Insight into the Adsorption Behaviors of Antimony onto Soils Using Multidisciplinary Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Chemical Reagents
2.1.2. Soil Samples
2.2. Adsorption Experiments
2.2.1. Kinetic Experiment
2.2.2. Isothermal Experiment
2.2.3. Error Functions
2.2.4. The Effects of pH on Sb Adsorption
2.3. Soil Analysis and Characterization
3. Results and Discussion
3.1. The Adsorption Behaviors of Sb onto Soils
3.1.1. The Effects of Contact Times on Adsorption Behaviors of Sb
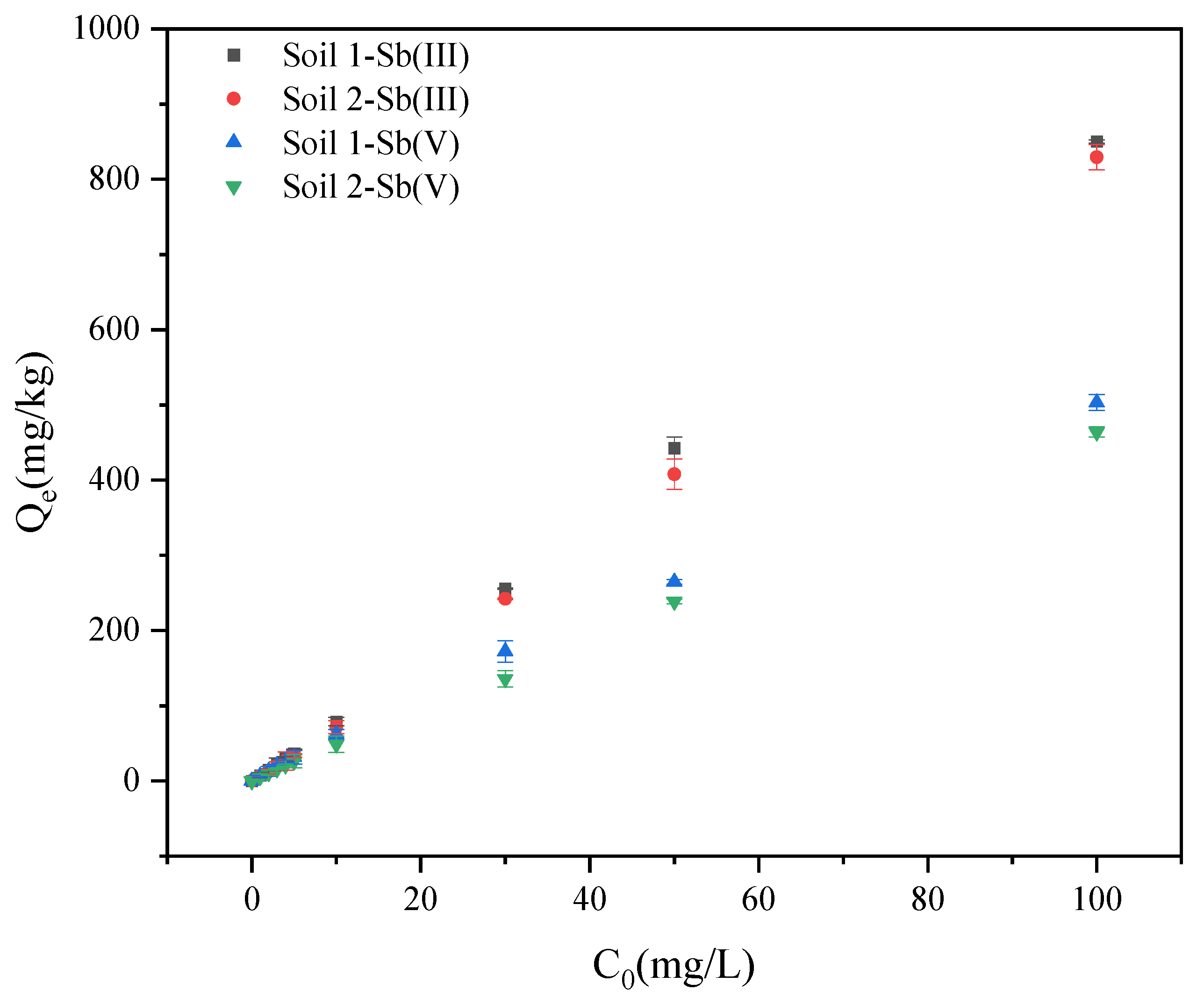
3.1.2. The Effects of Initial Concentration on Adsorption Behaviors of Sb
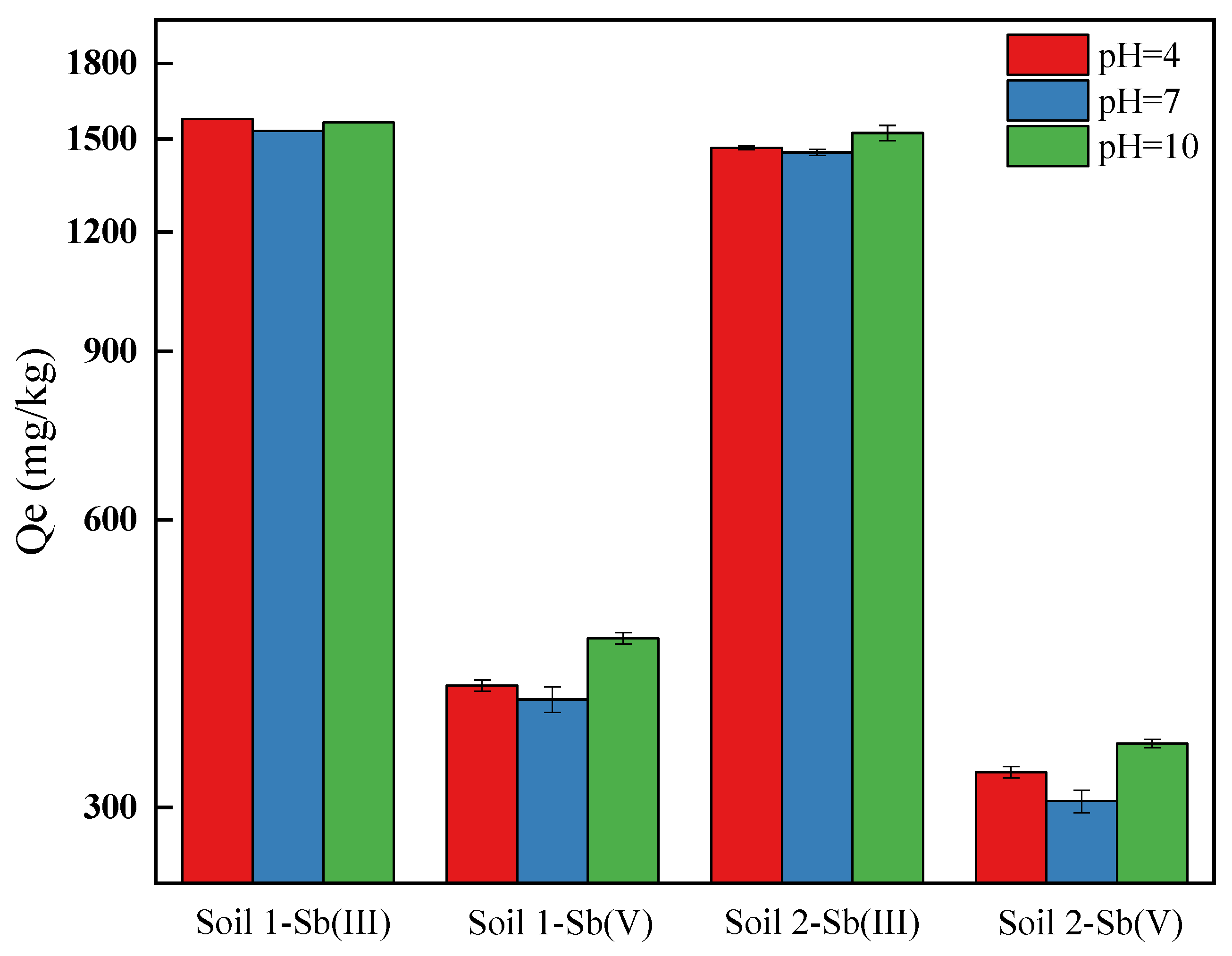
3.1.3. The Effects of pH on Adsorption Behaviors of Sb on Soils
3.2. The Adsorption Mechanism of Sb onto Soils
3.2.1. Adsorption Kinetic Experiments
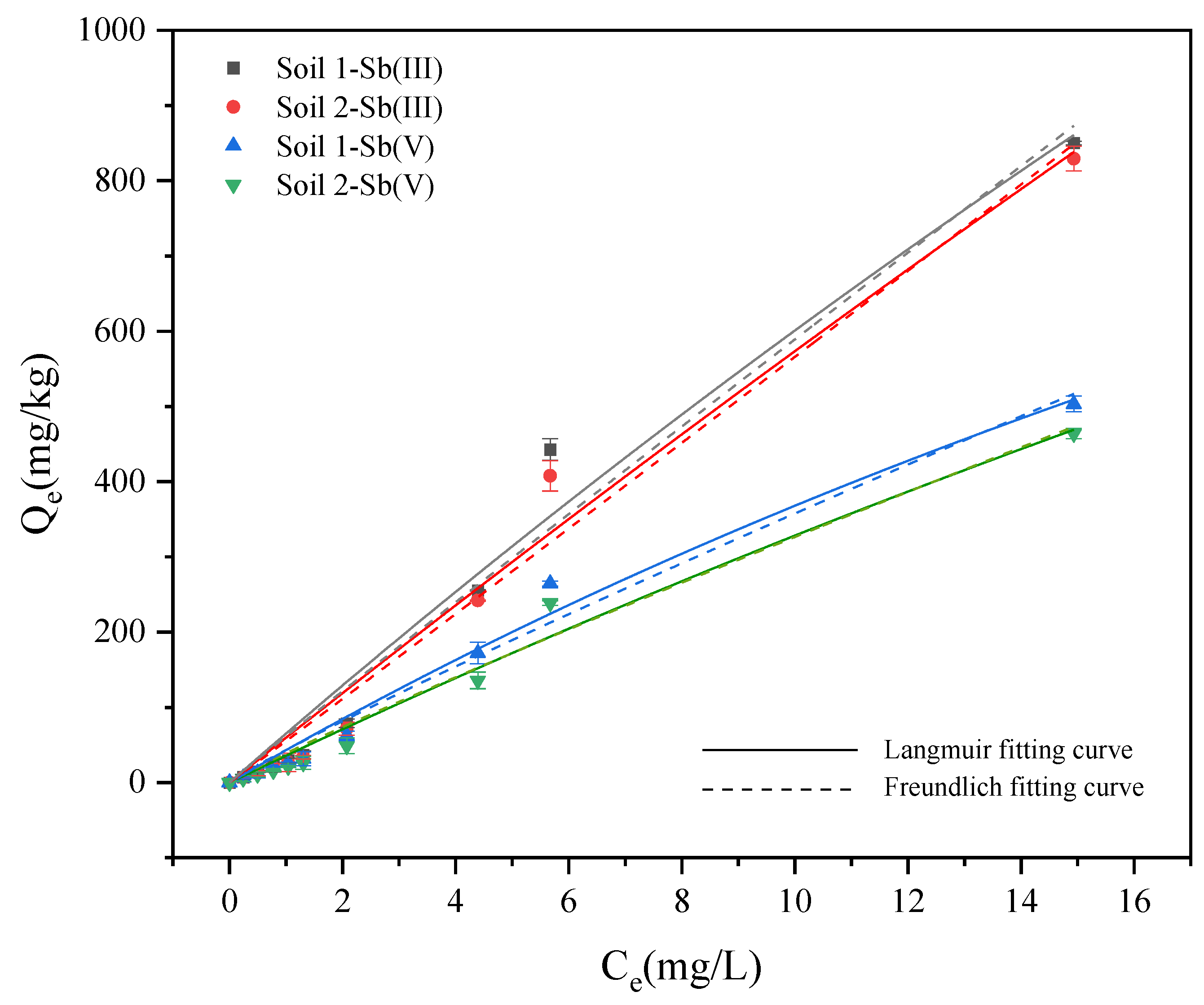
3.2.2. Adsorption Isotherm Experiments
3.3. The Transformation of Chemical Species of Sb in Soils
3.4. Insight into the Adsorption Behaviors of Sb on Soils
3.5. How to Understand the Migration and Transformation of Sb between Soil and Groundwater
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wang, Q.; Li, M.; Yang, B.; Shi, M.; Guo, W.; McDermott, T.R.; Rensing, C.; Wang, G. Proteomics and Genetics for Identi-fication of a Bacterial Antimonite Oxidase in Agrobacterium tumefaciens. Environ. Sci. Technol. 2015, 49, 5980–5989. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Zhu, Y.-G.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fu, Z.; Liu, B.; Mo, C.; Chen, B.; Corns, W.; Liao, H. Health risk associated with dietary co-exposure to high levels of antimony and arsenic in the world’s largest antimony mine area. Sci. Total Environ. 2011, 409, 3344–3351. [Google Scholar] [CrossRef]
- Wang, Q.; He, M.; Wang, Y. Influence of combined pollution of antimony and arsenic on culturable soil microbial populations and enzyme activities. Ecotoxicology 2011, 20, 9–19. [Google Scholar] [CrossRef]
- An, Y.-J.; Kim, M. Effect of antimony on the microbial growth and the activities of soil enzymes. Chemosphere 2009, 74, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Tripathy, S.; Kim, K.; Kim, S.-H. Arsenic fractions and enzyme activities in arsenic-contaminated soils by groundwater irrigation in West Bengal. Ecotoxicol. Environ. Saf. 2008, 71, 149–156. [Google Scholar] [CrossRef]
- Bordeleau, G.; Martel, R.; Ampleman, G.; Thiboutot, S. Environmental Impacts of Training Activities at an Air Weapons Range. J. Environ. Qual. 2008, 37, 308–317. [Google Scholar] [CrossRef]
- Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- Chang, C.; Li, F.; Wang, Q.; Hu, M.; Du, Y.; Zhang, X.; Zhang, X.; Chen, C.; Yu, H.-Y. Bioavailability of antimony and arsenic in a flowering cabbage–soil system: Controlling factors and interactive effect. Sci. Total Environ. 2022, 815, 152920. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Gao, P.; Yang, N.; Xu, R.; Sun, X.; Li, B.; Xu, F.; Wang, X.; Song, B.; et al. Variation in the diazotrophic community in a vertical soil profile contaminated with antimony and arsenic. Environ. Pollut. 2021, 291, 118248. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Li, L.; He, M.; Ouyang, W.; Lin, C.; Liu, X. Toxicity and bioavailability of antimony to the earthworm (Eisenia fetida) in different agricultural soils. Environ. Pollut. 2021, 291, 118215. [Google Scholar] [CrossRef] [PubMed]
- Ashley, P.M.; Craw, D.; Tighe, M.K.; Wilson, N.J. Magnitudes, spatial scales and processes of environmental antimony mobility from orogenic gold–antimony mineral deposits, Australasia. Environ. Earth Sci. 2006, 51, 499–507. [Google Scholar] [CrossRef]
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. How can we adapt to geological scarcity of antimony? Investigation of antimony’s substitutability and of other measures to achieve a sustainable use. Resour. Conserv. Recycl. 2016, 108, 54–62. [Google Scholar] [CrossRef]
- McNamara, D.D.; Sewell, S.; Buscarlet, E.; Wallis, I.C. A review of the Rotokawa Geothermal Field, New Zealand. Geothermics 2016, 59, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Simmons, S.F.; Brown, K.L.; Browne, P.R.; Rowland, J.V. Gold and silver resources in Taupo Volcanic Zone geothermal systems. Geothermics 2016, 59, 205–214. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Xi, J.; Lu, X. Antimony distribution and mobility in rivers around the world’s largest antimony mine of Xikuangshan, Hunan Province, China. Microchem. J. 2011, 97, 4–11. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Ma, J.; Zhao, W.; Chen, H.; Qu, Y. Antimony, beryllium, cobalt, and vanadium in urban park soils in Beijing: Machine learning-based source identification and health risk-based soil environmental criteria. Environ. Pollut. 2021, 293, 118554. [Google Scholar] [CrossRef]
- Busby, R.R.; Barbato, R.A.; Jung, C.M.; Bednar, A.J.; Douglas, T.A.; Ringelberg, D.B.; Indest, K.J. Alaskan plants and their assembled rhizosphere communities vary in their responses to soil antimony. Appl. Soil Ecol. 2021, 167, 104031. [Google Scholar] [CrossRef]
- He, M.; Wan, H. Distribution, speciation, toxicity and bioavailability of antimony in the environment. Prog. Chem. 2004, 16, 131–135. [Google Scholar]
- Rong, Q.; Nong, X.; Zhang, C.; Zhong, K.; Zhao, H. Immobilization mechanism of antimony by applying zirconium-manganese oxide in soil. Sci. Total Environ. 2022, 823, 153435. [Google Scholar] [CrossRef]
- Xia, B.; Yang, Y.; Li, F.; Liu, T. Kinetics of antimony biogeochemical processes under pre-definite anaerobic and aerobic con-ditions in a paddy soil. J. Environ. Sci. 2022, 113, 269–280. [Google Scholar] [CrossRef]
- He, Z.; Liu, R.; Liu, H.; Qu, J. Adsorption of Sb(III) and Sb(V) on Freshly Prepared Ferric Hydroxide (FeOxHy). Environ. Eng. Sci. 2015, 32, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ilgen, A.; Majs, F.; Barker, A.; Douglas, T.; Trainor, T. Oxidation and mobilization of metallic antimony in aqueous systems with simulated groundwater. Geochim. Et Cosmochim. Acta 2014, 132, 16–30. [Google Scholar] [CrossRef]
- Takaoka, M.; Fukutani, S.; Yamamoto, T.; Horiuchi, M.; Satta, N.; Takeda, N.; Oshita, K.; Yoneda, M.; Morisawa, C.; Tanaka, T. De-termination of chemical form of antimony in contaminated soil around a smelter using X-ray absorption fine structure. Anal. Sci. 2005, 21, 769–773. [Google Scholar] [CrossRef] [Green Version]
- Scheinost, A.C.; Rossberg, A.; Vantelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.-K.; Funke, H.; Johnson, C.A. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Et Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- Pilarski, J.; Waller, P.; Pickering, W. Sorption of antimony species by humic acid. Water Air Soil Pollut. 1995, 84, 51–59. [Google Scholar] [CrossRef]
- Lintschinger, J.; Michalke, B.; Schulte-Hostede, S.; Schramel, P. Studies on Speciation of Antimony in Soil Contaminated by Industrial Activity. Int. J. Environ. Anal. Chem. 1998, 72, 11–25. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Harada, T.; Takahashi, Y. Comparison of Antimony Behavior with that of Arsenic under Various Soil Redox Conditions. Environ. Sci. Technol. 2006, 40, 7270–7276. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Edyvean, R.G. Bioremoval of antimony(III) from contaminated water using several plant wastes: Optimization of batch and dynamic flow conditions for sorption by green bean husk (Vigna radiata). Chem. Eng. J. 2013, 225, 192–201. [Google Scholar] [CrossRef]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Xi, J.; He, M.; Lin, C. Adsorption of antimony(V) on kaolinite as a function of pH, ionic strength and humic acid. Environ. Earth Sci. 2009, 60, 715–722. [Google Scholar] [CrossRef]
- Brannon, J.; Patrick, W. Fixation and mobilization of antimony in sediments. Environ. Pollut. Ser. B Chem. Phys. 1985, 9, 107–126. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, J.; Xie, X.; Zhang, Y.; Jing, C. Acidity-dependent mobilization of antimony and arsenic in sediments near a mining area. J. Hazard. Mater. 2021, 426, 127790. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, T.; Jia, X.; Zhong, M.; Wang, N.; Li, N. Study on stabilization of antimony(Sb)in contaminated soil by primary explosives using iron-based and aluminum-based adsorbents. China Environ. Sci. 2020, 40, 3520–3529. [Google Scholar]
- Rakshit, S.; Sarkar, D.; Datta, R. Surface complexation of antimony on kaolinite. Chemosphere 2015, 119, 349–354. [Google Scholar] [CrossRef]
- Leuz, A.-K.; Mönch, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to Goethite: Influence on Sb(III) Oxidation and Mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef]
- Do, X.-H.; Lee, B.-K. Removal of Pb2+ using a biochar–alginate capsule in aqueous solution and capsule regeneration. J. Environ. Manag. 2013, 131, 375–382. [Google Scholar] [CrossRef]
- Tavakoli, H.; Sepehrian, H.; Cheraghali, R. Encapsulation of nanoporous MCM-41 in biopolymeric matrix of calcium alginate and its use as effective adsorbent for lead ions: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2013, 44, 343–348. [Google Scholar] [CrossRef]
- Hadi, M.; Samarghandi, M.R.; McKay, G. Equilibrium two-parameter isotherms of acid dyes sorption by activated carbons: Study of residual errors. Chem. Eng. J. 2010, 160, 408–416. [Google Scholar] [CrossRef]
- Ilgen, A.G.; Trainor, T.P. Sb(III) and Sb(V) Sorption onto Al-Rich Phases: Hydrous Al Oxide and the Clay Minerals Kaolinite KGa-1b and Oxidized and Reduced Nontronite NAu-1. Environ. Sci. Technol. 2011, 46, 843–851. [Google Scholar] [CrossRef]
- Liu, R.; Xu, W.; He, Z.; Lan, H.; Liu, H.; Qu, J.; Prasai, T. Adsorption of antimony(V) onto Mn(II)-enriched surfaces of manganese-oxide and Fe Mn binary oxide. Chemosphere 2015, 138, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Renard, F.; Putnis, C.V.; Montes-Hernandez, G.; King, H.E.; Breedveld, G.D.; Okkenhaug, G. Sequestration of Antimony on Calcite Observed by Time-Resolved Nanoscale Imaging. Environ. Sci. Technol. 2018, 52, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef]
- Tighe, M.; Lockwood, P.; Wilson, S. Adsorption of antimony(V) by floodplain soils, amorphous iron(III) hydroxide and humic acid. J. Environ. Monit. 2005, 7, 1177–1185. [Google Scholar] [CrossRef] [Green Version]
- Dousova, B.; Buzek, F.; Herzogova, L.; Machovič, V.; Lhotka, M. Effect of organic matter on arsenic(V) and antimony(V) adsorption in soils. Eur. J. Soil Sci. 2014, 66, 74–82. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef]
- Verbeeck, M.; Moens, C.; Gustafsson, J.P. Mechanisms of antimony ageing in soils: An XAS study. Appl. Geochem. 2021, 128, 104936. [Google Scholar] [CrossRef]
- Morel, M.C.; Spadini, L.; Brimo, K.; Martins, J.M. Speciation study in the sulfamethoxazole-copper-pH-soil system: Implications for retention prediction. Sci. Total Environ. 2014, 481, 266–273. [Google Scholar] [CrossRef]
- Xi, J.; He, M.; Kong, L. Adsorption of antimony on kaolinite as a function of time, pH, HA and competitive anions. Environ. Earth Sci. 2016, 75, 136. [Google Scholar] [CrossRef]
- Mittal, V.K.; Bera, S.; Narasimhan, S.; Velmurugan, S. Adsorption behavior of antimony(III) oxyanions on magnetite surface in aqueous organic acid environment. Appl. Surf. Sci. 2013, 266, 272–279. [Google Scholar] [CrossRef]
- Mason, S.E.; Trainor, T.P.; Goffinet, C.J. DFT study of Sb(III) and Sb(V) adsorption and heterogeneous oxidation on hydrated oxide surfaces. Comput. Theor. Chem. 2012, 987, 103–114. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Namasivayam, C. Activated carbon from coconut coirpith as metal adsorbent: Adsorption of Cd(II) from aqueous solution. Adv. Environ. Res. 2003, 7, 471–478. [Google Scholar] [CrossRef]
- Xi, J.; He, M.; Lin, C. Adsorption of antimony(III) and antimony(V) on bentonite: Kinetics, thermodynamics and anion competition. Microchem. J. 2011, 97, 85–91. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Copper, Nickel and Lead Single Component Systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Singh, N. Sorption Behavior of Triazole Fungicides in Indian Soils and Its Correlation with Soil Properties. J. Agric. Food Chem. 2002, 50, 6434–6439. [Google Scholar] [CrossRef]
- Dai, Y.; Nasir, M.; Zhang, Y.; Wu, H.; Guo, H.; Lv, J. Comparison of DGT with traditional methods for assessing cadmium bioavailability to Brassica chinensis in different soils. Sci. Rep. 2017, 7, 14206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelis, G.; Van Gerven, T.; Snellings, R.; Verbinnen, B.; Elsen, J.; Vandecasteele, C. Stability of pyrochlores in alkaline matrices: Solubility of calcium antimonate. Appl. Geochem. 2011, 26, 809–817. [Google Scholar] [CrossRef]
- Filella, M.; May, P.M. Computer simulation of the low-molecular-weight inorganic species distribution of antimony(III) and an-timony(V) in natural waters. Geochim. Et Cosmochim. Acta 2003, 67, 4013–4031. [Google Scholar] [CrossRef]
- Kirsch, R.; Scheinost, A.C.; Rossberg, A.; Banerjee, D.; Charlet, L. Reduction of antimony by nano-particulate magnetite and macki-nawite. Mineral. Mag. 2008, 72, 185–189. [Google Scholar] [CrossRef]
- Zhang, Y.; O’Loughlin, E.J.; Kwon, M.J. Antimony redox processes in the environment: A critical review of associated oxidants and reductants. J. Hazard. Mater. 2022, 431, 128607. [Google Scholar] [CrossRef]
- Cai, Y.; Mi, Y.; Zhang, H. Kinetic modeling of antimony(III) oxidation and sorption in soils. J. Hazard. Mater. 2016, 316, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Brugger, J.; Gieré, R.; Graeser, S.; Meisser, N. The crystal chemistry of roméite. Contrib. Mineral. Petrol. 1997, 127, 136–146. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]






| Soil Categories | Soil 1 | Soil 2 |
|---|---|---|
| pH | 8.19 | 8.61 |
| CEC (cmol/kg) | 47.35 | 14.45 |
| SOM (g/kg) | 24.96 | 4.53 |
| BET surface area (m2/g) | 14.07 | 6.52 |
| Pore volume (cm3/g) | 0.0199 | 0.0117 |
| Pore size (nm) | 5.646 | 7.172 |
| Particle sizes | <0.002 mm 45.3% 0.002–0.02 mm 50.1% >0.02 mm 4.6% | <0.002 mm 1% 0.002–0.02 mm 13.3% >0.02 mm 85.7% |
| Soil Sample | Valence State | First Order Kinetic Model | Second Kinetic Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe | k1 | R2 | ARE | RMSE | Qe | k2 | R2 | ARE | RMSE | ||
| soil 1 | Sb (III) | 1314.46 | 8.72 | 0.934 | 0.782 | 95.95 | 1354.07 | 0.013 | 0.969 | 0.927 | 118.66 |
| Sb (V) | 415.65 | 0.17 | 0.828 | 3.875 | 61.15 | 445.69 | 5.76 × 10−4 | 0.887 | 6.955 | 129.77 | |
| soil 2 | Sb (III) | 1359.25 | 2.88 | 0.872 | 1.370 | 150.69 | 1416.86 | 0.003 | 0.943 | 2.364 | 257.30 |
| Sb (V) | 533.97 | 0.09 | 0.870 | 4.380 | 69.80 | 606.06 | 1.78 × 10−4 | 0.916 | 9.733 | 177.77 | |
| Soil Categories | Valence State | Langmuir Model | Freundlich Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qm | KL | R2 | ARE | RMSE | KF | nF | R2 | ARE | RMSE | ||
| soil 1 | Sb (III) | 6949.72 | 0.009 | 0.975 | 7.192 | 40.52 | 61.55 | 1.019 | 0.974 | 6.522 | 42.23 |
| Sb (V) | 2285.78 | 0.019 | 0.984 | 3.991 | 18.76 | 43.26 | 1.090 | 0.981 | 4.292 | 21.50 | |
| soil 2 | Sb (III) | 12981.87 | 0.005 | 0.979 | 7.529 | 36.13 | 55.21 | 0.989 | 0.979 | 6.483 | 36.58 |
| Sb (V) | 3564.77 | 0.010 | 0.980 | 4.396 | 19.41 | 38.88 | 1.082 | 0.999 | 5.476 | 21.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Z.-Q.; Xu, D.-M.; Fu, R.-B. Insight into the Adsorption Behaviors of Antimony onto Soils Using Multidisciplinary Characterization. Int. J. Environ. Res. Public Health 2022, 19, 4254. https://doi.org/10.3390/ijerph19074254
Mu Z-Q, Xu D-M, Fu R-B. Insight into the Adsorption Behaviors of Antimony onto Soils Using Multidisciplinary Characterization. International Journal of Environmental Research and Public Health. 2022; 19(7):4254. https://doi.org/10.3390/ijerph19074254
Chicago/Turabian StyleMu, Zi-Qi, Da-Mao Xu, and Rong-Bing Fu. 2022. "Insight into the Adsorption Behaviors of Antimony onto Soils Using Multidisciplinary Characterization" International Journal of Environmental Research and Public Health 19, no. 7: 4254. https://doi.org/10.3390/ijerph19074254
APA StyleMu, Z.-Q., Xu, D.-M., & Fu, R.-B. (2022). Insight into the Adsorption Behaviors of Antimony onto Soils Using Multidisciplinary Characterization. International Journal of Environmental Research and Public Health, 19(7), 4254. https://doi.org/10.3390/ijerph19074254





