Residential Radon Exposure in Patients with Advanced Lung Cancer in Lublin Region, Poland
Abstract
:1. Introduction
2. Materials and Methods
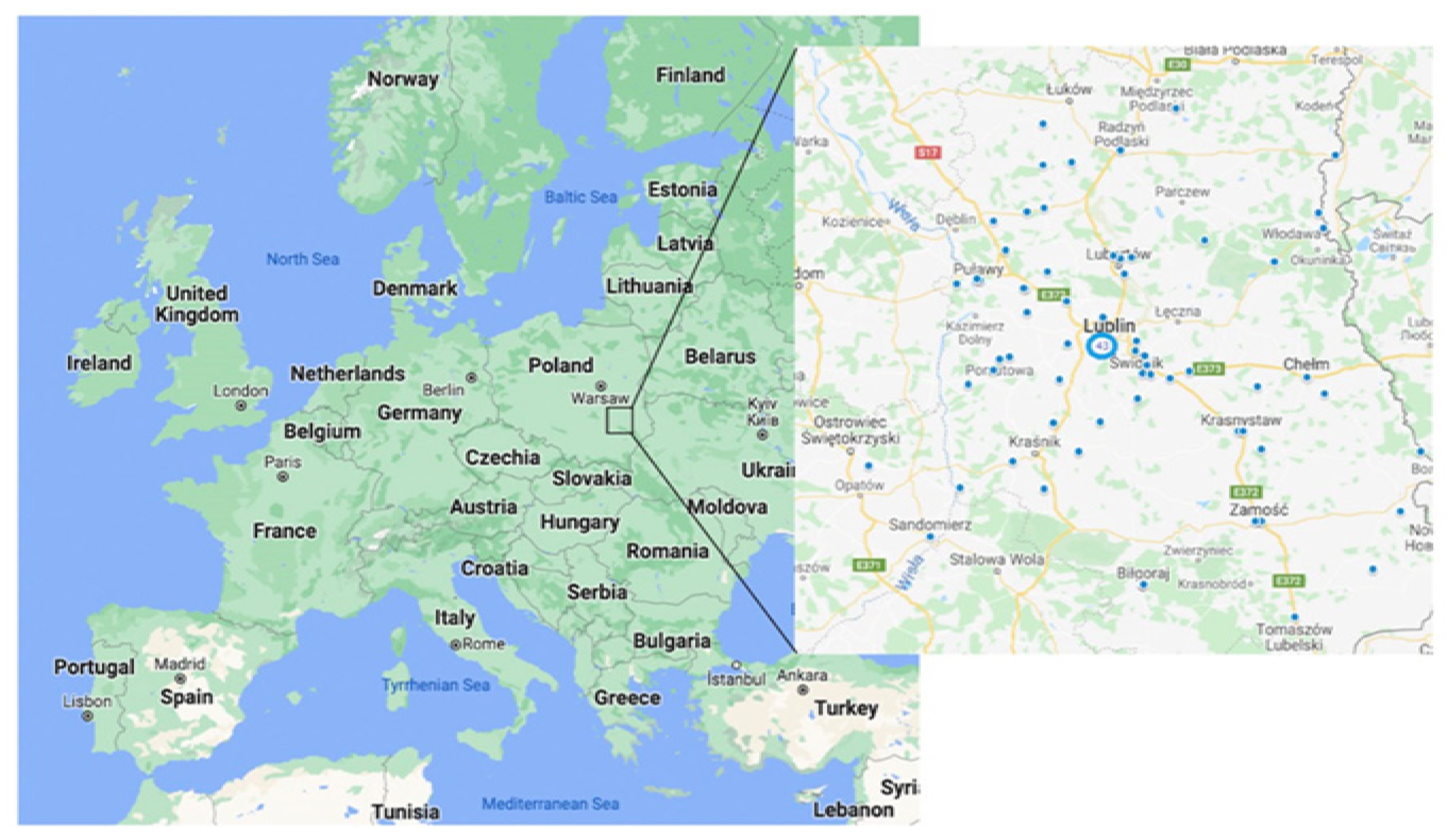
2.1. Study Group
2.2. Radon Exposure Measurements
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Grzywa-Celińska, A.; Krusiński, A.; Szewczyk, K.; Grzycka-Kowalczyk, L. A single-institution retrospective analysis of the differences between 7th and 8th edition of the UICC TNM staging system in patients with advanced lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8394–8401. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- De Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Read, S.; McGale, P.; Darby, S. Lung cancer deaths from indoor radon and the cost effectiveness and potential of policies to reduce them. BMJ 2009, 338, a3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Handbook on Indoor Radon: A Public Health Perspective/Edited by Hajo Zeeb and Ferid Shannoun; World Health Organization: Geneva, Switzerland, 2009.
- Pelosof, L.; Ahn, C.; Gao, A.; Horn, L.; Madrigales, A.; Cox, J.; McGavic, D.; Minna, J.D.; Gazdar, A.F.; Schiller, J. Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. J. Natl. Cancer Inst. 2017, 109, djw295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cufari, M.E.; Proli, C.; De Sousa, P.; Raubenheimer, H.; Al Sahaf, M.; Chavan, H.; Shedden, L.; Niwaz, Z.; Leung, M.; Nicholson, A.G.; et al. Increasing frequency of non-smoking lung cancer: Presentation of patients with early disease to a tertiary institution in the UK. Eur. J. Cancer 2017, 84, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hystad, P.; Demers, P.A.; Johnson, K.C.; Carpiano, R.M.; Brauer, M. Long-term residential exposure to air pollution and lung cancer risk. Epidemiology 2013, 24, 762–772. [Google Scholar] [CrossRef]
- Zheng, W.; Blot, W.J.; Liao, M.L.; Wang, Z.X.; Levin, L.I.; Zhao, J.J.; Fraumeni, J.F., Jr.; Gao, Y.T. Lung cancer and prior tuberculosis infection in Shanghai. Br. J. Cancer 1987, 56, 501–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayne, S.T.; Buenconsejo, J.; Janerich, D.T. Previous lung disease and risk of lung cancer among men and women nonsmokers. Am. J. Epidemiol. 1999, 149, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yang, M.; Li, P.; Su, Z.; Gao, P.; Zhang, J. Idiopathic pulmonary fibrosis will increase the risk of lung cancer. Chin. Med. J. 2014, 127, 3142–3149. [Google Scholar] [PubMed]
- Torres-Durán, M.; Ruano-Ravina, A.; Parente-Lamelas, I.; Leiro-Fernández, V.; Abal-Arca, J.; Montero-Martínez, C.; Pena-Álvarez, C.; Castro-Añón, O.; Golpe-Gómez, A.; Martínez, C.; et al. Residential radon and lung cancer characteristics in never smokers. Int. J. Radiat. Biol. 2015, 91, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; De Cort, M.; Tollefsen, T.; Achatz, M.; Ajtic, J.; Ballabio, C.; Barnet, I.; Bochicchio, F.; Borrelli, P.; Bossew, P.; et al. (Eds.) European Atlas of Natural Radiation; Publication Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Grządziel, D.; Kozak, K.; Mazur, J.; Połednik, B.; Dudzińska, M.R.; Bilska, I. The influence of air conditioning changes on the effective dose due to radon and its short-lived decay products. Nukleonika 2016, 61, 239–244. [Google Scholar] [CrossRef] [Green Version]
- National Center for Biotechnology Information. PubChem Database. Radon. CID=24857. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Radon (accessed on 23 March 2020).
- Grzywa-Celińska, A.; Krusiński, A.; Mazur, J.; Szewczyk, K.; Kozak, K. Radon—the element of risk. The impact of radon exposure on human health. Toxics 2020, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Balásházy, I.; Hofmann, W. Quantification of local deposition patterns of inhaled radon decay products in human bronchial airway bifurcations. Health Phys. 2000, 78, 147–158. [Google Scholar]
- Vähäkangas, K.H.; Samet, J.M.; Metcalf, R.A.; Welsh, J.A.; Bennett, W.P.; Lane, D.P.; Harris, C.C. Mutations of p53 and ras genes in radon-associated lung cancer from uranium miners. Lancet 1992, 339, 576–580. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Gu, C. Down-regulation of let-7 microRNA increased K-ras expression in lung damage induced by radon. Environ. Toxicol. Pharmacol. 2015, 40, 541–548. [Google Scholar] [CrossRef]
- Błach, J.; Frąk, M.; Krawczyk, P.; Pankowski, J.; Pankowski, A.; Buczkowski, J.; Szlubowski, A.; Siwiec, J.; Krudyś, P.; Michnar, M.; et al. Observational cross-sectional study of 5279 bronchoscopy results for the practical effectiveness of various biopsy techniques in the diagnosis of lung diseases with particular emphasis on lung cancer. BMJ Open 2021, 11, e043820. [Google Scholar] [CrossRef]
- Vogeltanz-Holm, N.; Schwartz, G.G. Radon and lung cancer: What does the public really know? J. Environ. Radioact. 2018, 192, 26–31. [Google Scholar] [CrossRef]
- Barros-Dios, J.M.; Ruano-Ravina, A.; Pérez-Ríos, M.; Castro-Bernárdez, M.; Abal-Arca, J.; Tojo-Castro, M. Residential radon exposure, histologic types, and lung cancer risk. A case-control study in Galicia. Spain. Cancer Epidemiol. Biomark. Prev. 2012, 21, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Biernacka, M.; Isajenko, K.; Lipiński, P.; Pietrzak-Flis, Z. Radiologiczny Atlas Polski 2005; Główny Inspektorat Ochrony Środowiska, Centralne Laboratorium Ochrony Radiologicznej, Biblioteka Monitoringu Środowiska: Warsaw, Poland, 2006; p. 64. [Google Scholar]
- Gawełek, E.; Drozdzowska, B.; Fuchs, A. Radon as a risk factor of lung cancer. Przegl. Epidemiol. 2017, 71, 90–98. [Google Scholar]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ 2005, 29, 223. [Google Scholar] [CrossRef] [Green Version]
- Hassfjell, C.S.; Grimsrud, T.K.; Standring, W.J.F.; Tretli, S. Lung cancer incidence associated with radon exposure in Norwegian homes. Tidsskr. Nor. Laegeforen. 2017, 137, 14–15. [Google Scholar]
- Khuder, S.A. Effect of cigarette smoking on major histological types of lung cancer: A meta-analysis. Lung Cancer 2001, 31, 139–148. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Yu, J.; Fan, Y.; Liu, D.; Zhou, W.; Shi, T. Residential radon and histological types of lung cancer: A meta-analysis of case—Control studies. Int. J. Environ. Res. Public Health 2020, 17, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Hwang, W.J.; Cho, J.S.; Kang, D.R. Attributable risk of lung cancer deaths due to indoor radon exposure. Ann. Occup. Environ. Med. 2016, 28, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesce, G.; Marcon, A.; Calciano, L.; Perret, J.L.; Abramson, M.J.; Bono, R.; Bousquet, J.; Fois, A.G.; Janson, C.; Jarvis, D.; et al. Time and age trends in smoking cessation in Europe. PLoS ONE 2019, 14, e0211976. [Google Scholar] [CrossRef] [Green Version]
| Variable | Study Group n = 102 (%) | |
|---|---|---|
| Gender | Female | 39 (38.2%) |
| Male | 63 (61.8%) | |
| Age | <65 | 39 (38.2%) |
| ≥65 | 63 (61.8%) | |
| Occupation | Engineer | 7 (6.9%) |
| Driver | 6 (5.9%) | |
| Teacher | 4 (3.9%) | |
| Worker | 17 (16.7%) | |
| White-collar worker | 19 (18.6%) | |
| Farmer | 17 (16.7%) | |
| Craftsmen | 12 (11.8%) | |
| Health professional | 8 (7.8%) | |
| Salesman | 5 (4.9%) | |
| Clerk | 4 (43.9%) | |
| Other | 3 (2.9%) | |
| Factor | Study Group n = 102 (%) | |
|---|---|---|
| Smoking status | current smoker | 35 (34.3%) |
| ex-smoker | 49 (48%) | |
| non-smoker | 18 (17.6%) | |
| Second-hand smoking exposure | Yes | 36 (35.3%) |
| No | 66 (64.7%) | |
| Place of second-hand smoking exposure | Home | 19 (54.3%) |
| Workplace | 10 (28.6%) | |
| Other | 6 (17.1%) | |
| Exposure to harmful substances | Yes | 55 (53.9%) |
| No | 47 (46.1%) | |
| Type of harmful substances | Biological | |
| chemicals * | 4 (7.4%) | |
| physical ** | 17 (30.7%) | |
| Dusts | 8 (14.8%) | |
| Pesticides | 10 (18.5%) | |
| no data (n = 48) | 15 (27.8%) | |
| Variable | Average Radon Concentration during Detector Exposure [Bq/m3] | Study Group n = 102 (%) | p | |
|---|---|---|---|---|
| Overall exposure to radon in the study group | 69.0 [37.0–117.0] | n = 102 | ||
| Representation of major types of lung cancer | Non-small cell lung cancer (NSCLC) | 72.5 [36.0–118.0] | 80 (78.4%) | 0.8547 |
| Small cell lung cancer (SCLC) | 66.5 [45.0–86.0] | 22 (21.6%) | ||
| Representation of subtypes of NSCLC | Adenocarcinoma | 70.5 [37.0–100.0] | 42 (41.2%) | 0.3696 |
| Squamous cell lung cancer | 69.0 [32.0–119.0] | 27 (26.5%) | ||
| Not-Otherwise-Specified (NOS) | 58.0 [35.5–108.7] | 7 (6.9%) | ||
| Other | 108.5 [95.0–147.5] | 4 (3.9%) | ||
| Genetic aberrations EGFR/ALK/ROS-1 | Yes | 74.0 [34.2–119.2] | 15 (34.9%) | 0.7499 |
| No | 66.0 [38.5–97.5] | 28 (65.1%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzywa-Celińska, A.; Chmielewska, I.; Krusiński, A.; Kozak, K.; Mazur, J.; Grządziel, D.; Dos Santos Szewczyk, K.; Milanowski, J. Residential Radon Exposure in Patients with Advanced Lung Cancer in Lublin Region, Poland. Int. J. Environ. Res. Public Health 2022, 19, 4257. https://doi.org/10.3390/ijerph19074257
Grzywa-Celińska A, Chmielewska I, Krusiński A, Kozak K, Mazur J, Grządziel D, Dos Santos Szewczyk K, Milanowski J. Residential Radon Exposure in Patients with Advanced Lung Cancer in Lublin Region, Poland. International Journal of Environmental Research and Public Health. 2022; 19(7):4257. https://doi.org/10.3390/ijerph19074257
Chicago/Turabian StyleGrzywa-Celińska, Anna, Izabela Chmielewska, Adam Krusiński, Krzysztof Kozak, Jadwiga Mazur, Dominik Grządziel, Katarzyna Dos Santos Szewczyk, and Janusz Milanowski. 2022. "Residential Radon Exposure in Patients with Advanced Lung Cancer in Lublin Region, Poland" International Journal of Environmental Research and Public Health 19, no. 7: 4257. https://doi.org/10.3390/ijerph19074257
APA StyleGrzywa-Celińska, A., Chmielewska, I., Krusiński, A., Kozak, K., Mazur, J., Grządziel, D., Dos Santos Szewczyk, K., & Milanowski, J. (2022). Residential Radon Exposure in Patients with Advanced Lung Cancer in Lublin Region, Poland. International Journal of Environmental Research and Public Health, 19(7), 4257. https://doi.org/10.3390/ijerph19074257