Comparison of the Toxicological Effects of Pesticides in Non-Tumorigenic MCF-12A and Tumorigenic MCF-7 Human Breast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Cell Viability and Proliferation
2.4. Apoptosis-Necrosis Assay
2.5. ATP Levels Assessment
2.6. Reactive Oxygen Species Assay
2.7. Cell Treatment
2.8. Estradiol ELISA Assay
2.9. Real-Time PCR
2.10. Statistical Analysis
3. Results
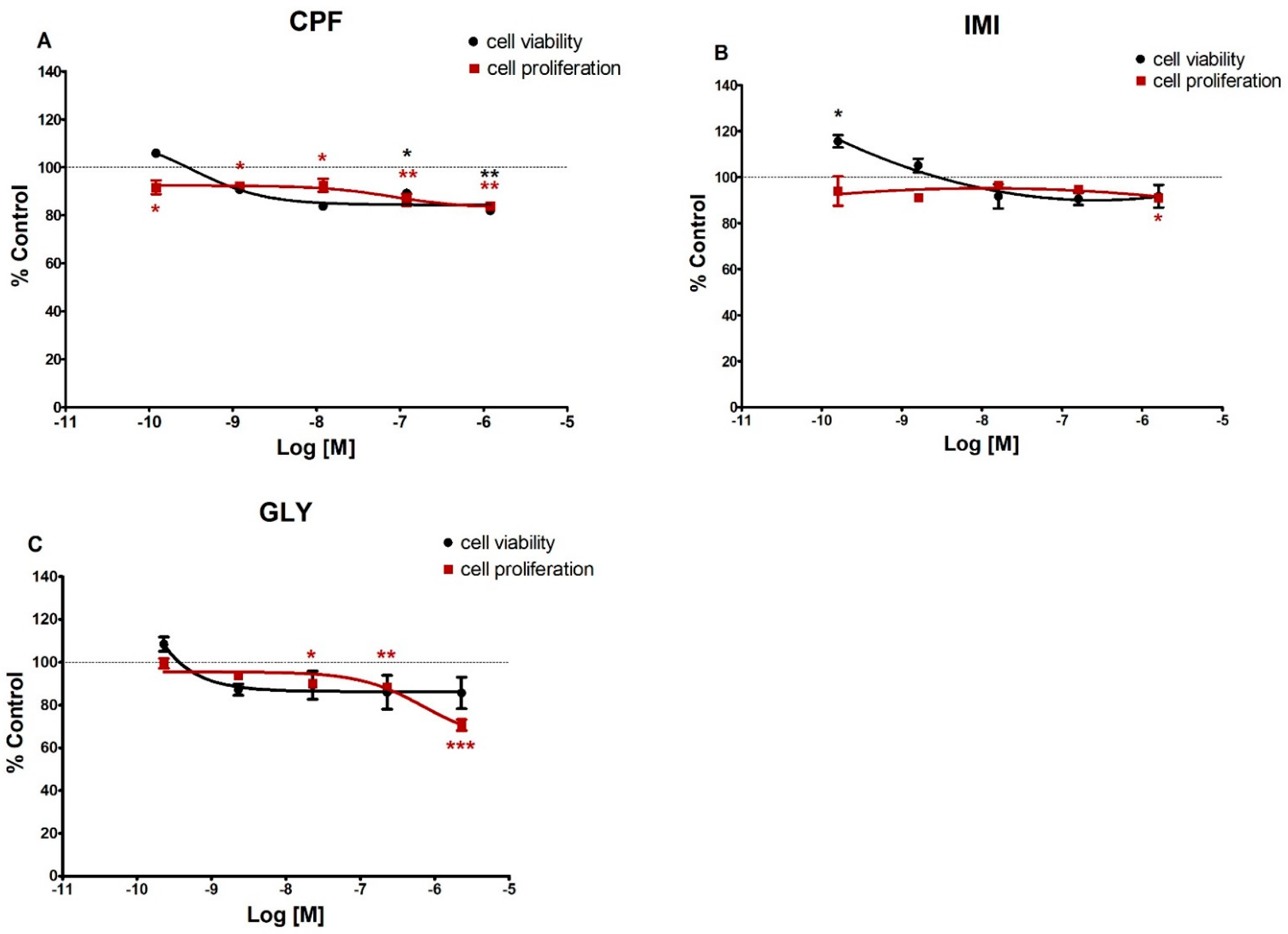
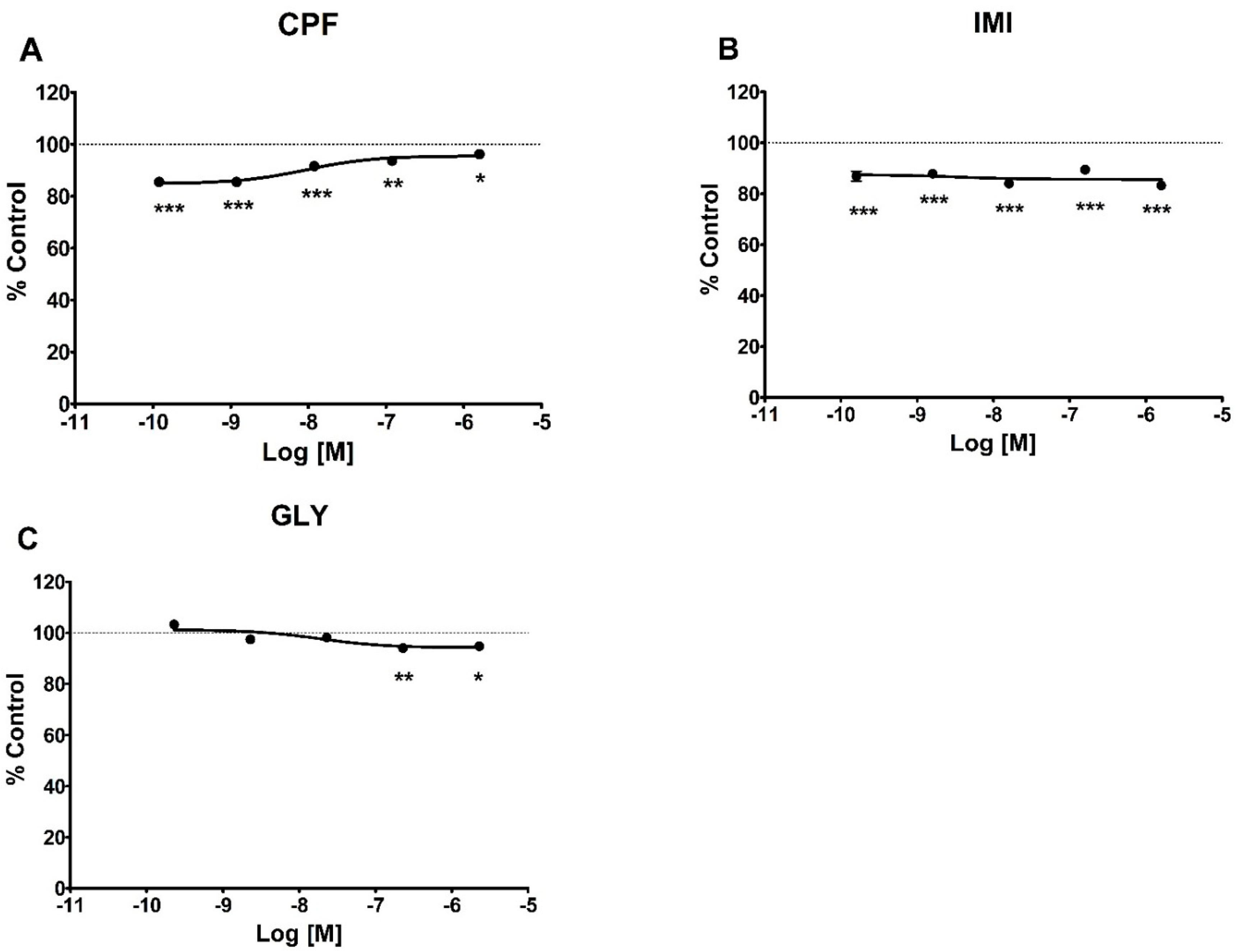
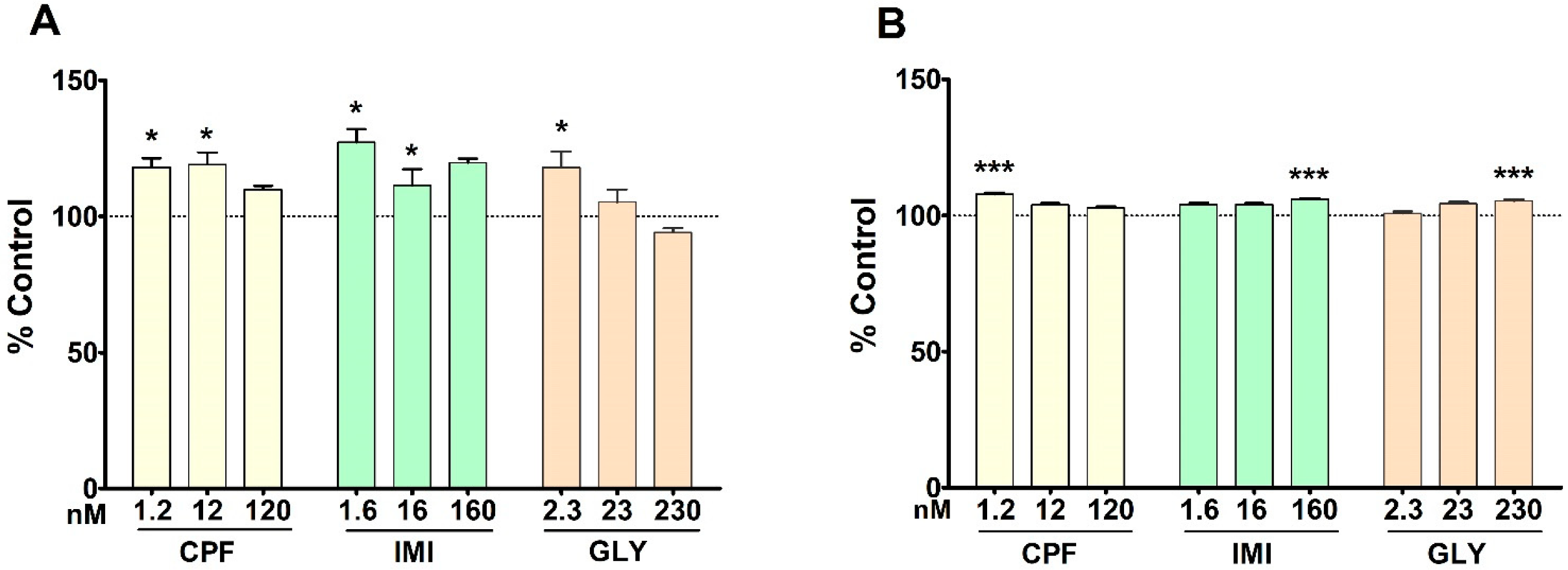
3.1. Cell Viability and Cell Proliferation
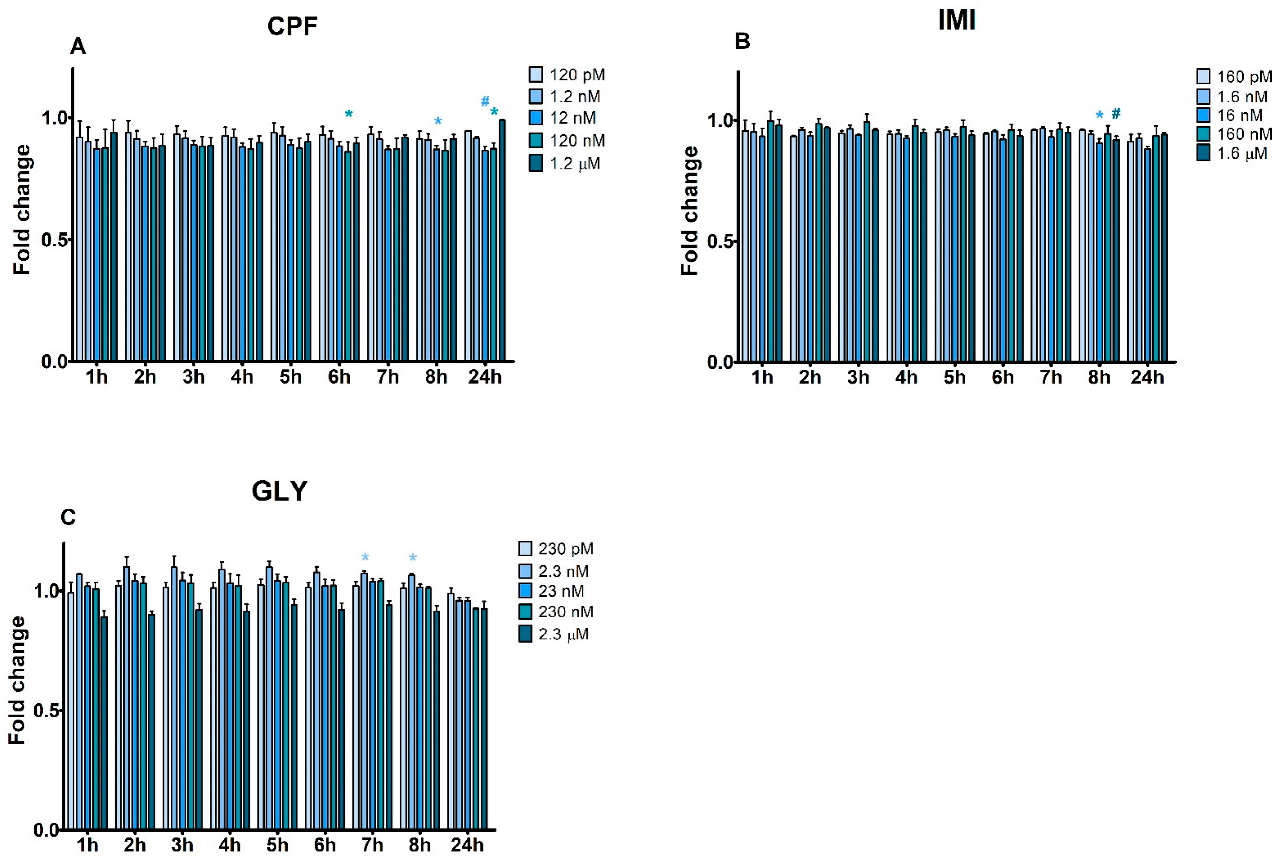
3.2. Apoptosis and Necrosis Time Course
3.3. ATP Levels
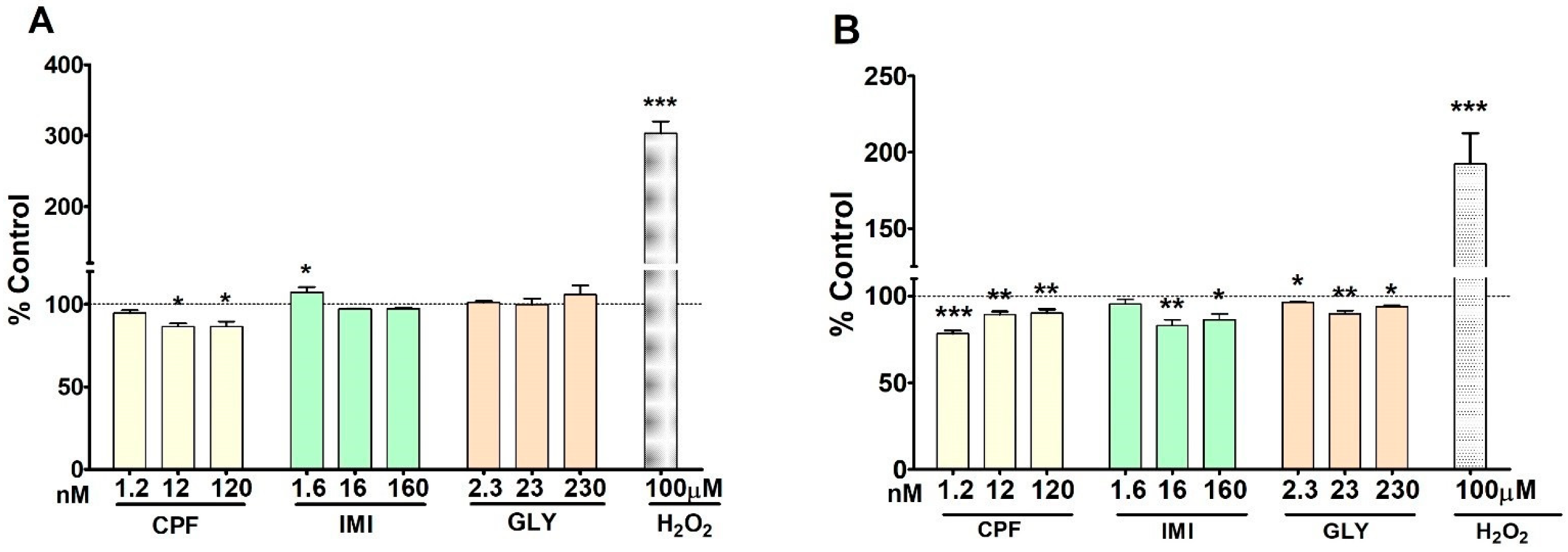
3.4. Reactive Oxygen Species
3.5. Estradiol Secretion
3.6. Nuclear Receptors’ Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aktar, M.W.; Chowdhury, A. Impact of Pesticides use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Encarnação, T.; Pais, A.A.; Campos, M.G.; Burrows, H.D. Endocrine Disrupting Chemicals: Impact on Human Health, Wildlife and the Environment. Sci. Prog. 2019, 102, 3–42. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides; European Parliament: Strasbourg, France, 2009; pp. 71–86. [Google Scholar]
- European Chemical Agency (ECHA) and European Food Safety Authority (EFSA) with the technical support of the Joint Research Centre (JRC); Andersson, N.; Arena, M.; Auteri, D.; Barmaz, S.; Grignard, E.; Kienzler, A.; Lepper, P.; Lostia, A.M.; Munn, S.; et al. Guidance for the Identification of Endocrine Disruptors in the Context of Regulations (EU) no 528/2012 and (EC) no 1107/2009. EFSA J. 2018, 16, e05311. [Google Scholar] [PubMed]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Ugarte, R.; Antoniou, M.N. Evaluation of Estrogen Receptor Alpha Activation by Glyphosate-Based Herbicide Constituents. Food Chem. Toxicol. 2017, 108, 30–42. [Google Scholar] [CrossRef]
- Rossetti, M.F.; Stoker, C.; Ramos, J.G. Agrochemicals and Neurogenesis. Mol. Cell. Endocrinol. 2020, 510, 110820. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An Update of Human Exposure and Toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Bianchi, S.; Nottola, S.A.; Torge, D.; Palmerini, M.G.; Necozione, S.; Macchiarelli, G. Association between Female Reproductive Health and Mancozeb: Systematic Review of Experimental Models. Int. J. Environ. Res. Public Health 2020, 17, 2580. [Google Scholar] [CrossRef]
- Greenspan, L.C.; Lee, M.M. Endocrine Disrupters and Pubertal Timing. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 49–54. [Google Scholar] [CrossRef]
- Farello, G.; Altieri, C.; Cutini, M.; Pozzobon, G.; Verrotti, A. Review of the Literature on Current Changes in the Timing of Pubertal Development and the Incomplete Forms of Early Puberty. Front. Pediatr. 2019, 7, 147. [Google Scholar] [CrossRef]
- Macias, H.; Hinck, L. Mammary Gland Development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.R.; Walters, K.A.; Desai, R.; Zhou, H.; Handelsman, D.J.; Simanainen, U. Androgen Receptor Inactivation Resulted in Acceleration in Pubertal Mammary Gland Growth, Upregulation of ERα Expression, and Wnt/Β-Catenin Signaling in Female Mice. Endocrinology 2014, 155, 4951–4963. [Google Scholar] [CrossRef] [PubMed]
- Le Provost, F.; Riedlinger, G.; Hee Yim, S.; Benedict, J.; Gonzalez, F.J.; Flaws, J.; Hennighausen, L. The Aryl Hydrocarbon Receptor (AhR) and its Nuclear Translocator (Arnt) are Dispensable for Normal Mammary Gland Development but are Required for Fertility. Genesis 2002, 32, 231–239. [Google Scholar] [CrossRef]
- Ohtake, F.; Takeyama, K.; Matsumoto, T.; Kitagawa, H.; Yamamoto, Y.; Nohara, K.; Tohyama, C.; Krust, A.; Mimura, J.; Chambon, P. Modulation of Oestrogen Receptor Signalling by Association with the Activated Dioxin Receptor. Nature 2003, 423, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Grünfeld, H.; Bonefeld-Jorgensen, E. Effect of in Vitro Estrogenic Pesticides on Human Oestrogen Receptor A and Β mRNA Levels. Toxicol. Lett. 2004, 151, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Venerosi, A.; Tait, S.; Stecca, L.; Chiarotti, F.; De Felice, A.; Cometa, M.F.; Volpe, M.T.; Calamandrei, G.; Ricceri, L. Effects of Maternal Chlorpyrifos Diet on Social Investigation and Brain Neuroendocrine Markers in the Offspring–a Mouse Study. Environ. Health 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, G.; Chatterjee, S.; Dabral, S.; Nanguneri, S.R.; Divya, G.; Roy, P. Anti-Androgenic Endocrine Disrupting Activities of Chlorpyrifos and Piperophos. J. Steroid Biochem. Mol. Biol. 2010, 120, 22–29. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, S.; Tassinari, R.; Maranghi, F.; Eusepi, A.; Di Virgilio, A.; Chiarotti, F.; Ricceri, L.; Pesciolini, A.V.; Gilardi, E.; Moracci, G. Developmental Exposure to Chlorpyrifos Induces Alterations in Thyroid and Thyroid Hormone Levels without Other Toxicity Signs in Cd1 Mice. Toxicol. Sci. 2009, 108, 311–319. [Google Scholar] [CrossRef]
- Takeuchi, S.; Iida, M.; Yabushita, H.; Matsuda, T.; Kojima, H. In Vitro Screening for Aryl Hydrocarbon Receptor Agonistic Activity in 200 Pesticides using a Highly Sensitive Reporter Cell Line, DR-EcoScreen Cells, and in Vivo Mouse Liver Cytochrome P450-1A Induction by Propanil, Diuron and Linuron. Chemosphere 2008, 74, 155–165. [Google Scholar] [CrossRef]
- Ventura, C.; Núñez, M.; Miret, N.; Lamas, D.M.; Randi, A.; Venturino, A.; Rivera, E.; Cocca, C. Differential Mechanisms of Action are Involved in Chlorpyrifos Effects in Estrogen-Dependent Or-Independent Breast Cancer Cells Exposed to Low or High Concentrations of the Pesticide. Toxicol. Lett. 2012, 213, 184–193. [Google Scholar] [CrossRef]
- Moyano, P.; García, J.; García, J.M.; Pelayo, A.; Muñoz-Calero, P.; Frejo, M.T.; Anadon, M.J.; Lobo, M.; Del Pino, J. Chlorpyrifos-Induced Cell Proliferation in Human Breast Cancer Cell Lines Differentially Mediated by Estrogen and Aryl Hydrocarbon Receptors and KIAA1363 Enzyme After 24 H and 14 Days Exposure. Chemosphere 2020, 251, 126426. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Nieto, M.R.R.; Bourguignon, N.; Lux-Lantos, V.; Rodriguez, H.; Cao, G.; Randi, A.; Cocca, C.; Núñez, M. Pesticide Chlorpyrifos Acts as an Endocrine Disruptor in Adult Rats Causing Changes in Mammary Gland and Hormonal Balance. J. Steroid Biochem. Mol. Biol. 2016, 156, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Hundal, S.S. Chlorpyrifos Induced Toxicity in Reproductive Organs of Female Wistar Rats. Food Chem. Toxicol. 2013, 62, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Zárate, L.V.; Pontillo, C.A.; Español, A.; Miret, N.V.; Chiappini, F.; Cocca, C.; Álvarez, L.; de Pisarev, D.K.; Sales, M.E.; Randi, A.S. Angiogenesis Signaling in Breast Cancer Models is Induced by Hexachlorobenzene and Chlorpyrifos, Pesticide Ligands of the Aryl Hydrocarbon Receptor. Toxicol. Appl. Pharmacol. 2020, 401, 115093. [Google Scholar] [CrossRef]
- Zhang, C.; Schilirò, T.; Gea, M.; Bianchi, S.; Spinello, A.; Magistrato, A.; Gilardi, G.; Di Nardo, G. Molecular Basis for Endocrine Disruption by Pesticides Targeting Aromatase and Estrogen Receptor. Int. J. Environ. Res. Public Health 2020, 17, 5664. [Google Scholar] [CrossRef]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.; Séralini, G. Glyphosate-Based Herbicides are Toxic and Endocrine Disruptors in Human Cell Lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate Induces Human Breast Cancer Cells Growth via Estrogen Receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Zanardi, M.V.; Schimpf, M.G.; Gastiazoro, M.P.; Milesi, M.M.; Muñoz-de-Toro, M.; Varayoud, J.; Durando, M. Glyphosate-Based Herbicide Induces Hyperplastic Ducts in the Mammary Gland of Aging Wistar Rats. Mol. Cell. Endocrinol. 2020, 501, 110658. [Google Scholar] [CrossRef]
- Coppola, L.; Tait, S.; Ciferri, L.; Frustagli, G.; Merola, C.; Perugini, M.; Fabbrizi, E.; La Rocca, C. Integrated Approach to Evaluate the Association between Exposure to Pesticides and Idiopathic Premature Thelarche in Girls: The PEACH Project. Int. J. Mol. Sci. 2020, 21, 3282. [Google Scholar] [CrossRef]
- Knudsen, L.E.; Hansen, P.W.; Mizrak, S.; Hansen, H.K.; Mørck, T.A.; Nielsen, F.; Siersma, V.; Mathiesen, L. Biomonitoring of Danish School Children and Mothers Including Biomarkers of PBDE and Glyphosate. Rev. Environ. Health 2017, 32, 279–290. [Google Scholar] [CrossRef]
- Curwin, B.D.; Hein, M.J.; Sanderson, W.T.; Striley, C.; Heederik, D.; Kromhout, H.; Reynolds, S.J.; Alavanja, M.C. Pesticide Dose Estimates for Children of Iowa Farmers and Non-Farmers. Environ. Res. 2007, 105, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Diaz, E.; Celis-de la Rosa, A.J.; Lozano-Kasten, F.; Trasande, L.; Peregrina-Lucano, A.A.; Sandoval-Pinto, E.; Gonzalez-Chavez, H. Urinary Pesticide Levels in Children and Adolescents Residing in Two Agricultural Communities in Mexico. Int. J. Environ. Res. Public Health 2019, 16, 562. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Sheldon, L.S.; Croghan, C.W.; Jones, P.A.; Robertson, G.L.; Chuang, J.C.; Wilson, N.K.; Lyu, C.W. Exposures of Preschool Children to Chlorpyrifos and its Degradation Product 3, 5, 6-Trichloro-2-Pyridinol in their Everyday Environments. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 297–309. [Google Scholar] [CrossRef]
- Ospina, M.; Wong, L.; Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Calafat, A.M. Exposure to Neonicotinoid Insecticides in the US General Population: Data from the 2015–2016 National Health and Nutrition Examination Survey. Environ. Res. 2019, 176, 108555. [Google Scholar] [CrossRef]
- Larsson, P.; Engqvist, H.; Biermann, J.; Rönnerman, E.W.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; Helou, K.; Parris, T.Z. Optimization of Cell Viability Assays to Improve Replicability and Reproducibility of Cancer Drug Sensitivity Screens. Sci. Rep. 2020, 10, 5798. [Google Scholar] [CrossRef]
- Marchese, S.; Silva, E. Disruption of 3D MCF-12A Breast Cell Cultures by Estrogens–An in Vitro Model for ER-Mediated Changes Indicative of Hormonal Carcinogenesis. PLoS ONE 2012, 7, e45767. [Google Scholar] [CrossRef][Green Version]
- Mesnage, R.; Biserni, M.; Genkova, D.; Wesolowski, L.; Antoniou, M.N. Evaluation of Neonicotinoid Insecticides for Oestrogenic, Thyroidogenic and Adipogenic Activity Reveals Imidacloprid Causes Lipid Accumulation. J. Appl. Toxicol. 2018, 38, 1483–1491. [Google Scholar] [CrossRef]
- Stander, X.X.; Stander, B.A.; Joubert, A.M. Synergistic Anticancer Potential of Dichloroacetate and Estradiol Analogue Exerting their Effect Via ROS-JNK-Bcl-2-Mediated Signalling Pathways. Cell. Physiol. Biochem. 2015, 35, 1499–1526. [Google Scholar] [CrossRef]
- Singh, N.; Lawana, V.; Luo, J.; Phong, P.; Abdalla, A.; Palanisamy, B.; Rokad, D.; Sarkar, S.; Jin, H.; Anantharam, V. Organophosphate Pesticide Chlorpyrifos Impairs STAT1 Signaling to Induce Dopaminergic Neurotoxicity: Implications for Mitochondria Mediated Oxidative Stress Signaling Events. Neurobiol. Dis. 2018, 117, 82–113. [Google Scholar] [CrossRef]
- Yamada, S.; Kubo, Y.; Yamazaki, D.; Sekino, Y.; Kanda, Y. Chlorpyrifos Inhibits Neural Induction via Mfn1-Mediated Mitochondrial Dysfunction in Human Induced Pluripotent Stem Cells. Sci. Rep. 2017, 7, 40925. [Google Scholar] [CrossRef]
- Thakur, S.; Dhiman, M.; Mantha, A.K. APE1 Modulates Cellular Responses to Organophosphate Pesticide-Induced Oxidative Damage in Non-Small Cell Lung Carcinoma A549 Cells. Mol. Cell. Biochem. 2018, 441, 201–216. [Google Scholar] [CrossRef]
- Chen, T.; Tan, J.; Wan, Z.; Zou, Y.; Kessete Afewerky, H.; Zhang, Z.; Zhang, T. Effects of Commonly used Pesticides in China on the Mitochondria and Ubiquitin-Proteasome System in Parkinson’s Disease. Int. J. Mol. Sci. 2017, 18, 2507. [Google Scholar] [CrossRef]
- Bizerra, P.F.; Guimarães, A.R.; Maioli, M.A.; Mingatto, F.E. Imidacloprid Affects Rat Liver Mitochondrial Bioenergetics by Inhibiting FoF1-ATP Synthase Activity. J. Toxicol. Environ. Health A 2018, 81, 229–239. [Google Scholar] [CrossRef]
- Guo, J.; Shi, R.; Cao, Y.; Luan, Y.; Zhou, Y.; Gao, Y.; Tian, Y. Genotoxic Effects of Imidacloprid in Human Lymphoblastoid TK6 Cells. Drug Chem. Toxicol. 2020, 43, 208–212. [Google Scholar] [CrossRef]
- de Liz Oliveira Cavalli, V.L.; Cattani, D.; Heinz Rieg, C.E.; Pierozan, P.; Zanatta, L.; Benedetti Parisotto, E.; Wilhelm Filho, D.; Mena Barreto Silva, F.R.; Pessoa-Pureur, R.; Zamoner, A. Roundup Disrupts Male Reproductive Functions by Triggering Calcium-Mediated Cell Death in Rat Testis and Sertoli Cells. Free Radic. Biol. Med. 2013, 65, 335–346. [Google Scholar] [CrossRef]
- Olorunsogo, O.O. Modification of the Transport of Protons and Ca2 Ions across Mitochondrial Coupling Membrane by N-(Phosphonomethyl) Glycine. Toxicology 1990, 61, 205–209. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Jarosiewicz, P.; Michałowicz, J.; Koter-Michalak, M.; Huras, B.; Bukowska, B. The Impact of Glyphosate, its Metabolites and Impurities on Viability, ATP Level and Morphological Changes in Human Peripheral Blood Mononuclear Cells. PLoS ONE 2016, 11, e0156946. [Google Scholar] [CrossRef]
- Gigante, P.; Berni, M.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ramoni, R.; Basini, G. Glyphosate Affects Swine Ovarian and Adipose Stromal Cell Functions. Anim. Reprod. Sci. 2018, 195, 185–196. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Shi, F.; Zhang, S.; Wang, S.; Feng, X. Melatonin Alleviates the Deterioration of Oocytes and Hormonal Disorders from Mice Subjected to Glyphosate. Mol. Cell. Endocrinol. 2021, 520, 111073. [Google Scholar] [CrossRef]
- Andersen, H.R.; Vinggaard, A.M.; Rasmussen, T.H.; Gjermandsen, I.M.; Bonefeld-Jørgensen, E.C. Effects of Currently used Pesticides in Assays for Estrogenicity, Androgenicity, and Aromatase Activity in Vitro. Toxicol. Appl. Pharmacol. 2002, 179, 1–12. [Google Scholar] [CrossRef]
- Kojima, H.; Katsura, E.; Takeuchi, S.; Niiyama, K.; Kobayashi, K. Screening for Estrogen and Androgen Receptor Activities in 200 Pesticides by in Vitro Reporter Gene Assays using Chinese Hamster Ovary Cells. Environ. Health Perspect. 2004, 112, 524–531. [Google Scholar] [CrossRef]
- Doan, T.; Connolly, L.; Igout, A.; Nott, K.; Muller, M.; Scippo, M. In Vitro Profiling of the Potential Endocrine Disrupting Activities Affecting Steroid and Aryl Hydrocarbon Receptors of Compounds and Mixtures Prevalent in Human Drinking Water Resources. Chemosphere 2020, 258, 127332. [Google Scholar]
- Long, M.; Laier, P.; Vinggaard, A.M.; Andersen, H.R.; Lynggaard, J.; Bonefeld-Jørgensen, E.C. Effects of Currently used Pesticides in the AhR-CALUX Assay: Comparison between the Human TV101L and the Rat H4IIE Cell Line. Toxicology 2003, 194, 77–93. [Google Scholar] [CrossRef]
- Kugathas, S.; Audouze, K.; Ermler, S.; Orton, F.; Rosivatz, E.; Scholze, M.; Kortenkamp, A. Effects of Common Pesticides on Prostaglandin D2 (PGD2) Inhibition in SC5 Mouse Sertoli Cells, Evidence of Binding at the COX-2 Active Site, and Implications for Endocrine Disruption. Environ. Health Perspect. 2016, 124, 452–459. [Google Scholar] [CrossRef]
- Mzid, M.; Ghlissi, Z.; Salem, M.B.; Khedir, S.B.; Chaabouni, K.; Ayedi, F.; Sahnoun, Z.; Hakim, A.; Rebai, T. Chemoprotective Role of Ethanol Extract of Urtica Urens L. Against the Toxicity of Imidacloprid on Endocrine Disruption and Ovarian Morphometric in Female Rats, GC/MS Analysis. Biomed. Pharmacother. 2018, 97, 518–527. [Google Scholar] [CrossRef]
- Nimako, C.; Ikenaka, Y.; Okamatsu-Ogura, Y.; Bariuan, J.V.; Kobayashi, A.; Yamazaki, R.; Taira, K.; Hoshi, N.; Hirano, T.; Nakayama, S.M. Chronic Low-Dose Exposure to Imidacloprid Potentiates High Fat Diet-Mediated Liver Steatosis in C57BL/6J Male Mice. J. Vet. Med. Sci. 2021, 83, 487–500. [Google Scholar] [CrossRef]
- Lorenz, V.; Pacini, G.; Luque, E.H.; Varayoud, J.; Milesi, M.M. Perinatal Exposure to Glyphosate or a Glyphosate-Based Formulation Disrupts Hormonal and Uterine Milieu during the Receptive State in Rats. Food Chem. Toxicol. 2020, 143, 111560. [Google Scholar] [CrossRef]
- Stingl, J. Estrogen and Progesterone in Normal Mammary Gland Development and in Cancer. Horm. Cancer 2011, 2, 85–90. [Google Scholar] [CrossRef]
- Morani, A.; Warner, M.; Gustafsson, J. Biological Functions and Clinical Implications of Oestrogen Receptors Alfa and Beta in Epithelial Tissues. J. Intern. Med. 2008, 264, 128–142. [Google Scholar] [CrossRef]
- Nakamura, J.; Lu, Q.; Aberdeen, G.; Albrecht, E.; Brodie, A. The Effect of Estrogen on Aromatase and Vascular Endothelial Growth Factor Messenger Ribonucleic Acid in the Normal Nonhuman Primate Mammary Gland. J. Clin. Endocrinol. Metab. 1999, 84, 1432–1437. [Google Scholar]
- Arendt, L.M.; Kuperwasser, C. Form and Function: How Estrogen and Progesterone Regulate the Mammary Epithelial Hierarchy. J. Mammary Gland Biol. Neoplasia 2015, 20, 9–25. [Google Scholar] [CrossRef]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A Paracrine Role for the Epithelial Progesterone Receptor in Mammary Gland Development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076–5081. [Google Scholar] [CrossRef]



| Gene | RefSeq Accession | Sequence 5′ to 3′ | |
|---|---|---|---|
| GAPDH | NM_002046.4 | fw | ACTCCTCCACCTTTGACGCT |
| rev | CTTCAAGGGGTCTACATGGC | ||
| ACTB | NM_001101.5 | fw | CAGCAAGCAGGAGTATGACG |
| rev | GTGAACTTTGGGGGATGCTC | ||
| ERα | NM_000125.3 | fw | ACTGCGGGCTCTACTTCATC |
| rev | GGCTGTTCCCAACAGAAGAC | ||
| ERβ | NM_001040275.1 | fw | CTCTTTTGCCTGAAGCAACG |
| rev | CTGGGCAGTTAAGGAGACCA | ||
| AR | NM_000044.3 | fw | CCCATCTATTTCCACACCCA |
| rev | GCAAAGTCTGAAGGTGCCAT | ||
| AhR | NM_001621.4 | fw | TTCCACCTCAGTTGGCTTTG |
| rev | GGACTCGGCACAATAAAGCA | ||
| PgR | NM_000926.4 | fw | AGGTCTACCCGCCCTATCTC |
| rev | AGTAGTTGTGCTGCCCTTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, L.; Tait, S.; Fabbrizi, E.; Perugini, M.; La Rocca, C. Comparison of the Toxicological Effects of Pesticides in Non-Tumorigenic MCF-12A and Tumorigenic MCF-7 Human Breast Cells. Int. J. Environ. Res. Public Health 2022, 19, 4453. https://doi.org/10.3390/ijerph19084453
Coppola L, Tait S, Fabbrizi E, Perugini M, La Rocca C. Comparison of the Toxicological Effects of Pesticides in Non-Tumorigenic MCF-12A and Tumorigenic MCF-7 Human Breast Cells. International Journal of Environmental Research and Public Health. 2022; 19(8):4453. https://doi.org/10.3390/ijerph19084453
Chicago/Turabian StyleCoppola, Lucia, Sabrina Tait, Enrica Fabbrizi, Monia Perugini, and Cinzia La Rocca. 2022. "Comparison of the Toxicological Effects of Pesticides in Non-Tumorigenic MCF-12A and Tumorigenic MCF-7 Human Breast Cells" International Journal of Environmental Research and Public Health 19, no. 8: 4453. https://doi.org/10.3390/ijerph19084453
APA StyleCoppola, L., Tait, S., Fabbrizi, E., Perugini, M., & La Rocca, C. (2022). Comparison of the Toxicological Effects of Pesticides in Non-Tumorigenic MCF-12A and Tumorigenic MCF-7 Human Breast Cells. International Journal of Environmental Research and Public Health, 19(8), 4453. https://doi.org/10.3390/ijerph19084453








