 (25 October 2019) |
| Vitamin B2 (riboflavin) | 40 mg | 13/2019 (25 October 2019) |
| Niacin | 830 mg as nicotinamide 16 mg as nicotinic acid | 8/2019 (11 June 2019) |
| Vitamin B6 | 18 mg | 18/2019 (13 December 2019) |
| Folic acid | 600 g 800 g in supplements marked as dedicated to pregnant women | 7/2019 (11 June 2019) |
| Vitamin B12 | 100 g | 14/2019 (25 October 2019) |
| Pantothenic acid | 10 mg of pantethine, 200 mg in other chemical forms as pantothenic acid | 11/2019 (25 October 2019) |
| Boron | 3 mg | 3/2020 (7 February 2020) |
| Chromium | 200 g | 4/2020 (7 February 2020) |
| Zinc | 15 mg | 10/2019 (11 June 2019) |
| Fluorine | 3.5 mg | 5/2020 (7 February 2020) |
| Phosphorus | 450 mg | 6/2020 (7 February 2020) |
| Iodine | 150 g> 200 g in dietary supplements marked as dedicated for pregnant and lactating women | 15/2019 (25 October 2019) |
| Magnesium | 400 mg | 19/2019 (13 December 2019) |
| Manganese | 1.8 mg | 9/2019 (11 June 2019) |
| Copper | 2 mg | 21/2019 (13 December 2019) |
| Iron | 20 mg 30 mg in supplements marked as dedicated to pregnant women | 20/2019 (13 December 2019) |
| Isoflavones | Soy isoflavones can be used in an amount up to 100 mg/day, in divided portions (at least two); The remaining isoflavones (from other plant sources) can be used up to 50 mg/day. | 1/2019 (14 January 2019) |
| Caffeine | Caffeine can be used in an amount up to 400 mg a day, in divided portions, provided that the product does not contain other ingredients with a synergistic effect; A portion of caffeine consumed at one time must not exceed 200 mg. | 16/2019 (25 October 2019) |
| White mulberry (Morus alba L.) | The maximum content of 1-deoxynojirmycin (DNJ)-at the level of 10 mg in the recommended daily portion of the product; An entity placing a given food on the market should have information on the content of DNJ, per the recommended daily portion of the product. | 17/2019 (25 October 2019) |
| Aloe | Only inner leaf gel/pulp may be used; Must not contain hydroxyanthracene compounds; Aloin A and B levels below 10 mg/kg (10 ppm); Cannot be used in products for children, pregnant and lactating women. | 2/2019 (14 January 2019) |
| Ashwagandha (Withania somnifera) | Withania somnifera (L.) Dunal root powder can be used in an amount less than 3 g per day; The maximum content of withanolides cannot exceed 10 mg in the recommended daily portion of the product; An entity placing a given food on the market should attach a quantitative specification confirming the content of the sum of withanolides per the recommended daily portion of the product. | 7/2020 (7 February 2020) |
| Beta-alanine | Beta-alanine can be used in the amount of up to 2400 mg/day in divided portions; A portion of beta-alanine consumed at one time cannot exceed 800 mg. | 3/2019 (14 January 2019) |
Appendix B




































References
- Figueiredo, A.; Costa, I.M.; Fernandes, T.A.; Gonçalves, L.L.; Brito, J. Food Supplements for Weight Loss: Risk Assessment of Selected Impurities. Nutrients 2020, 12, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, J.; Coates, P.; Smith, M. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory Landscape of Dietary Supplements and Herbal Medicines from a Global Perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef] [PubMed]
- Muela-Molina, C.; Perell-Oliver, S.; García-Arranz, A. Health-Related Claims in Food Supplements Endorsements: A Content Analysis from the Perspective of EU Regulation. Public Health 2021, 190, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Zovko Končić, M. Getting More Than You Paid For: Unauthorized “Natural” Substances in Herbal Food Supplements on EU Market. Planta Med. 2018, 84, 394–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunsford, K.E.; Bodzin, A.S.; Reino, D.C.; Wang, H.L.; Busuttil, R.W. Dangerous Dietary Supplements: Garcinia Cambogia—Associated Hepatic Failure Requiring Transplantation. World J. Gastroenterol. 2016, 22, 10071. [Google Scholar] [CrossRef]
- Pourshahidi, L.K.; Mullan, R.; Collins, N.; O’Mahony, S.; Slevin, M.M.; Magee, P.J.; Kerr, M.A.; Sittlington, J.J.; Simpson, E.E.A.; Hughes, C.F. Reporting Adverse Events Linked to the Consumption of Food Supplements and Other Food Products: An Audit of Nutrivigilance Newsletters. Proc. Nutr. Soc. 2021, 80, E133. [Google Scholar] [CrossRef]
- Hys, K. Identification of the Reasons Why Individual Consumers Purchase Dietary Supplements. In Perspectives on Consumer Behaviour: Theoretical Aspects and Practical Applications; Sroka, W., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 193–209. ISBN 978-3-030-47380-8. [Google Scholar]
- De Backer, G.G. Food Supplements with Red Yeast Rice: More Regulations Are Needed. Eur. J. Prev. Cardiol. 2017, 24, 1429–1430. [Google Scholar] [CrossRef] [Green Version]
- Act on Food and Nutrition Safety. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20200002021/U/D20202021Lj.pdf (accessed on 25 June 2021).
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02002L0046-20210320 (accessed on 25 June 2021).
- Österreichische Agentur fr Gesundheit und Ernährungssicherheit GmbH. Available online: https://www.ages.at/themen/lebensmittelsicherheit/nahrungsergaenzungsmittel/ (accessed on 25 June 2021).
- Wet van 8 Februari 2007 Tot Vaststelling van Een Nieuwe Geneesmiddelenwet. Available online: https://wetten.overheid.nl/BWBR0021505/2021-05-26#Hoofdstukl (accessed on 25 June 2021).
- Livsmedelsverket Swedish Food Agency. Available online: https://www.livsmedelsverket.se/en/production-control-and-trade/food-production/food-supplements?AspxAutoDetectCookieSupport=1#Registration,_approval_and_notification_of_food_supplements (accessed on 25 June 2021).
- Food Standards Agency. Available online: https://www.food.gov.uk/business-guidance/food-supplements#registration (accessed on 26 June 2021).
- Mattilsynet Norwegian Food Safety Authority. Available online: https://www.mattilsynet.no/language/english/food_and_water/food_supplements/regulations_on_food_supplements.16383 (accessed on 25 June 2021).
- Federal Food Safety and Veterinary Office FSVO. Available online: https://www.blv.admin.ch/blv/en/home/lebensmittel-und-ernaehrung/rechts-und-vollzugsgrundlagen/bewilligung-und-meldung.html (accessed on 26 June 2021).
- Guidelines on How to Apply the New Mutual Recognition Regulation (EU) 2019/515 to Food Supplements in the EU. Available online: https://foodsupplementseurope.org/wp-content/themes/fse-theme/documents/publications-and-guidelines/FSE-Mutual_Recognition-April2021.pdf (accessed on 25 June 2021).
- Bekendtgrelse om Kosttilskud. Available online: https://www.retsinformation.dk/eli/lta/2017/1239 (accessed on 25 June 2021).
- Agriculture and Food Board. Available online: https://pta.agri.ee/en/food/food-supplements (accessed on 25 June 2021).
- Agence Nationale de Sécurité Sanitaire de l’alimentation, de l’environnement et Du Travail. Available online: https://www.anses.fr/en/content/food-supplements-need-informed-consumption (accessed on 26 June 2021).
- Verordnung ber Nahrungsergänzungsmittel (Nahrungsergänzungsmittelverordnung-NemV). Available online: https://www.gesetze-im-internet.de/nemv/BJNR101110004.html (accessed on 25 June 2021).
- Food Safety Authority of Ireland’s. Available online: https://notifications.fsai.ie/login (accessed on 25 June 2021).
- Sécurité Alimentaire Grand-Duché de Luxembourg. Available online: https://securite-alimentaire.public.lu/fr/professionnel/Denrees-alimentaires/Complements-alimentaires.html (accessed on 25 June 2021).
- Procedimento de Notificação de Suplementos Alimentares. Available online: https://www.dgav.pt/alimentos/conteudo/generos-alimenticios/regras-especificas-por-tipo-de-alimentos/suplementos-alimentares/procedimento-de-notificacao-de-suplementos-alimentares/ (accessed on 25 June 2021).
- AESAN-Agencia Española de Seguridad Alimentaria y Nutricin. Available online: https://www.aesan.gob.es/en/AECOSAN/web/para_el_consumidor/ampliacion/complementos_alimenticios.htm (accessed on 25 June 2021).
- Głwny Inspektorat Sanitarny. Available online: https://www.gov.pl/web/gis/elektroniczny-system-powiadomien-powiadomienie-o-wprowadzeniu-po-raz-pierwszy-do-obrotu-suplementy-diety-zywnosc-wzbogacana-zywnosc-dla-okreslonych-grup (accessed on 13 July 2021).
- Ministarstvo Zdravstva Republike Hrvatske. Available online: https://zdravlje.gov.hr/djelokrug-1297/javnozdravstvena-zastita/hrana-1359/dodaci-prehrani-1841/stavljanje-na-trziste/2205 (accessed on 25 June 2021).
- Ministry of Health. Available online: https://www.moh.gov.cy/Moh/mphs/phs.nsf/All/F42EAF0B77B05102C225851B0043D52E?OpenDocument (accessed on 25 June 2021).
- Guideline for Submittal of Food Supplement Notification. Available online: https://www.ruokavirasto.fi/globalassets/tietoa-meista/asiointi/oppaat-ja-lomakkeet/yritykset/elintarvikeala/ravintolisat_taydennetyt/ohje_ravintolisailmoituksen_tekemisesta-17006_4_en.pdf (accessed on 25 June 2021).
- Food Law and Food Supplements Legislation. Available online: https://www.eof.gr/c/document_library/get_file?uuid=843dab13-c61f-4e51-9fd1-5784dae76aac&groupId=12225 (accessed on 25 June 2021).
- Nuova <Span Class = SpellE> Notifica di Integratore Alimentare. Available online: https://www.salute.gov.it/portale/ministro/p4_8_0.jsp?lingua=italiano&label=servizionline&idMat=APINF&idAmb=IA&idSrv=FSNN&flag=P (accessed on 25 June 2021).
- Įsakymas Dėl Pranešimo (Notifikavimo) Apie Lietuvos Respublikos Rinkai Tiekiamus Maisto Papildus Teikimo Ir Vertinimo Tvarkos Aprašo Patvirtinimo. Available online: https://www.e-tar.lt/rs/actualedition/bf979470836311e3a89fd7598ca5c9ab/LoPQJqCCqW/format/ISO_PDF/ (accessed on 25 June 2021).
- MCCAA-Malta Competition and Consumer Affairs Authority. Available online: https://mccaa.org.mt/Section/Content?contentId=1143 (accessed on 25 June 2021).
- Public Health Authority of the Slovak Republic. Available online: https://www.uvzsr.sk/en/index.php?option=com_content&view=article&id=25&Itemid=10 (accessed on 26 June 2021).
- Országos Gygyszerészeti Intézet. Available online: https://ogyei.gov.hu/etrendkiegeszitok_bejelentesehez_szukseges_tudnivalok (accessed on 13 July 2021).
- Pārtikas un Veterinārais Dienests. Available online: https://latvija.lv/en/PPK/uznemejdarbiba/partika-veterinarija/p572/ProcesaApraksts (accessed on 13 July 2021).
- Law Firm Ruskov & Colleagues. Available online: https://www.ruskov-law.eu/bulgaria/article/registration-food-supplements.html (accessed on 25 June 2021).
- FPS Public Health. Available online: https://www.health.belgium.be/nl/voeding/specifieke-voedingsmiddelen/voedingssupplementen-en-verrijkte-voedingsmiddelen (accessed on 25 June 2021).
- ISZTAR. Available online: https://ext-isztar4.mf.gov.pl/taryfa_celna/searchResults.xhtml?text=suplement&page=1&type=ALL&t=102&l=205&lang=PL&date=20210731 (accessed on 31 July 2021).
- Horgan, R.; Gavinelli, A. The Expanding Role of Animal Welfare within EU Legislation and Beyond. Livest. Sci. 2006, 103, 303–307. [Google Scholar] [CrossRef]
- Silano, M.; Silano, V. The Fifth Anniversary of the European Food Safety Authority (EFSA): Mission, Organization, Functioning and Main Results. Fitoterapia 2008, 79, 149–160. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Bovine Lactoferrin. EFSA J. 2012, 10, 2701. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the Evaluation of the Safety in Use of Yohimbe (Pausinystalia Yohimbe (K. Schum.) Pierre Ex Beille). EFSA J. 2013, 11, 3302. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Statement on the Post-Marketing Monitoring of the Use of Lycopene. EFSA J. 2015, 13, 3955. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of Cranberry Extract Powder as a Novel Food Ingredient Pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of Dried Aerial Parts of Hoodia Parviflora as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e05002. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the Safety of Green Tea Catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of Synthetic Trans-resveratrol as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4368. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the Safety of Monacolins in Red Yeast Rice. EFSA J. 2018, 16, e05368. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Astaxanthin for Its Use as a Novel Food in Food Supplements. EFSA J. 2020, 18, e05993. [Google Scholar] [CrossRef] [Green Version]
- Wojtyła, A.; Biliński, P.; Jaworska-Łuczak, B. Regulatory Strategies to Ensure Food and Feed Safety in Poland—Update Review. Ann. Agric. Environ. Med. 2010, 17, 215–220. [Google Scholar]
- State Sanitary Inspectorate Act of 14 March 1985. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20190000059/U/D20190059Lj.pdf (accessed on 21 June 2021).
- Urbaniak, M.; Wojtyła-Buciora, P.; Posobkiewicz, M.; Nowak-Ślusarz, K.; Marcinkowski, J.T. Status prawny Głwnego Inspektora Sanitarnego. Hygeia 2014, 49, 405–412. [Google Scholar]
- Grabowski, M.L.; Kosińska, B.; Knap, J.P. Evolution of Sanitary-Epidemiological Services in Poland in the Years 1944–2014. Adv. Hyg. Exp. Med./Postepy Hig. I Med. Dosw. 2017, 71, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, B.G. Research Trends in Text Mining: Semantic Network and Main Path Analysis of Selected Journals. Expert Syst. Appl. 2020, 162, 113851. [Google Scholar] [CrossRef]
- Joinpoint Help Manual 4.8.0.1. Available online: https://surveillance.cancer.gov/joinpoint/Joinpoint_Help_4.8.0.1.pdf (accessed on 10 December 2021).
- Ingram, D.D.; Malec, D.J.; Makuc, D.M.; Kruszon-Moran, D.; Gindi, R.M.; Albert, M.; Beresovsky, V.; Hamilton, B.E.; Holmes, J.; Schiller, J.S.; et al. National Center for Health Statistics Guidelines for Analysis of Trends; National Center for Health Statistics (U.S.), Ed.; Vital and Health Statistics, Series 2, Data Evaluation and Methods Research; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2018; ISBN 978-0-8406-0689-1.
- Sharma, L.L.; Teret, S.P.; Brownell, K.D. The Food Industry and Self-Regulation: Standards to Promote Success and to Avoid Public Health Failures. Am. J. Public Health 2010, 100, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, O.; Kruse, M. Food Industry Self-Regulation Scheme “EU Pledge” Cannot Prevent the Marketing of Unhealthy Foods to Children. Obes. Med. 2016, 1, 24–28. [Google Scholar] [CrossRef]
- Blaznik, U.; Krušič, S.; Hribar, M.; Kušar, A.; Žmitek, K.; Pravst, I. Use of Food Additive Titanium Dioxide (E171) before the Introduction of Regulatory Restrictions Due to Concern for Genotoxicity. Foods 2021, 10, 1910. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Frst, P.; Gundert-Remy, U.; Grtler, R.; et al. Safety Assessment of Titanium Dioxide (E171) as a Food Additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E 171). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R0063 (accessed on 25 January 2022).
- Scientific Committee on Food; Scientific Panel on Dietetic Products, Nutrition and Allergies. Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority, Europäische Kommission, Scientific Panel on Dietetic Products, Nutrition and Allergies, Eds.; European Food Safety Authority: Parma, Italy, 2006; ISBN 978-92-9199-014-6. [Google Scholar]
- Szentesi, S.; Tamás, P.; Illés, B. Supplier Selection Methods of Consignment Seller Dietary Supplements Manufacturing Companies. Transp. Logist. 2017, 17, 7. [Google Scholar]
- Franz, C.; Chizzola, R.; Novak, J.; Sponza, S. Botanical Species Being Used for Manufacturing Plant Food Supplements (PFS) and Related Products in the EU Member States and Selected Third Countries. Food Funct. 2011, 2, 720. [Google Scholar] [CrossRef]
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. The Impact of the COVID-19 Pandemic on the Composition of Dietary Supplements and Functional Foods Notified in Poland. Int. J. Environ. Res. Public. Health 2021, 18, 11751. [Google Scholar] [CrossRef]









| Model of Registration | State * |
|---|---|
| Free placement on the market | Austria, The Netherlands, Sweden, Great Britain, Norway, Switzerland, Slovenia |
| Notification free of charge; authorization not required | Denmark (3), Estonia (1), Poland (2), France (3), Germany, Ireland, Luxembourg, Portugal, Spain |
| Paid notification; authorization not required | Croatia (1500 HRK), Cyprus (50 EUR), Finland, Greece (300–600 EUR), Hungary (50,000 HUF) (1), Italy (160.20 EUR) (1), Latvia (125–380 EUR) (3), Lithuania (98–156 EUR) (1), Malta (10 EUR), Slovakia (50 EUR) (1) |
| Notification free of charge; authorization required | Bulgaria (1) |
| Paid notification; authorization required | Belgium (200 EUR) (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Assessment of the Impact of Scientific Reports Published by EFSA and GIS on Functional Foods Newly Placed on the Market in Poland. Int. J. Environ. Res. Public Health 2022, 19, 4057. https://doi.org/10.3390/ijerph19074057
Wróbel K, Milewska AJ, Marczak M, Kozłowski R. Assessment of the Impact of Scientific Reports Published by EFSA and GIS on Functional Foods Newly Placed on the Market in Poland. International Journal of Environmental Research and Public Health. 2022; 19(7):4057. https://doi.org/10.3390/ijerph19074057
Chicago/Turabian StyleWróbel, Kacper, Anna Justyna Milewska, Michał Marczak, and Remigiusz Kozłowski. 2022. "Assessment of the Impact of Scientific Reports Published by EFSA and GIS on Functional Foods Newly Placed on the Market in Poland" International Journal of Environmental Research and Public Health 19, no. 7: 4057. https://doi.org/10.3390/ijerph19074057
APA StyleWróbel, K., Milewska, A. J., Marczak, M., & Kozłowski, R. (2022). Assessment of the Impact of Scientific Reports Published by EFSA and GIS on Functional Foods Newly Placed on the Market in Poland. International Journal of Environmental Research and Public Health, 19(7), 4057. https://doi.org/10.3390/ijerph19074057







