Volatile Organic Compounds in Finnish Office Environments in 2010–2019 and Their Relevance to Adverse Health Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Data
2.2. Sampling and Analysing VOCs
2.3. Sampling and Analysing Formaldehyde
2.4. Statistical Methods
2.5. Health Risk Assessment
3. Results
3.1. Statistics of Entire Ten-Year Data
3.2. Differences between Indoor Environments
3.3. Trends
4. Discussion
4.1. Indoor Air Concentrations of VOCs and Formaldehyde Are Generally Low and Pose No Health Risks
4.2. Differences between VOC Patterns in Different Types of Indoor Environments Have No Practical Relevance
4.3. Interpretation of VOC Trends Involves Uncertainties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, R.D.; Jurvelin, J.; Koistinen, K.; Saarela, K.; Jantunen, M. VOC source identification from personal and residential indoor, outdoor and workplace microenvironment samples in EXPOLIS-Helsinki, Finland. Atmos. Environ. 2001, 35, 4829–4841. [Google Scholar] [CrossRef]
- Geiss, O.; Giannopoulos, G.; Tirendi, S.; Barrero-Moreno, J.; Larsen, B.R.; Kotzias, D. The AIRMEX study—VOC measurements in public buildings and schools/kindergartens in eleven European cities: Statistical analysis of the data. Atmos. Environ. 2011, 45, 3676–3684. [Google Scholar] [CrossRef]
- Paciencia, I.; Madureira, J.; Rufo, J.; Moreira, A.; Fernandes, E.d.O. A systematic review of evidence and implications of spatial and seasonal variations of volatile organic compounds (VOC) in indoor human environments. J. Toxicol. Environ. Health B Crit. Rev. 2016, 19, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Su, F.-C.; Batterman, S. Volatile Organic Compounds (VOCs) in Conventional and High Performance School Buildings in the U.S. Int. J. Environ. Res. Public Health 2017, 14, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbatt, J.P.D.; Wang, C. The atmospheric chemistry of indoor environments. Environ. Sci. Process. Impacts 2019, 22, 25–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinazzè, A.; Campagnolo, D.; Cattaneo, A.; Urso, P.; Sakellaris, I.A.; Saraga, D.; Mandin, C.; Canha, N.; Mabilia, R.; Perreca, E.; et al. Indoor gaseous air pollutants determinants in office buildings—The OFFICAIR project. Indoor Air 2019, 30, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Mandin, C.; Trantallidi, M.; Cattaneo, A.; Canha, N.; Mihucz, V.G.; Szigeti, T.; Mabilia, R.; Perreca, E.; Spinazzè, A.; Fossati, S.; et al. Assessment of indoor air quality in office buildings across Europe—The OFFICAIR study. Sci. Total Environ. 2017, 579, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloch, R.M.; Maesano, C.N.; Christoffersen, J.; Banerjee, S.; Gabriel, M.; Csobod, É.; de Oliveira Fernandes, E.; Annesi-Maesano, I.; SINPHONIE Study Group. Indoor air pollution, physical and comfort parameters related to schoolchildren’s health: Data from the European SINPHONIE study. Sci. Total Environ. 2020, 739, 139870. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.L.; Katsoyiannis, A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T. Emerging indoor pollutants. Int. J. Hyg. Environ. Health 2020, 224, 113423. [Google Scholar] [CrossRef] [PubMed]
- Sundell, J. Reflections on the history of indoor air science, focusing on the last 50 years. Indoor Air 2017, 27, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Weschler, C.J. Changes in indoor pollutants since the 1950s. Atmos. Environ. 2009, 43, 153–169. [Google Scholar] [CrossRef]
- Fromme, H.; Debiak, M.; Sagunski, H.; Röhl, C.; Kraft, M.; Kolossa-Gehring, M. The German approach to regulate indoor air contaminants. Int. J. Hyg. Environ. Health 2019, 222, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Umweltbundesamt—UBA, German Committee on Indoor Guide Values. 2021. Available online: https://www.umweltbundesamt.de/en/topics/health/commissions-working-groups/german-committee-on-indoor-guide-values#guide-values-i-and-ii (accessed on 7 December 2021).
- EU-LCI Working Group. EU-LCI Values. 2020. Available online: https://ec.europa.eu/growth/sectors/construction/eu-lci-subgroup_en (accessed on 7 December 2021).
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment. Part E: Risk Characterisation, Version 3.0. ECHA-2016-G-04-EN. 2016. Available online: https://echa.europa.eu/documents/10162/17224/information_requirements_part_e_en.pdf/ (accessed on 9 February 2022).
- Madureira, J.; Paciência, I.; Rufo, J.; Ramos, E.; Barros, H.; Teixeira, J.P.; de Oliveira Fernandes, E. Indoor air quality in schools and its relationship with children’s respiratory symptoms. Atmos. Environ. 2015, 118, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, M.I.; Curtis, J.A.; Vearrier, D. The perception of odor is not a surrogate marker for chemical exposure: A review of factors influencing human odor perception. Clin. Toxicol. 2013, 51, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, D.; Saraga, D.E.; Cattaneo, A.; Spinazzè, A.; Mandin, C.; Mabilia, R.; Perreca, E.; Sakellaris, I.; Canha, N.; Mihucz, V.G.; et al. VOCs and aldehydes source identification in European office buildings—The OFFICAIR study. Build. Environ. 2017, 115, 18–24. [Google Scholar] [CrossRef]
- M1 Classification for Low-Emission Building Materials. The Building Information Foundation RTS sr. 2021. Available online: https://cer.rts.fi/en/ (accessed on 7 December 2021).
| Year | Office | School | Kindergarten | Healthcare Office |
|---|---|---|---|---|
| 2010 | 384 | 253 | 52 | 119 |
| 2011 | 416 | 310 | 68 | 182 |
| 2012 | 333 | 298 | 67 | 295 |
| 2013 | 385 | 309 | 69 | 190 |
| 2014 | 447 | 344 | 102 | 105 |
| 2015 | 313 | 163 | 42 | 99 |
| 2016 | 324 | 489 | 81 | 195 |
| 2017 | 379 | 548 | 87 | 139 |
| 2018 | 446 | 449 | 90 | 144 |
| 2019 | 445 | 420 | 69 | 139 |
| Year | Office | School | Kindergarten | Healthcare Office |
|---|---|---|---|---|
| 2010 | 19 | 14 | 5 | 6 |
| 2011 | 47 | 37 | 7 | 14 |
| 2012 | 72 | 37 | 5 | 26 |
| 2013 | 35 | 42 | 5 | 28 |
| 2014 | 58 | 95 | 6 | 7 |
| 2015 | 73 | 79 | 9 | 19 |
| 2016 | 38 | 182 | 10 | 10 |
| 2017 | 57 | 131 | 10 | 25 |
| 2018 | 77 | 208 | 5 | 39 |
| 2019 | 45 | 113 | 6 | 10 |
| Group | Analyte | CAS | All Samples n = 9789 (From Offices, 3872; Schools, 3583; Kindergartens, 727; and Healthcare Offices, 1607 Samples) | Health-Based Reference Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency in Samples 2010–2019 (>LOQ) | Md (µg/m3) | P90 (µg/m3) | P95 (µg/m3) | P99 (µg/m3) | Max (µg/m3) | RW I/II (µg/m3) | EU-LCI (µg/m3) | |||
| Alkanes LOQ = 0.3–0.5 µg/m3 | Heptane | 142-82-5 | 15% | <LOQ | 0.5 | 0.9 | 3.0 | 110 | – | 15,000 |
| Nonane | 111-84-2 | 13% | <LOQ | 0.4 | 0.7 | 1.0 | 35 | – | – | |
| Octane | 111-65-9 | 13% | <LOQ | 0.4 | 0.6 | 1.0 | 12 | – | – | |
| 2,2,4,6,6-Pentamethylheptane | 13475-82-6 | 11% | <LOQ | 0.4 | 1.0 | 6.0 | 260 | – | – | |
| Aromatic hydrocarbons LOQ = 0.5 µg/m3 | Benzene | 71-43-2 | 65% | 0.5 | 1.0 | 2.0 | 3.0 | 31 | – 1 | – |
| Ethylbenzene | 100-41-4 | 23% | <LOQ | 0.7 | 1.0 | 4.0 | 380 | 200/2000 1 | 850 | |
| 1,2,4-Trimethyl-benzene | 95-63-6 | 15% | <LOQ | 0.5 | 0.8 | 2.0 | 62 | 400/4000 | 450 | |
| Xylenes (p, m) | 108-38-3, 106-42-3 | 60% | 0.5 | 2.0 | 3.0 | 12 | 1110 | 100/800 2 | 500 1 | |
| Xylene (o) | 95-47-6 | 27% | <LOQ | 0.8 | 1.0 | 5.0 | 370 | 100/800 2 | 500 3 | |
| Toluene | 108-88-3 | 81% | 0.7 | 3.0 | 5.0 | 17 | 620 | 300/3000 2 | 2900 | |
| Terpenes LOQ = 0.5 µg/m3 | 3-Carene | 498-15-7 | 32% | <LOQ | 2.0 | 3.0 | 10 | 620 | 200/2000 | 1500 |
| Limonene | 138-86-3, 5989-27-5, 5989-54-8 | 25% | <LOQ | 1.0 | 3.0 | 14 | 1020 | 1000/10,000 | 5000 | |
| α-Pinene | 80-56-8 | 64% | 0.7 | 4.0 | 8.0 | 24 | 250 | 200/2000 | 2500 | |
| β-Pinene | 127-91-3 | 10% | <LOQ | <LOQ | 0.7 | 2.0 | 16 | 200/2000 | 1400 | |
| Alcohols LOQ = 0.5 µg/m3 except for 1,2-propanediol 0.8–1 µg/m3 | Benzyl alcohol | 100-51-6 | 19% | <LOQ | 0.9 | 2.0 | 13 | 170 | 400/4000 | 440 |
| 1-Butanol | 71-36-3 | 73% | 0.7 | 3.0 | 5.0 | 13 | 790 | 700/2000 | 3000 | |
| 2-Ethyl-1-hexanol | 104-76-7 | 64% | 0.6 | 3.0 | 7.0 | 21 | 230 | 100/1000 1 | 300 | |
| 2-Methyl-1-propanol | 78-83-1 | 21% | <LOQ | 0.8 | 1.0 | 4.0 | 180 | – | 11,000 | |
| 1,2-Propanediol | 57-55-6 | 56% | 0.8 | 7.0 | 13 | 45 | 720 | 60/600 | 2100 | |
| Phenols LOQ = 0.5 µg/m3 | Phenol | 108-95-2 | 20% | <LOQ | 0.8 | 1.0 | 4.0 | 40 | 20/200 | 70 |
| Alcohol and phenol ethers LOQ = 0.5–1 µg/m3 | 2-(2-Butoxy ethoxy)ethanol | 112-34-5 | 17% | <LOQ | 2.0 | 3.0 | 11 | 97 | 400/2000 4 | 350 |
| 2-Butoxyethanol | 111-76-2 | 19% | <LOQ | 0.8 | 2.0 | 8.0 | 140 | 100/1000 | 1600 | |
| 2-(2-Ethoxyethoxy) ethanol | 111-90-0 | 21% | <LOQ | 2.0 | 4.0 | 25 | 730 | 700/2000 4 | 350 | |
| 2-Phenoxyethanol | 122-99-6 | 22% | <LOQ | 0.9 | 2.0 | 5.0 | 110 | 30/100 | 60 | |
| 1-Methoxy-2-propanol | 107-98-2 | 14% | <LOQ | 0.7 | 1.0 | 6.0 | 370 | 1000/10,000 | 7900 | |
| Aldehydes LOQ = 0.5 µg/m3 | Benzaldehyde | 100-52-7 | 78% | 1.0 | 2.0 | 3.0 | 5.0 | 76 | 20/200 4 | – |
| Decanal | 112-31-2 | 62% | 0.7 | 2.0 | 3.0 | 5.0 | 19 | 100/2000 | 900 | |
| Hexanal | 66-25-1 | 56% | 0.6 | 3.0 | 5.0 | 14 | 310 | 100/2000 | 900 | |
| Heptanal | 111-71-7 | 17% | <LOQ | 0.6 | 0.9 | 2.0 | 20 | 100/2000 | 900 | |
| Nonanal | 124-19-6 | 80% | 1.0 | 4.0 | 6.0 | 11 | 75 | 100/2000 | 900 | |
| Octanal | 124-13-0 | 38% | <LOQ | 1.0 | 1.0 | 3.0 | 36 | 100/2000 | 900 | |
| Pentanal | 110-62-3 | 32% | <LOQ | 1.0 | 2.0 | 4.0 | 96 | 100/2000 | 800 | |
| Ketones LOQ = 0.5 µg/m3 | Acetophenone | 98-86-2 | 23% | <LOQ | 0.7 | 0.9 | 2.0 | 25 | – | 490 |
| Acids LOQ = 0.5–1 µg/m3 | Hexanoic acid | 142-62-1 | 39% | <LOQ | 5.0 | 7.0 | 14 | 330 | – | 2100 |
| Pentanoic acid | 109-52-4 | 12% | <LOQ | 0.6 | 2.0 | 4.0 | 98 | – | 2100 | |
| Propinoic acid | 79-09-4 | 19% | <LOQ | 2.0 | 3.0 | 8.0 | 110 | – | 1500 | |
| Esters LOQ = 0.3–0.5 µg/m3 | n-Butyl acetate | 123-86-4 | 14% | <LOQ | 0.6 | 1.0 | 5.0 | 150 | – | 4800 |
| 2-(2-Butoxy ethoxy)ethyl acetate | 124-17-4 | 10% | <LOQ | 0.3 | 1.0 | 6.0 | 72 | – | 850 | |
| Ethyl acetate | 141-78-6 | 14% | <LOQ | 0.7 | 2.0 | 8.0 | 450 | 600/6000 | – | |
| Texanol | 25265-77-4 | 23% | <LOQ | 1.0 | 3.0 | 15 | 320 | – | 850 | |
| TXIB | 6846-50-0 | 23% | <LOQ | 2.0 | 3.0 | 10 | 100 | – | 1300 | |
| Si-compounds LOQ = 0.5 µg/m3 | Decamethylcyclo-pentasiloxane | 541-02-6 | 72% | 1.0 | 6.0 | 11 | 33 | 680 | 100/1000 | pending |
| TVOC | 100% | 30 | 90 | 137 | 290 | 4700 | – | – | ||
| Analyte | CAS | All Samples n = 1711 (From Offices, 521; Schools, 938; Kindergartens, 68; and Healthcare Offices, 184 Samples) | Health-Based Reference Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency in Samples 2010–2019 (>LOQ) | Md (µg/m3) | P90 (µg/m3) | P95 (µg/m3) | P99 (µg/m3) | Max (µg/m3) | RW I/II (µg/m3) | EU-LCI (µg/m3) | ||
| Formaldehyde LOQ = 1 µg/m3 | 50-00-0 | 94% | 3.8 | 12 | 18 | 46 | 88 | 100/- | 100 |
| All Samples n = 9789 (From Offices, 3872; Schools, 3583; Kindergartens, 727; and Healthcare Offices, 1607 Samples) | Health-Based Reference Values | |||||
|---|---|---|---|---|---|---|
| Analyte | CAS | Frequency in Samples 2010–2019 (>LOQ) | P99 (µg/m3) | Max (µg/m3) | RW I/II (µg/m3) | EU-LCI (µg/m3) |
| Carbon tetrachloride | 56-23-5 | 1.0% | <LOQ | 12 | – | pending |
| Chloroform | 67-66-3 | 0.4% | <LOQ | 9 | – | – |
| 1,4-Dichlorobenzene | 106-46-7 | 0.03% | <LOQ | 0.7 | – | 150 |
| Trichloroethene | 79-01-6 | 0.4% | <LOQ | 10 | – 5 | – |
| Tetrachloroethene | 127-18-4 | 1.1% | 0.4 | 170 | 100/1000 | 80 |
| Styrene | 100-42-5 | 5.7% | 1.0 | 18 | 30/300 | 250 |
| Group | Analyte | CAS | Office n = 3872 | School n = 3583 | Kindergarten n = 727 | Healthcare Office n = 1607 | |
|---|---|---|---|---|---|---|---|
| Alkanes LOQ = 0.3–0.5 µg/m3 | Heptane | 142-82-5 | Frequency | 18% | 13% * | 11% * | 12% * |
| P95 (µg/m3) | 1.0 | 0.8 * | 0.7 * | 0.9 * | |||
| Nonane | 111-84-2 | Frequency | 17% | 11% * | 6% * | 11% * | |
| P95 (µg/m3) | 0.8 | 0.6 * | 0.5 * | 0.6 * | |||
| Octane | 111-65-9 | Frequency | 17% | 10% * | 12% * | 10% * | |
| P95 (µg/m3) | 0.7 | 0.5 * | 0.6 * | 0.5 * | |||
| 2,2,4,6,6-Pentamethylheptane | 13475-82-6 | Frequency | 10% | 10% | 10% | 16% * | |
| P95 (µg/m3) | 1.0 | 1.0 | 1.0 | 2.0 * | |||
| Aromatic hydrocarbons LOQ = 0.5 µg/m3 | Benzene | 71-43-2 | Frequency | 68% | 63% * | 67% | 59% * |
| P95 (µg/m3) | 2.0 | 2.0 | 2.0 | 1.6 * | |||
| Ethylbenzene | 100-41-4 | Frequency | 28% | 22% * | 18% * | 18% * | |
| P95 (µg/m3) | 1.0 | 1.0 | 0.8 * | 1.0 | |||
| 1,2,4-Trimethylbenzene | 95-63-6 | Frequency | 18% | 14% * | 9% * | 12% * | |
| P95 (µg/m3) | 0.8 | 0.8 | 0.6 * | 0.9 * | |||
| Xylenes (p, m) | 108-38-3, 106-42-3 | Frequency | 69% | 55% * | 47% * | 55% * | |
| P95 (µg/m3) | 3.0 | 4.0 * | 2.0 * | 3.0 | |||
| Xylene (o) | 95-47-6 | Frequency | 32% | 25% * | 18% * | 22% * | |
| P95 (µg/m3) | 1.0 | 2.0 * | 0.9 * | 1.0 | |||
| Toluene | 108-88-3 | Frequency | 87% | 77% * | 79% * | 79% * | |
| P95 (µg/m3) | 5.0 | 4.0 * | 5.0 | 4.0 * | |||
| Terpenes LOQ = 0.5 µg/m3 | 3-Carene | 498-15-7 | Frequency | 31% | 33% | 42% * | 28% * |
| P95 (µg/m3) | 3.0 | 4.0 * | 3.0 | 2.0 * | |||
| Limonene | 138-86-3, 5989-27-5, 5989-54-8 | Frequency | 33% | 19% * | 24% * | 20% * | |
| P95 (µg/m3) | 3.4 | 2.0 * | 3.0 * | 2.0 * | |||
| α-Pinene | 80-56-8 | Frequency | 65% | 62% * | 76% * | 60% * | |
| P95 (µg/m3) | 8.0 | 9.0 * | 7.0 * | 6.0 * | |||
| β-Pinene | 127-91-3 | Frequency | 10% | 10% | 10% | 8% * | |
| P95 (µg/m3) | 0.7 | 0.7 | 0.7 * | 0.5 * | |||
| Alcohols LOQ = 0.5 µg/m3 except for 1,2-propanediol 0.8–1 µg/m3 | Benzyl alcohol | 100-51-6 | Frequency | 19% | 20% | 18% | 15% * |
| P95 (µg/m3) | 2.0 | 3.0 * | 1.0 * | 1.0 * | |||
| 1-Butanol | 71-36-3 | Frequency | 77% | 71% * | 75% | 68% * | |
| P95 (µg/m3) | 4.0 | 5.0 * | 5.6 * | 5.0 * | |||
| 2-Ethyl-1-hexanol | 104-76-7 | Frequency | 65% | 62% * | 67% | 68% * | |
| P95 (µg/m3) | 7.0 | 6.0 * | 6.0 * | 8.0 * | |||
| 2-Methyl-1-propanol | 78-83-1 | Frequency | 24% | 20% * | 20% * | 18% * | |
| P95 (µg/m3) | 1.0 | 1.0 | 2.0 * | 1.0 | |||
| 1,2-Propanediol | 57-55-6 | Frequency | 60% | 51% * | 63% | 53% * | |
| P95 (µg/m3) | 13 | 12 * | 14 * | 13 | |||
| Phenols LOQ = 0.5 µg/m3 | Phenol | 108-95-2 | Frequency | 22% | 18% * | 17% * | 21% |
| P95 (µg/m3) | 1.0 | 1.0 | 1.0 | 1.0 | |||
| Alcohol and phenol ethers LOQ = 0.5–1 µg/m3 | 2-(2-Butoxy ethoxy)ethanol | 112-34-5 | Frequency | 18% | 18% | 16% | 14% * |
| P95 (µg/m3) | 3.0 | 4.0 * | 3.0 | 3.0 | |||
| 2-Butoxyethanol | 111-76-2 | Frequency | 20% | 19% | 19% | 12% * | |
| P95 (µg/m3) | 2.0 | 2.0 | 2.0 | 1.0 * | |||
| 2-(2-Ethoxyethoxy)ethanol | 111-90-0 | Frequency | 21% | 24% * | 23% | 10% * | |
| P95 (µg/m3) | 4.0 | 7.0 * | 5.0 * | 1.0 * | |||
| 2-Phenoxyethanol | 122-99-6 | Frequency | 22% | 21% | 24% | 21% | |
| P95 (µg/m3) | 1.0 | 2.0 * | 2.0 * | 2.0 * | |||
| 1-Methoxy-2-propanol | 107-98-2 | Frequency | 14% | 17% * | 15% | 9% * | |
| P95 (µg/m3) | 1.0 | 2.0 * | 2.0 * | 1.0 | |||
| Aldehydes LOQ = 0.5 µg/m3 | Benzaldehyde | 100-52-7 | Frequency | 80% | 77% * | 78% | 77% * |
| P95 (µg/m3) | 3.0 | 3.0 | 3.0 | 2.0 * | |||
| Decanal | 112-31-2 | Frequency | 60% | 61% | 71% * | 66% * | |
| P95 (µg/m3) | 3.0 | 3.0 | 4.0 * | 4.0 * | |||
| Hexanal | 66-25-1 | Frequency | 54% | 55% | 79% * | 50% * | |
| P95 (µg/m3) | 5.0 | 6.0 * | 8.0 * | 4.0 * | |||
| Heptanal | 111-71-7 | Frequency | 16% | 17% | 31% * | 13% * | |
| P95 (µg/m3) | 0.8 | 0.9 * | 1.0 * | 0.7 * | |||
| Nonanal | 124-19-6 | Frequency | 77% | 80% * | 93% * | 81% * | |
| P95 (µg/m3) | 5.0 | 6.0 * | 11 * | 5.0 | |||
| Octanal | 124-13-0 | Frequency | 39% | 36% * | 49% * | 34% * | |
| P95 (µg/m3) | 1.0 | 1.0 | 2.0 * | 1.0 | |||
| Pentanal | 110-62-3 | Frequency | 33% | 30% * | 44% * | 25% * | |
| P95 (µg/m3) | 2.0 | 2.0 | 3.0 * | 1.0 * | |||
| Ketones LOQ = 0.5 µg/m3 | Acetophenone | 98-86-2 | Frequency | 21% | 25% * | 23% | 24% * |
| P95 (µg/m3) | 0.8 | 0.9 * | 0.8 * | 1.0 * | |||
| Acids LOQ = 0.5–1 µg/m3 | Hexanoic acid | 142-62-1 | Frequency | 44% | 38% * | 43% | 31% * |
| P95 (µg/m3) | 8.0 | 7.0 * | 8.0 | 5.0 * | |||
| Pentanoic acid | 109-52-4 | Frequency | 15% | 11% * | 13% | 8% * | |
| P95 (µg/m3) | 2.0 | 1.0 * | 2.0 | 0.8 * | |||
| Propinoic acid | 79-09-4 | Frequency | 23% | 18% * | 20% | 12% * | |
| P95 (µg/m3) | 4.0 | 3.0 * | 4.0 | 2.0 * | |||
| Esters LOQ = 0.3–0.5 µg/m3 | n-Butyl acetate | 123-86-4 | Frequency | 15% | 13% * | 20% * | 11% * |
| P95 (µg/m3) | 1.0 | 1.0 | 2.0 * | 0.9 * | |||
| 2-(2-Butoxy ethoxy)ethyl acetate | 124-17-4 | Frequency | 12% | 9% * | 11% | 8% * | |
| P95 (µg/m3) | 1.0 | 1.0 | 1.0 | 0.8 * | |||
| Ethyl acetate | 141-78-6 | Frequency | 16% | 13% * | 14% | 14% | |
| P95 (µg/m3) | 2.0 | 1.0 * | 2.0 | 2.0 | |||
| Texanol | 25265-77-4 | Frequency | 22% | 26% * | 24% | 17% * | |
| P95 (µg/m3) | 3.0 | 5.0 * | 3.0 | 2.0 * | |||
| TXIB | 6846-50-0 | Frequency | 21% | 20% | 57% * | 19% | |
| P95 (µg/m3) | 3.0 | 2.0 * | 6.0 * | 3.0 | |||
| Si-compounds LOQ = 0.5 µg/m3 | Decamethylcyclo-pentasiloxane | 541-02-6 | Frequency | 76% | 65% * | 70% * | 76% |
| P95 (µg/m3) | 13 | 9.0 * | 12 * | 12 * | |||
| TVOC | Frequency | 100% | 100% | 100% | 100% | ||
| Md (µg/m3) | 30 | 20 * | 30 | 23 * | |||
| P90 (µg/m3) | 90 | 90 | 100 * | 90 | |||
| P95 (µg/m3) | 140 | 130 * | 136 * | 130 * |
| Analyte | CAS | Office n = 521 | School n = 938 | Kindergarten n = 68 | Healthcare Office n = 184 | |
|---|---|---|---|---|---|---|
| Formaldehyde LOQ = 1 µg/m3 | 50-00-0 | Frequency | 95% | 93% | 96% | 91% |
| Md (µg/m3) | 5 | 3 * | 4 | 4 | ||
| P90 (µg/m3) | 18 | 10 * | 14 * | 13 * | ||
| P95 (µg/m3) | 25 | 14 * | 20 * | 17 * |
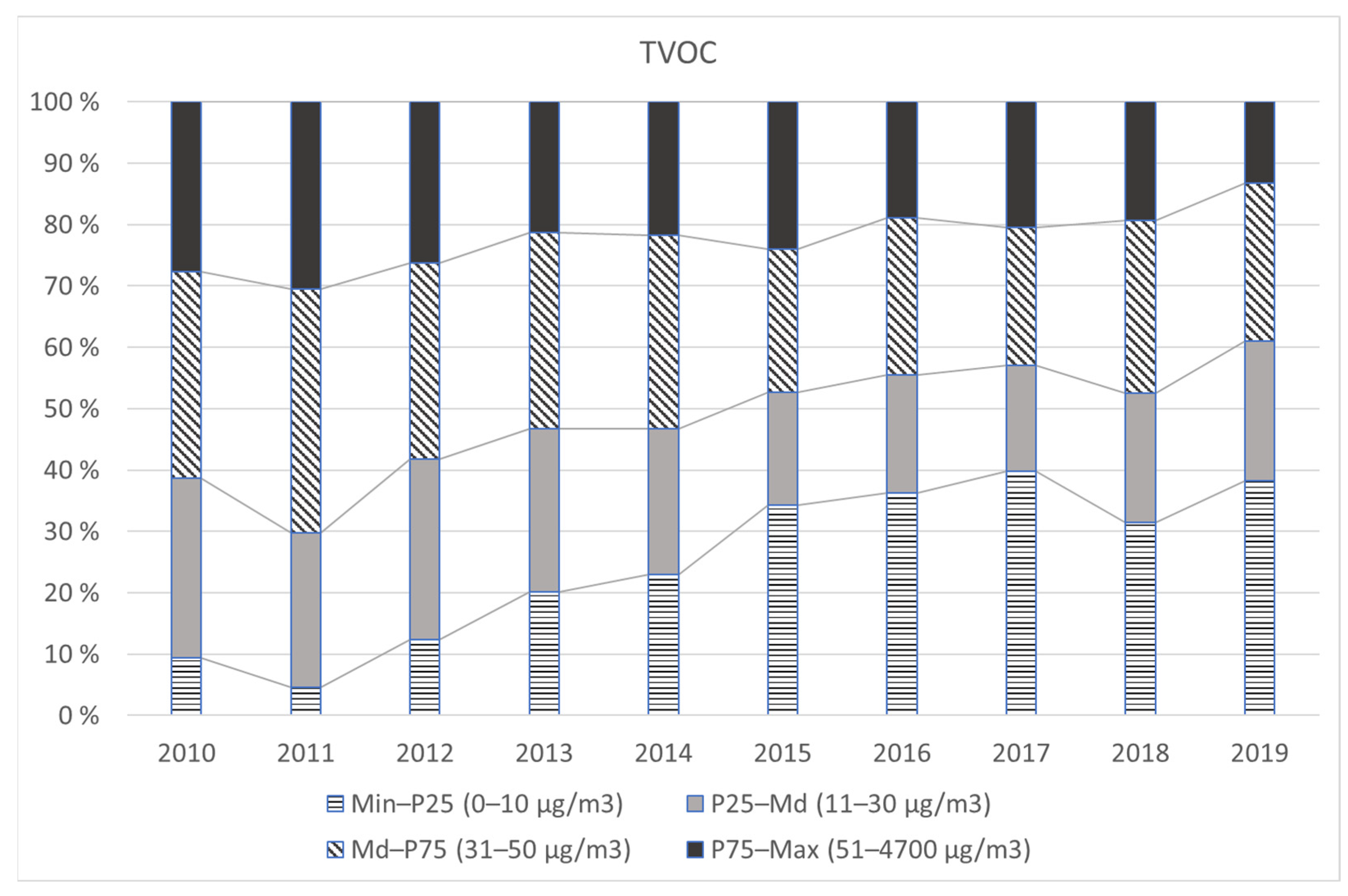
| Group | Analyte CAS | Trend (freq.) Z | Trend (conc.) Z | Year | 2010 n = 807 | 2011 n = 971 | 2012 n = 975 | 2013 n = 977 | 2014 n = 995 | 2015 n = 620 | 2016 n = 1089 | 2017 n = 1153 | 2018 n = 1129 | 2019 n = 1073 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkanes | Heptane 142-82-5 |  |  | Frequency | 20% | 20% | 22% | 16% | 12% | 13% | 12% | 9% | 11% | 13% |
| −10 | −10 | P95 (µg/m3) | 1.0 | 1.0 | 2.0 | 1.0 | 0.8 | 0.8 | 0.6 | 0.5 | 0.6 | 0.7 | ||
| Nonane 111-84-2 |  |  | Frequency | 24% | 25% | 23% | 20% | 15% | 9% | 5% | 3% | 6% | 5% | |
| −23 | −23 | P95 (µg/m3) | 0.8 | 0.8 | 0.9 | 1.0 | 0.8 | 0.6 | 0.4 | <LOQ | 0.3 | <LOQ | ||
| Octane 111-65-9 |  |  | Frequency | 24% | 32% | 24% | 15% | 14% | 5% | 4% | 3% | 5% | 4% | |
| −26 | −26 | P95 (µg/m3) | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 | 0.4 | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 2,2,4,6,6-Pentamethylhep-tane 13475-82-6 |  |  | Frequency | 13% | 21% | 20% | 19% | 9% | 12% | 8% | 5% | 6% | 5% | |
| −16 | −16 | P95 (µg/m3) | 1.0 | 4.0 | 2.0 | 2.0 | 0.7 | 0.7 | 0.6 | <LOQ | 0.4 | 0.4 | ||
| Aromatic hydrocarbons | Benzene 71-43-2 |  |  | Frequency | 98% | 89% | 73% | 70% | 59% | 52% | 61% | 49% | 56% | 49% |
| −32 | −33 | P95 (µg/m3) | 2.0 | 2.0 | 2.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Ethylbenzene 100-41-4 |  |  | Frequency | 47% | 36% | 22% | 21% | 17% | 24% | 20% | 14% | 20% | 18% | |
| −15 | −15 | P95 (µg/m3) | 1.6 | 1.0 | 2.0 | 2.0 | 0.9 | 3.0 | 1.0 | 0.7 | 1.0 | 0.8 | ||
| 1,2,4-Trimethylbenzene 95-63-6 |  |  | Frequency | 31% | 23% | 13% | 15% | 9% | 14% | 17% | 9% | 11% | 12% | |
| −11 | −11 | P95 (µg/m3) | 1.0 | 0.9 | 0.7 | 1.0 | 0.6 | 1.0 | 1.0 | 0.6 | 0.6 | 0.5 | ||
| Xylenes (p, m) 106-42-3 108-38-3 |  |  | Frequency | 84% | 73% | 66% | 54% | 53% | 60% | 58% | 49% | 60% | 52% | |
| −15 | −16 | P95 (µg/m3) | 4.0 | 4.0 | 4.0 | 4.0 | 3.0 | 9.0 | 3.0 | 2.0 | 3.0 | 2.0 | ||
| Xylene (o) 95-47-6 |  |  | Frequency | 48% | 38% | 25% | 24% | 21% | 26% | 26% | 17% | 26% | 22% | |
| −12 | −11 | P95 (µg/m3) | 2.0 | 1.0 | 1.0 | 2.0 | 1.0 | 4.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Toluene 108-88-3 |  |  | Frequency | 98% | 90% | 89% | 83% | 77% | 76% | 78% | 71% | 82% | 75% | |
| −12 | −19 | P95 (µg/m3) | 6.0 | 4.4 | 6.0 | 7.0 | 4.0 | 5.0 | 4.0 | 3.0 | 3.0 | 4.0 | ||
| Terpenes | 3-Carene 498-15-7 |  |  | Frequency | 26% | 24% | 23% | 29% | 26% | 37% | 44% | 39% | 39% | 36% |
| +13 | +12 | P95 (µg/m3) | 2.0 | 2.0 | 2.0 | 4.0 | 3.0 | 4.0 | 4.0 | 3.0 | 4.0 | 3.0 | ||
| Limonene 138-86-3, 5989-27-5, 5989-54-8 | - | - | Frequency | 25% | 29% | 25% | 22% | 22% | 26% | 29% | 25% | 29% | 25% | |
| P95 (µg/m3) | 5.0 | 6.0 | 3.0 | 2.0 | 3.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | ||||
| α-Pinene 80-56-8 |  | - | Frequency | 65% | 63% | 57% | 61% | 61% | 65% | 67% | 71% | 68% | 64% | |
| +4 | P95 (µg/m3) | 7.0 | 7.0 | 7.0 | 10 | 8.0 | 9.0 | 8.0 | 9.0 | 9.0 | 6.0 | |||
| β-Pinene 127-91-3 |  |  | Frequency | 7% | 5% | 7% | 11% | 8% | 12% | 14% | 11% | 13% | 12% | |
| +8 | +8 | P95 (µg/m3) | 0.7 | 0.4 | 0.5 | 0.7 | 0.7 | 0.7 | 0.7 | 0.6 | 0.8 | 0.7 | ||
| Alcohols | Benzyl alcohol 100-51-6 |  |  | Frequency | 17% | 11% | 16% | 19% | 23% | 22% | 24% | 20% | 19% | 19% |
| +5 | +4 | P95 (µg/m3) | 3.0 | 1.0 | 1.2 | 2.0 | 3.0 | 5.0 | 4.0 | 2.3 | 1.0 | 2.0 | ||
| 1-Butanol 71-36-3 |  | - | Frequency | 81% | 78% | 71% | 55% | 72% | 73% | 70% | 74% | 80% | 80% | |
| +5 | P95 (µg/m3) | 5.0 | 5.0 | 4.0 | 5.0 | 5.0 | 5.0 | 5.0 | 6.0 | 4.0 | 3.0 | |||
| 2-Ethyl-1-hexanol 104-76-7 |  |  | Frequency | 80% | 67% | 63% | 60% | 74% | 59% | 64% | 60% | 60% | 59% | |
| −9 | −15 | P95 (µg/m3) | 5.0 | 9.0 | 9.0 | 6.0 | 4.0 | 5.0 | 4.0 | 7.0 | 4.0 | 13 | ||
| 2-Methyl-1-propanol 78-83-1 |  | - | Frequency | 26% | 24% | 16% | 12% | 14% | 26% | 29% | 22% | 24% | 24% | |
| +4 | P95 (µg/m3) | 1.6 | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 2.0 | 1.0 | 2.0 | 1.0 | |||
| 1,2-Propanediol 57-55-6 |  | - | Frequency | 50% | 50% | 55% | 55% | 59% | 64% | 52% | 52% | 57% | 66% | |
| +6 | P95 (µg/m3) | 19 | 12 | 12 | 13 | 15 | 12 | 14 | 9.0 | 17 | 9.0 | |||
| Phenols | Phenol 108-95-2 |  |  | Frequency | 18% | 11% | 11% | 10% | 12% | 11% | 34% | 27% | 26% | 30% |
| +16 | +15 | P95 (µg/m3) | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 | 1.0 | 2.0 | 2.0 | 0.9 | 1.0 | ||
| Alcohol and phenol ethers | 2-(2-Butoxy ethoxy)ethanol 112-34-5 | - | - | Frequency | 14% | 18% | 17% | 18% | 21% | 20% | 19% | 12% | 17% | 17% |
| P95 (µg/m3) | 4.6 | 4.0 | 3.0 | 3.0 | 4.0 | 4.0 | 4.0 | 3.3 | 3.0 | 1.0 | ||||
| 2-Butoxyethanol 111-76-2 |  |  | Frequency | 8% | 12% | 13% | 14% | 16% | 27% | 26% | 29% | 23% | 17% | |
| +13 | +12 | P95 (µg/m3) | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 | 9.0 | 2.0 | 2.0 | 2.0 | 1.0 | ||
| 2-(2-Ethoxy ethoxy)ethanol 111-90-0 |  |  | Frequency | 16% | 22% | 24% | 27% | 25% | 23% | 20% | 13% | 17% | 17% | |
| −5 | −6 | P95 (µg/m3) | 6.0 | 4.4 | 7.0 | 9.0 | 5.0 | 5.0 | 5.0 | 2.0 | 2.0 | 2.0 | ||
| 2-Phenoxyethanol 122-99-6 | - |  | Frequency | 13% | 22% | 26% | 29% | 22% | 27% | 25% | 17% | 19% | 18% | |
| −5 | P95 (µg/m3) | 2.0 | 2.0 | 2.0 | 2.1 | 2.0 | 2.0 | 1.0 | 0.9 | 0.9 | 0.9 | |||
| 1-Metoxy-2-propanol 107-98-2 | - | - | Frequency | 13% | 15% | 13% | 16% | 15% | 16% | 16% | 13% | 15% | 13% | |
| P95 (µg/m3) | 2.0 | 2.0 | 1.0 | 2.0 | 1.2 | 3.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Aldehydes | Benzaldehyde 100-52-7 |  |  | Frequency | 67% | 58% | 65% | 76% | 77% | 86% | 87% | 87% | 85% | 90% |
| +23 | −9 | P95 (µg/m3) | 4.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 2.0 | 2.0 | ||
| Decanal 112-31-2 |  | - | Frequency | 47% | 48% | 52% | 53% | 60% | 72% | 71% | 64% | 75% | 75% | |
| +20 | P95 (µg/m3) | 4.0 | 4.0 | 4.0 | 3.0 | 4.0 | 3.0 | 2.0 | 2.0 | 2.0 | 3.0 | |||
| Hexanal 66-25-1 |  |  | Frequency | 49% | 61% | 50% | 54% | 52% | 43% | 55% | 54% | 66% | 68% | |
| +8 | +7 | P95 (µg/m3) | 4.0 | 4.0 | 4.0 | 5.0 | 6.2 | 6.0 | 6.0 | 7.0 | 6.0 | 5.0 | ||
| Heptanal 111-71-7 |  |  | Frequency | 12% | 20% | 13% | 14% | 15% | 12% | 10% | 13% | 25% | 31% | |
| +9 | +8 | P95 (µg/m3) | 0.9 | 1.0 | 0.8 | 0.9 | 0.9 | 0.8 | 0.7 | 0.8 | 0.8 | 0.8 | ||
| Nonanal 124-19-6 |  | - | Frequency | 60% | 70% | 70% | 73% | 75% | 87% | 90% | 89% | 90% | 88% | |
| +23 | P95 (µg/m3) | 5.0 | 6.0 | 7.0 | 5.1 | 7.0 | 6.0 | 5.0 | 6.0 | 5.0 | 5.0 | |||
| Octanal 124-13-0 |  | - | Frequency | 23% | 50% | 27% | 28% | 38% | 35% | 37% | 37% | 50% | 45% | |
| +10 | P95 (µg/m3) | 1.0 | 2.0 | 2.0 | 1.0 | 2.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||
| Pentanal 110-62-3 |  |  | Frequency | 23% | 27% | 18% | 20% | 16% | 39% | 40% | 38% | 49% | 44% | |
| +20 | +16 | P95 (µg/m3) | 2.0 | 2.0 | 1.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | ||
| Ketones | Acetophenone 98-86-2 |  |  | Frequency | 2% | 8% | 18% | 31% | 28% | 15% | 26% | 25% | 36% | 31% |
| +20 | +18 | P95 (µg/m3) | <LOQ | 0.7 | 1.0 | 1.0 | 1.0 | 0.8 | 0.8 | 0.8 | 0.9 | 0.8 | ||
| Acids | Hexanoic acid 142-62-1 |  |  | Frequency | 21% | 31% | 22% | 23% | 28% | 47% | 51% | 56% | 51% | 55% |
| +27 | +19 | P95 (µg/m3) | 7.0 | 8.0 | 7.0 | 9.0 | 11 | 7.0 | 6.0 | 6.3 | 5.0 | 5.0 | ||
| Pentanoic acid 109-52-4 |  |  | Frequency | 8% | 7% | 3% | 11% | 10% | 17% | 17% | 20% | 16% | 14% | |
| +12 | +11 | P95 (µg/m3) | 3.0 | 2.0 | <LOQ | 3.0 | 3.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Propionic acid 79-09-4 |  |  | Frequency | 10% | 12% | 6% | 12% | 6% | 20% | 21% | 22% | 28% | 46% | |
| +24 | +22 | P95 (µg/m3) | 4.0 | 4.0 | 2.0 | 4.0 | 2.2 | 3.0 | 4.0 | 3.0 | 3.0 | 2.0 | ||
| Esters | n-Butyl acetate 123-86-4 | - | - | Frequency | 15% | 15% | 12% | 13% | 16% | 18% | 12% | 12% | 17% | 16% |
| P95 (µg/m3) | 2.0 | 0.9 | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 | 1.0 | 1.0 | 0.9 | ||||
| 2-(2-Butoxy ethoxy)ethyl acetate 124-17-4 |  |  | Frequency | 7% | 10% | 12% | 10% | 13% | 12% | 13% | 9% | 6% | 6% | |
| −4 | −5 | P95 (µg/m3) | 1.0 | 2.0 | 1.0 | 1.1 | 2.0 | 0.8 | 1.0 | 0.9 | 0.5 | 0.5 | ||
| Ethyl acetate 141-78-6 |  |  | Frequency | 12% | 15% | 15% | 9% | 8% | 16% | 13% | 14% | 18% | 23% | |
| +6 | +6 | P95 (µg/m3) | 2.0 | 2.0 | 2.0 | 1.0 | 1.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | ||
| Texanol 25265-77-4 |  |  | Frequency | 11% | 17% | 19% | 22% | 28% | 28% | 25% | 27% | 31% | 20% | |
| +9 | +8 | P95 (µg/m3) | 2.0 | 3.0 | 3.0 | 3.0 | 4.0 | 4.0 | 3.0 | 3.3 | 8.0 | 2.0 | ||
| TXIB 6846-50-0 |  | - | Frequency | 16% | 19% | 21% | 24% | 27% | 23% | 31% | 20% | 25% | 22% | |
| +4 | P95 (µg/m3) | 3.0 | 4.0 | 2.0 | 3.0 | 5.0 | 4.0 | 3.0 | 2.0 | 2.0 | 2.0 | |||
| Si-compounds | Decamethylcyclo-pentasiloxane 541-02-6 |  |  | Frequency | 57% | 69% | 75% | 70% | 70% | 74% | 73% | 73% | 76% | 74% |
| +7 | −5 | P95 (µg/m3) | 12 | 12 | 11 | 12 | 16 | 16 | 12 | 10 | 9.0 | 7.0 | ||
| TVOC | - |  | Frequency | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| −20 | Md (µg/m3) | 30 | 40 | 30 | 30 | 30 | 28 | 20 | 20 | 20 | 20 | |||
| P90 (µg/m3) | 100 | 100 | 100 | 90 | 100 | 130 | 80 | 80 | 80 | 70 | ||||
| P95 (µg/m3) | 150 | 140 | 150 | 150 | 140 | 220 | 125 | 110 | 110 | 100 |
| Analyte CAS | Trend (freq.) | Trend (conc.) | Year | 2010 n = 44 | 2011 n = 105 | 2012 n = 140 | 2013 n = 110 | 2014 n = 166 | 2015 n = 180 | 2016 n = 240 | 2017 n = 223 | 2018 n = 329 | 2019 n = 174 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formaldehyde 50-00-0 | - |  −6 | Frequency | 100% | 91% | 96% | 92% | 89% | 93% | 94% | 91% | 95% | 99% |
| Md (µg/m3) | 9 | 10 | 5 | 4 | 3 | 4 | 4 | 3 | 4 | 3 | |||
| P90 (µg/m3) | 25 | 26 | 15 | 14 | 11 | 11 | 10 | 10 | 12 | 9 | |||
| P95 (µg/m3) | 43 | 37 | 19 | 23 | 20 | 15 | 14 | 17 | 16 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallenius, K.; Hovi, H.; Remes, J.; Mahiout, S.; Liukkonen, T. Volatile Organic Compounds in Finnish Office Environments in 2010–2019 and Their Relevance to Adverse Health Effects. Int. J. Environ. Res. Public Health 2022, 19, 4411. https://doi.org/10.3390/ijerph19074411
Wallenius K, Hovi H, Remes J, Mahiout S, Liukkonen T. Volatile Organic Compounds in Finnish Office Environments in 2010–2019 and Their Relevance to Adverse Health Effects. International Journal of Environmental Research and Public Health. 2022; 19(7):4411. https://doi.org/10.3390/ijerph19074411
Chicago/Turabian StyleWallenius, Kaisa, Hanna Hovi, Jouko Remes, Selma Mahiout, and Tuula Liukkonen. 2022. "Volatile Organic Compounds in Finnish Office Environments in 2010–2019 and Their Relevance to Adverse Health Effects" International Journal of Environmental Research and Public Health 19, no. 7: 4411. https://doi.org/10.3390/ijerph19074411
APA StyleWallenius, K., Hovi, H., Remes, J., Mahiout, S., & Liukkonen, T. (2022). Volatile Organic Compounds in Finnish Office Environments in 2010–2019 and Their Relevance to Adverse Health Effects. International Journal of Environmental Research and Public Health, 19(7), 4411. https://doi.org/10.3390/ijerph19074411






