Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Elderberry Wastewater
2.2. Analytical Determinations
2.3. Plant-Based Coagulants Preparation
2.4. Plant-Based Coagulants Powder Characterization
2.5. Wastewater Treatment Experiments
2.5.1. Coagulation–Flocculation–Decantation Experimental Set Up
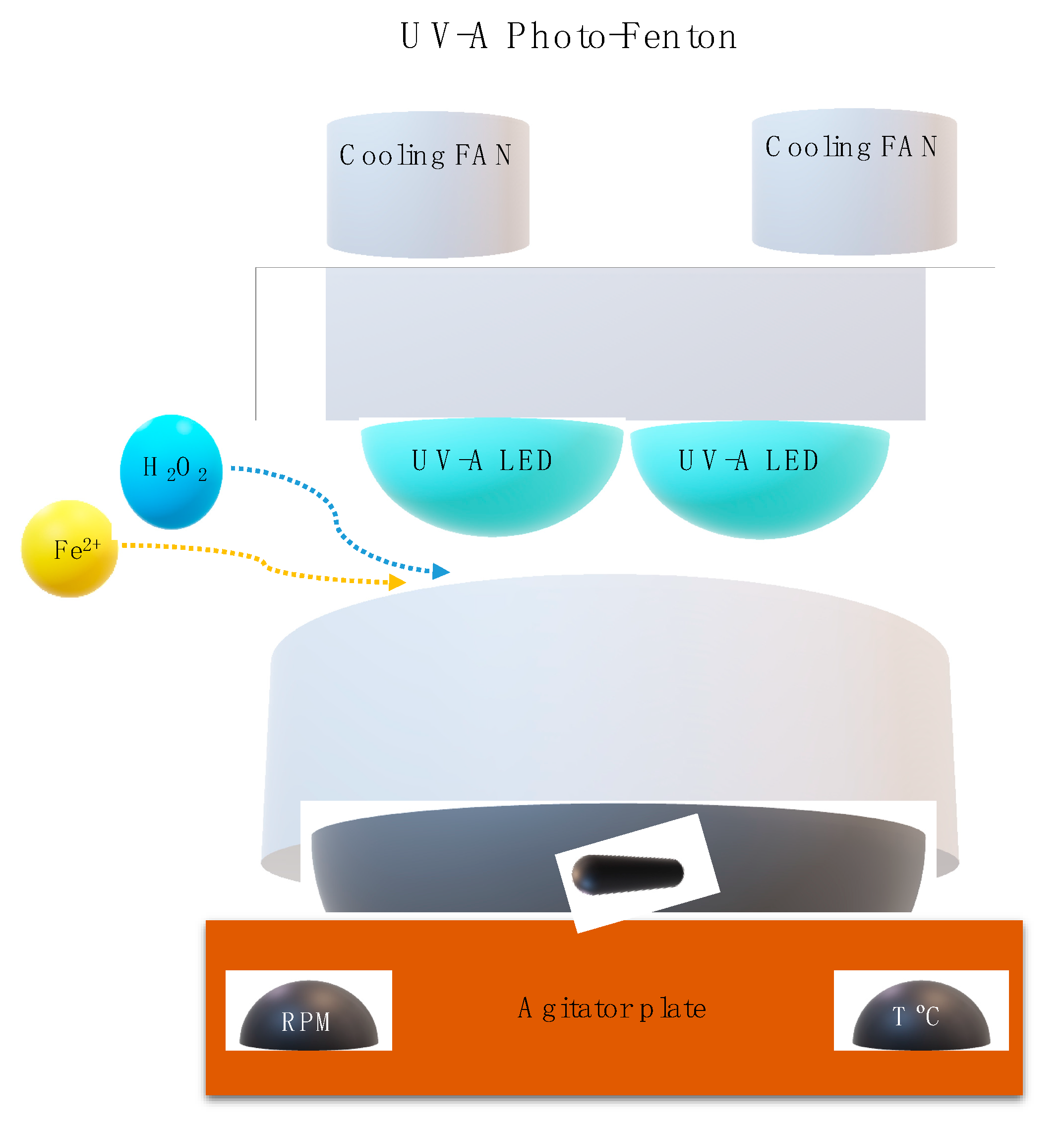
2.5.2. Photo-Fenton Experimental Set Up
2.6. Kinetic Analysis
2.7. Statistical Analysis
3. Results and Discussion
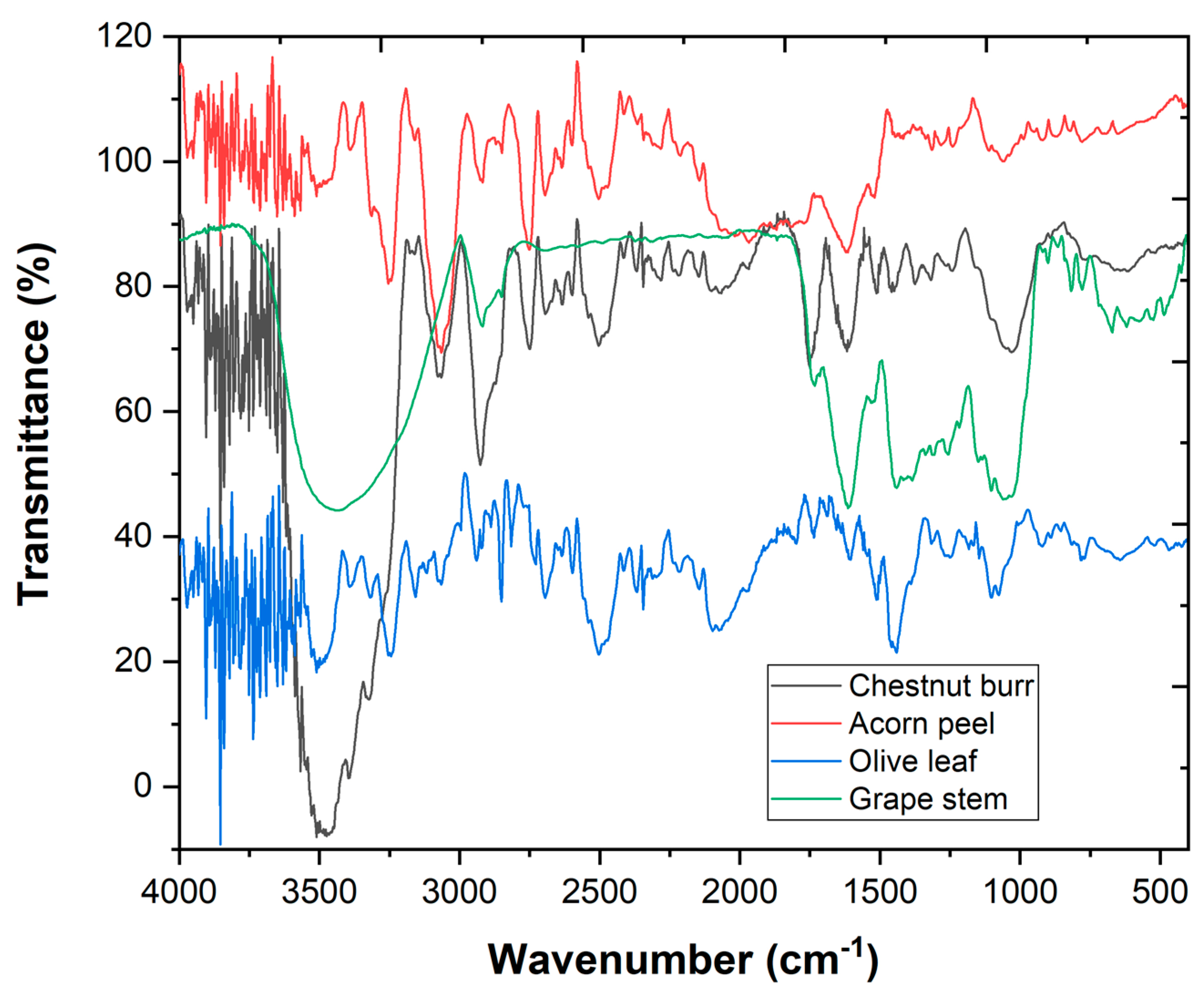
3.1. Plant-Based Coagulants Powder Characterization
3.2. Coagulation–Flocculation–Decantation Experiments
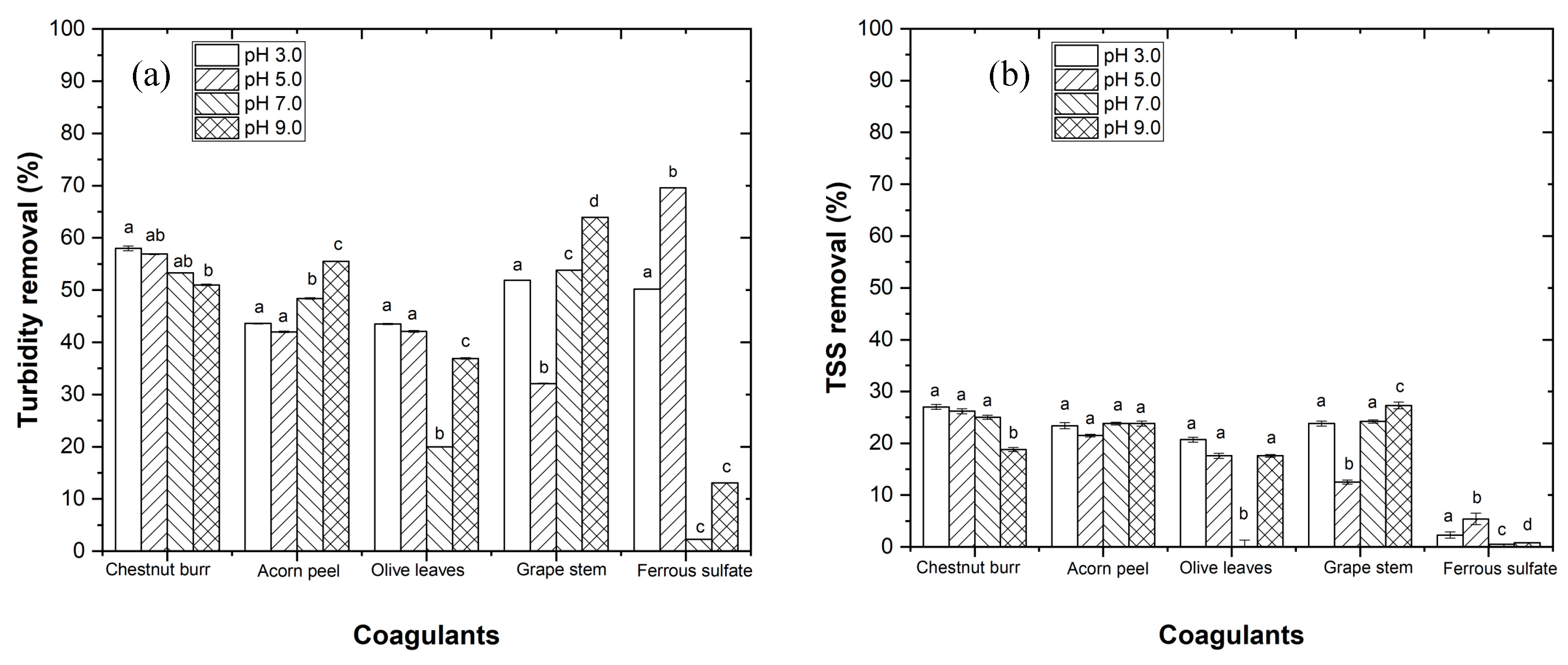
3.2.1. Effect of pH
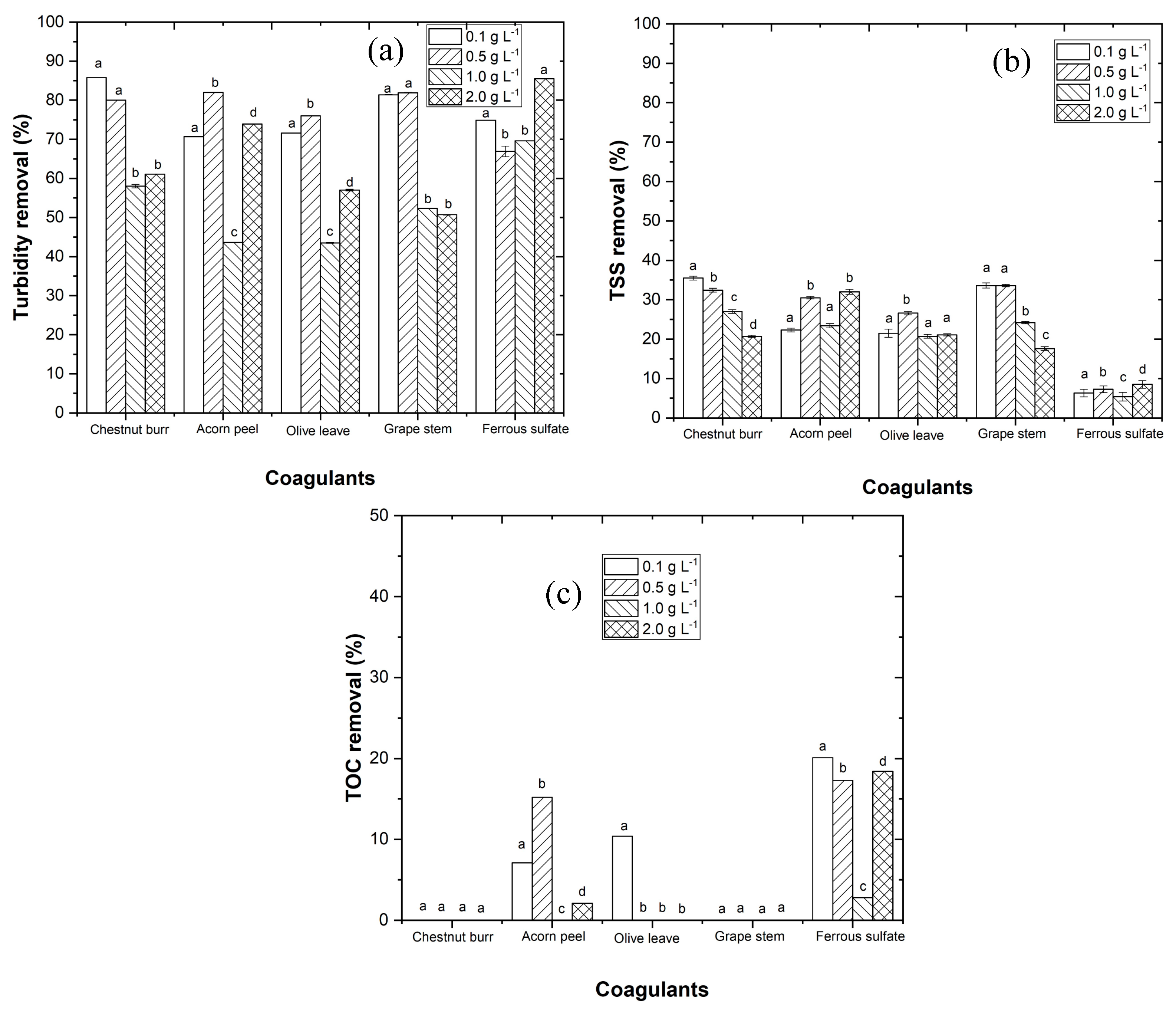
3.2.2. Dosage Effect
3.3. Photo-Fenton Experiments
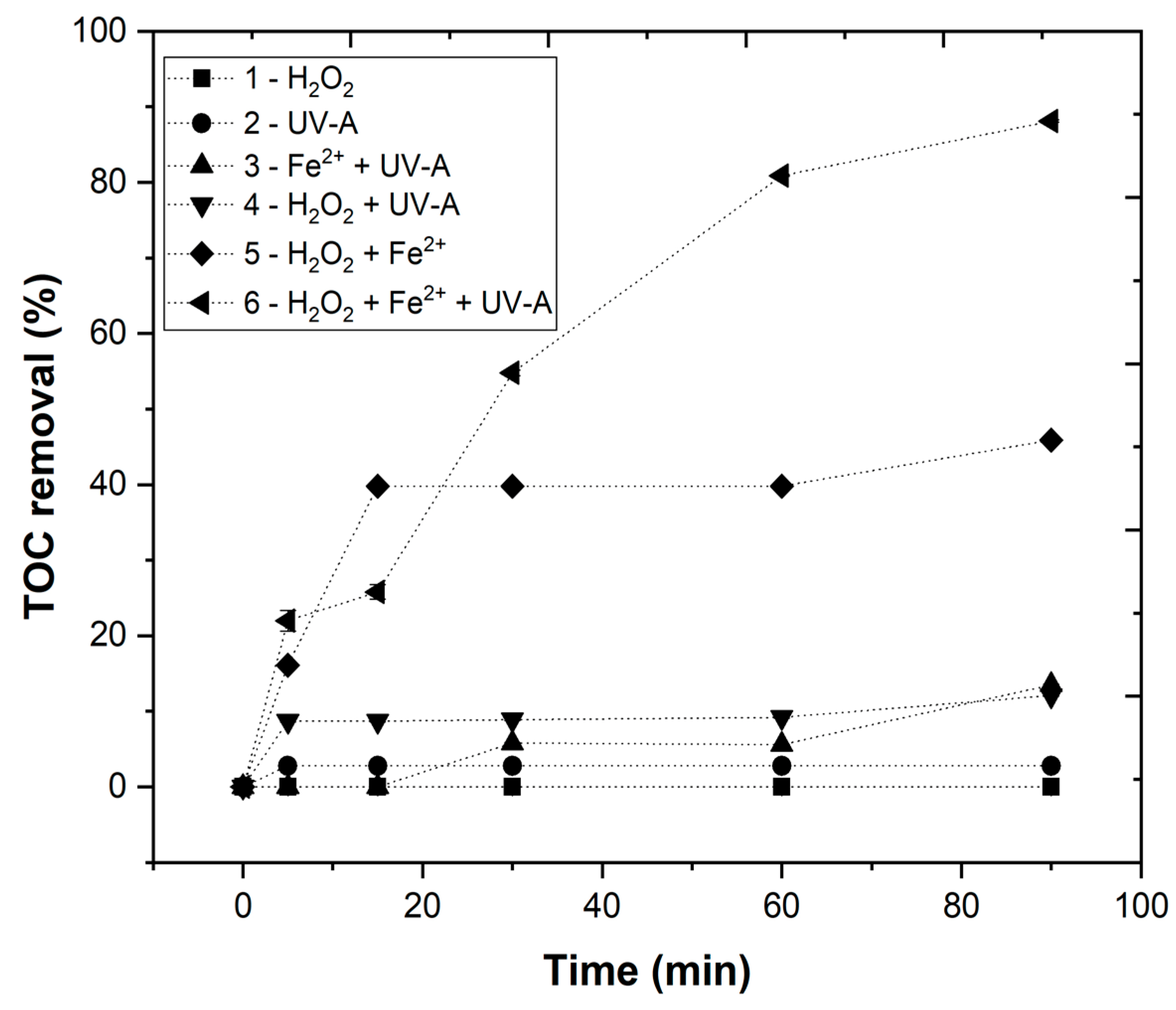
3.3.1. Chemical Degradability of Elderberry Wastewater
3.3.2. Effect of pH
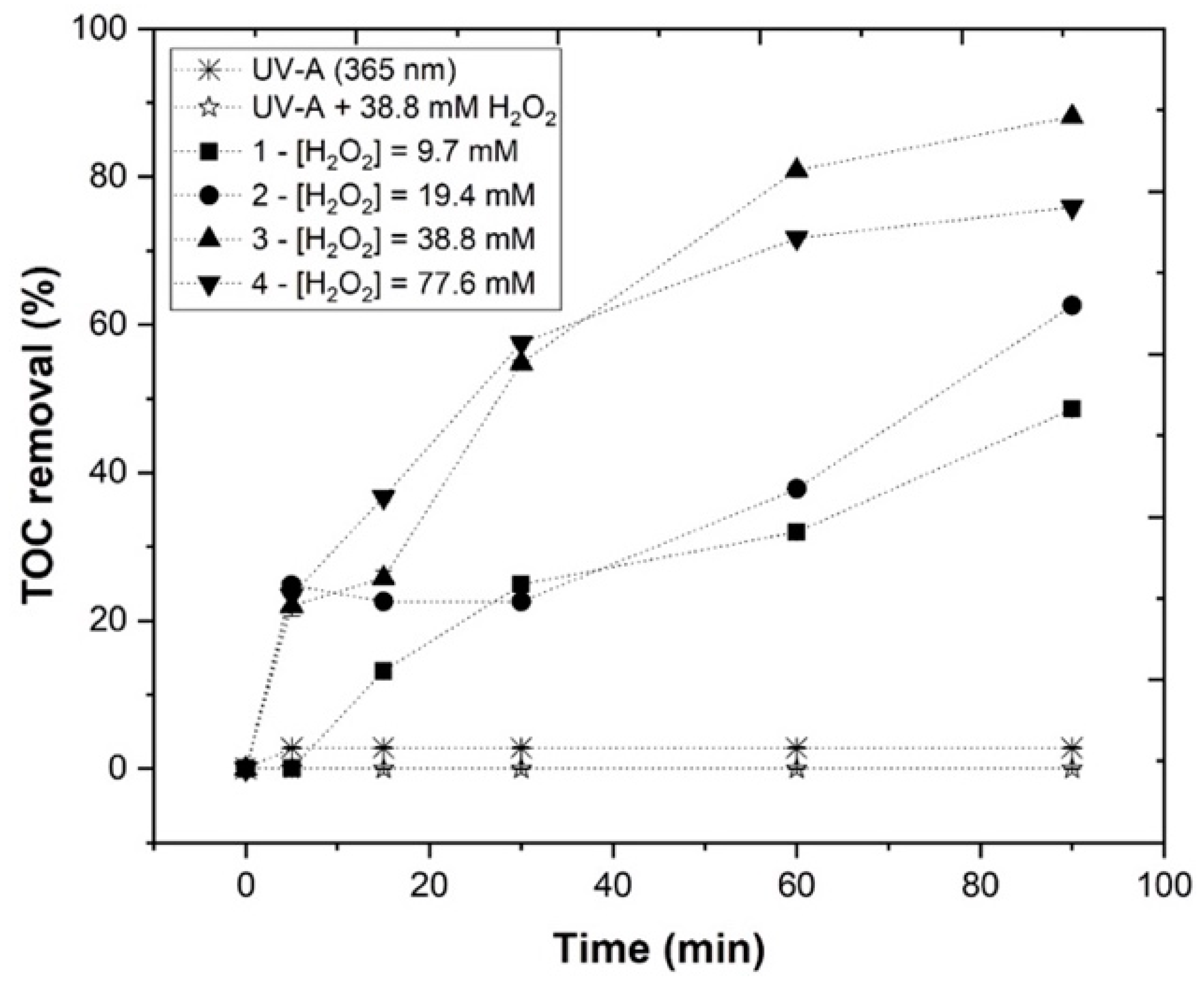
3.3.3. Effect of H2O2 Concentration
3.3.4. Effect of Fe2+ Concentration
3.3.5. Effect of Irradiance Power (IUV)
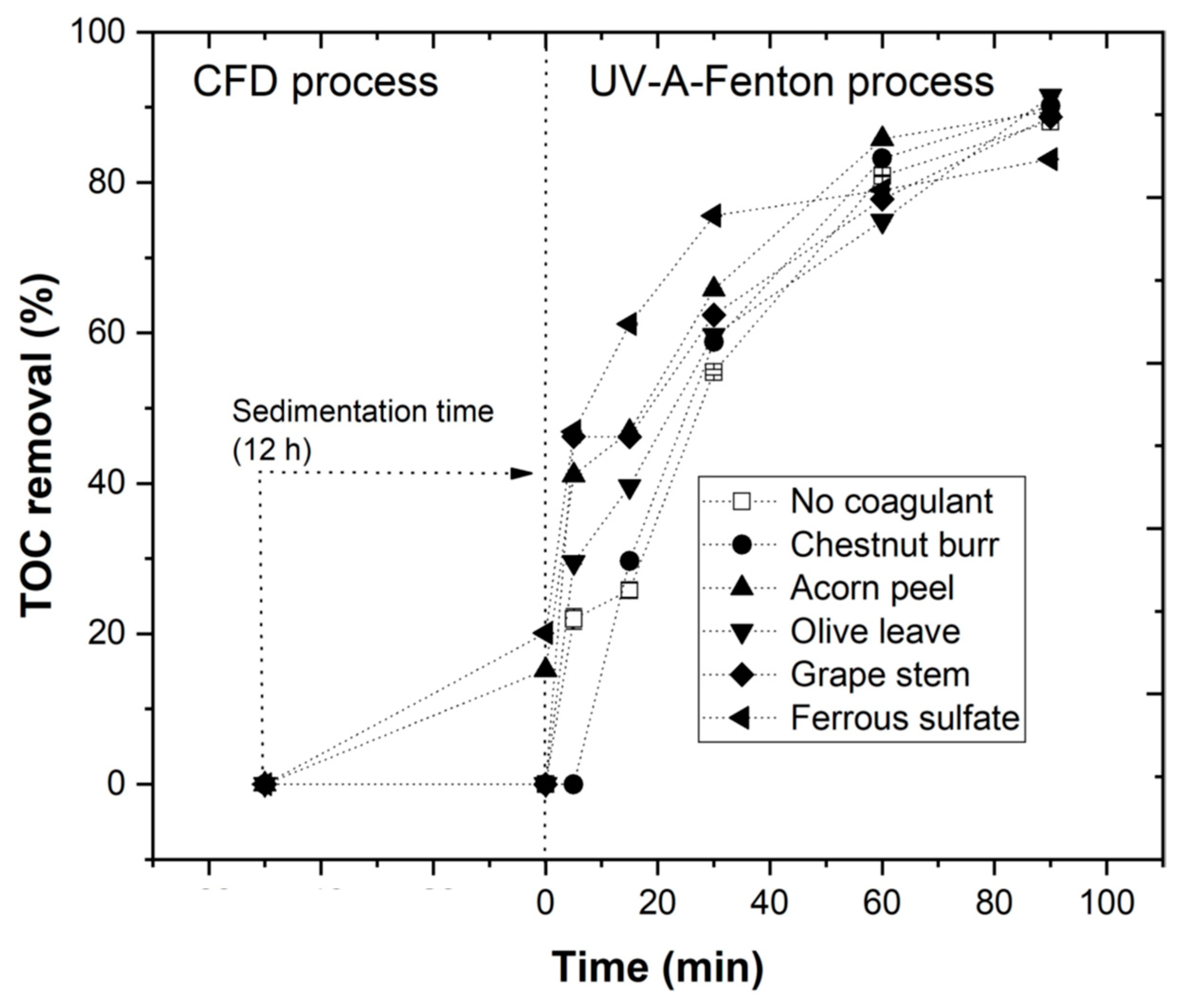
3.4. Combination of Coagulation–Flocculation–Decantation and UV-A-Fenton
4. Conclusions
- The plant-based coagulants are a carbon-based material with proteins, fatty acids, carbohydrates, cellulose, and lignin in their constitution.
- The plant-based coagulants achieve higher efficiency at pH 3.0. The EW pre-treatment using the CFD process is important to reduce turbidity and total suspended solids in order to improve the efficiency of the photo-Fenton process.
- Under the best operational conditions, the photo-Fenton process achieves 88.1% TOC removal, and the synergistic effect is more evident with the application of higher irradiance power.
- The performance of pre-treatment using the CFD process increases the efficiency of the photo-Fenton process, with higher TOC removal rates.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards circular economy in the agri-food sector. A systematic literature review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 6 December 2021).
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Tsani, S.; Koundouri, P.; Akinsete, E. Resource management and sustainable development: A review of the European water policies in accordance with the United Nations’ Sustainable Development Goals. Environ. Sci. Policy 2020, 114, 570–579. [Google Scholar] [CrossRef]
- Peres, J.A.; de Heredia, J.B.; Domınguez, J.R. Integrated Fenton’s reagent—coagulation/flocculation process for the treatment of cork processing wastewaters. J. Hazard. Mater. 2004, 107, 115–121. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Peres, J.A. Improvement of the flocculation process in water treatment by using Moringa oleifera seeds extract. Braz. J. Chem. Eng. 2012, 29, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Gautam, S.; Saini, G. Use of natural coagulants for industrial wastewater treatment. Glob. J. Environ. Sci. Manag. 2020, 6, 553–578. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N. Potential of local plant leaves as natural coagulant for turbidity removal. Environ. Sci. Pollut. Res. 2022, 29, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Venkateswarlu, K.; Thavamani, P.; Lee, Y.B.; Naidu, R.; Megharaj, M. Quercus robur acorn peel as a novel coagulating adsorbent for cationic dye removal from aquatic ecosystems. Ecol. Eng. 2017, 101, 3–8. [Google Scholar] [CrossRef]
- Ibrahim, T.H.; Sabri, M.A.; Khamis, M.I.; Elsayed, Y.A.; Sara, Z.; Hafez, B. Produced water treatment using olive leaves. Desalin. Water Treat. 2017, 60, 129–136. [Google Scholar] [CrossRef]
- Elsherif, K.M.; El-Dali, A.; Alkarewi, A.A.; Ewlad-ahmed, A.M.; Treban, A. Adsorption of crystal violet dye onto olive leaves powder: Equilibrium and kinetic studies. Chem. Int. 2021, 7, 79–89. [Google Scholar]
- Fawzy, M.; Nasr, M.; Abdel-Rahman, A.M.; Hosny, G.; Odhafa, B.R. Techno-economic and environmental approaches of Cd2+ adsorption by olive leaves (Olea europaea L.) waste. Int. J. Phytoremediat. 2019, 21, 1205–1214. [Google Scholar] [CrossRef]
- Šćiban, M.; Klašnja, M.; Antov, M.; Škrbić, B. Removal of water turbidity by natural coagulants obtained from chestnut and acorn. Bioresour. Technol. 2009, 100, 6639–6643. [Google Scholar] [CrossRef]
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of advanced oxidation processes for the treatment of recalcitrant agro-industrial wastewater: A review. Water 2019, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Amor, C.; Lucas, M.S.; Pirra, A.J.; Peres, J.A. Treatment of concentrated fruit juice wastewater by the combination of biological and chemical processes. J. Environ. Sci. Heal. Part A 2012, 47, 1809–1817. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Dhib, R.; Mehrvar, M. Recent Advances in Dynamic Modeling and Process Control of PVA Degradation by Biological and Advanced Oxidation Processes: A Review on Trends and Advances. Environments 2021, 8, 116. [Google Scholar] [CrossRef]
- Litter, M.I.; Slodowicz, M. An overview on heterogeneous Fenton and photo-Fenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20, 20160173. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Pulgarin, C.; Benítez, N. Degradation of DBPs’ precursors in river water before and after slow sand filtration by photo-Fenton process at pH 5 in a solar CPC reactor. Water Res. 2008, 42, 4125–4132. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Liu, D.; Huston, P. Evidence for an Additional Oxidant in the Photoassisted Fenton Reaction. Environ. Sci. Technol. 1999, 33, 1832–1839. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Salmerón, I.; Peres, J.A.; Tavares, P.B.; Lucas, M.S.; Malato, S. Advanced Oxidation Processes as sustainable technologies for the reduction of elderberry agro-industrial water impact. Water Resour. Ind. 2020, 24, 100137. [Google Scholar] [CrossRef]
- Amaral-Silva, N.; Martins, R.C.; Castro-Silva, S.; Quinta-Ferreira, R.M. Fenton’s treatment as an effective treatment for elderberry effluents: Economical evaluation. Environ. Technol. 2016, 37, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 1999. [Google Scholar]
- Durán, A.; Monteagudo, J.M.; Carnicer, A. Photo-Fenton mineralization of synthetic apple-juice wastewater. Chem. Eng. J. 2011, 168, 102–107. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Matos, C.C.; Lucas, M.S.; Peres, J.A. Combination of Coagulation–Flocculation–Decantation and Ozonation Processes for Winery Wastewater Treatment. Int. J. Environ. Res. Public Heal. 2021, 18, 8882. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.M.; Farhan, A.M. Equilibrium, Kinetic and Thermodynamics of Biosorption of Lead (II) Copper (II) and Cadmium (II) Ions from Aqueous Solutions onto Olive Leaves Powder. Am. J. Chem. 2012, 2, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.À.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical characterization of different granulometric fractions of grape stalks waste. Ind. Crop. Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Mébarki, M.; Hachem, K.; Harche, M.K. Lignocellulosic fraction of the pericarps of the acorns of Quercus suber and Quercus ilex: Isolation, characterization, and biosorption studies in the removal of copper from aqueous solutions. Polish J. Chem. Technol. 2019, 21, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Malkoc, E.; Nuhoglu, Y. Determination of kinetic and equilibrium parameters of the batch adsorption of Cr(VI) onto waste acorn of Quercus ithaburensis. Chem. Eng. Process. Process Intensif. 2007, 46, 1020–1029. [Google Scholar] [CrossRef]
- Wani, I.A.; Hamid, H.; Hamdani, A.M.; Gani, A.; Ashwar, B.A. Physico-chemical, rheological and antioxidant properties of sweet chestnut (Castanea sativa Mill.) as affected by pan and microwave roasting. J. Adv. Res. 2017, 8, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Evtuguin, D.V.; Silva, A.M.; Lopes, L.P. Structural Characterization of Lignin from Grape Stalks (Vitis vinifera L.). J. Agric. Food Chem. 2014, 62, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Yadav, A.; Ghosh, P.C.; Adeloju, S.B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017, 90, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Boulaadjoul, S.; Zemmouri, H.; Bendjama, Z.; Drouiche, N. A novel use of Moringa oleifera seed powder in enhancing the primary treatment of paper mill effluent. Chemosphere 2018, 206, 142–149. [Google Scholar] [CrossRef]
- Howe, K.J.; Hand, D.W.; Crittenden, J.C.; Trussell, R.R.; Tchobanoglous, G. Principles of Water Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 0470405384. [Google Scholar]
- Amor, C.; De Torres-Socías, E.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef]
- Ishak, A.R.; Hamid, F.S.; Mohamad, S.; Tay, K.S. Stabilized landfill leachate treatment by coagulation-flocculation coupled with UV-based sulfate radical oxidation process. Waste Manag. 2018, 76, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Vunain, E.; Mike, P.; Mpeketula, G.; Monjerezi, M.; Etale, A. Evaluation of coagulating efficiency and water borne pathogens reduction capacity of Moringa oleifera seed powder for treatment of domestic wastewater from Zomba, Malawi. J. Environ. Chem. Eng. 2019, 7, 103118. [Google Scholar] [CrossRef]
- Santos, C.; Lucas, M.S.; Dias, A.A.; Bezerra, R.M.F.; Peres, J.A. Winery wastewater treatment by combination of Cryptococcus laurentii and Fenton’s reagent. Chemosphere 2014, 117, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Amor, C.; Fernandes, J.R.; Tavares, P.B.; Lucas, M.S.; Peres, J.A. Treatment of crystallized-fruit wastewater by UV-A LED photo-Fenton and coagulation-flocculation. Chemosphere 2016, 145, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Wang, C.; Yu, G.; Chen, H.; Wang, J. Degradation of norfloxacin by hydroxylamine enhanced fenton system: Kinetics, mechanism and degradation pathway. Chemosphere 2021, 270, 129408. [Google Scholar] [CrossRef]
- Tekin, H.; Bilkay, O.; Ataberk, S.S.; Balta, T.H.; Ceribasi, I.H.; Sanin, F.D.; Dilek, F.B.; Yetis, U. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 2006, 136, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Silva, T.; Fernandes, J.R.; Li Puma, G.; Lucas, M.S.; Peres, J.A.; Sampaio, A. Inactivation of pathogenic microorganisms in freshwater by HSO5−/UV-A LED and HSO5−/Mn+/UV-A LED oxidation processes. Water Res. 2017, 123, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontistis, Z. Sonoelectrochemical degradation of propyl paraben: An examination of the synergy in different water matrices. Int. J. Environ. Res. Public Health 2020, 17, 2621. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Muñoz-Serrano, A.; Peres, J.A. Towards overcoming TOC increase in wastewater treated with Moringa oleifera seed extract. Chem. Eng. J. 2012, 188, 40–46. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Treatment of olive mill wastewater by a combined process: Fenton’s reagent and chemical coagulation. J. Environ. Sci. Health Part A 2009, 44, 198–205. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Elderberry Wastewater | Portuguese Law Decree n° 236/98 |
|---|---|---|
| pH | 4.39 ± 0.04 | 6.0–9.0 |
| Electrical conductivity (μS cm−1) | 54.4 ± 10.2 | |
| Turbidity (NTU) | 16.0 ± 2.6 | |
| Total suspended solids—TSS (mg L−1) | 64.0 ± 2.5 | 60 |
| Chemical Oxygen Demand—COD (mg O2 L−1) | 773 ± 7.0 | 150 |
| Biochemical Oxygen Demand—BOD5 (mg O2 L−1) | 175 ± 18.0 | 40 |
| BOD5/COD | 0.23 ± 0.02 | |
| Total Organic Carbon—TOC (mg C L−1) | 144 ± 0.1 | |
| Total polyphenols (mg gallic acid L−1) | 37.8 ± 0.2 | 0.5 |
| Plant Species | Fruit Common Name | Re-Used Portion |
|---|---|---|
| Castanea sativa | Chestnut | Burr |
| Quercus ilex and Quercus rotundifolia | Acorn | Peel |
| Olea europaea | Olive | Leaf |
| Vitis vinifera | Grape | Stem |
| Processes | k (min−1) | S (%) | |
|---|---|---|---|
| H2O2 + UV-A | 1.3 × 10−3 ± 0.4 × 10−3 | a | |
| Fe2+ + UV-A | 1.6 × 10−3 ± 0.3 × 10−3 | a | |
| H2O2 + Fe2+ (Fenton) | 6.4 × 10−3 ± 1.1 × 10−3 | b | |
| H2O2 + Fe2+ + UV-A (5.2 W m−2) | 8.0 × 10−3 ± 1.9 × 10−3 | c | −11.3 ± 1.163 a |
| H2O2 + Fe2+ + UV-A (18.3 W m−2) | 13.6 × 10−3 ± 0.9 × 10−3 | d | 36.4 ± 0.624 b |
| H2O2 + Fe2+ + UV-A (32.7 W m−2) | 25.0 × 10−3 ± 1.5 × 10−3 | e | 64.4 ± 0.687 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.B.; Jorge, N.; Lucas, M.S.; Raymundo, A.; Barros, A.I.R.N.A.; Peres, J.A. Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment. Int. J. Environ. Res. Public Health 2022, 19, 4134. https://doi.org/10.3390/ijerph19074134
Martins RB, Jorge N, Lucas MS, Raymundo A, Barros AIRNA, Peres JA. Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment. International Journal of Environmental Research and Public Health. 2022; 19(7):4134. https://doi.org/10.3390/ijerph19074134
Chicago/Turabian StyleMartins, Rita Beltrão, Nuno Jorge, Marco S. Lucas, Anabela Raymundo, Ana I. R. N. A. Barros, and José A. Peres. 2022. "Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment" International Journal of Environmental Research and Public Health 19, no. 7: 4134. https://doi.org/10.3390/ijerph19074134
APA StyleMartins, R. B., Jorge, N., Lucas, M. S., Raymundo, A., Barros, A. I. R. N. A., & Peres, J. A. (2022). Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment. International Journal of Environmental Research and Public Health, 19(7), 4134. https://doi.org/10.3390/ijerph19074134










