The Removal of Pb2+ from Aqueous Solution by Using Navel Orange Peel Biochar Supported Graphene Oxide: Characteristics, Response Surface Methodology, and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BGO Composites
2.2. Experimental Methods
2.2.1. Single-Factor Adsorption Experiment
2.2.2. Response Surface Experiment
2.2.3. Adsorption Isotherm and Kinetic Fitting Experiment
2.2.4. Batch Adsorption–Desorption Experiments
2.3. Analytical Instruments and Methods
2.4. Quality Control
2.5. Data Processing and Analysis
3. Results and Discussion
3.1. Characterization of Biochar Composites
3.1.1. The Analysis of Physical Properties
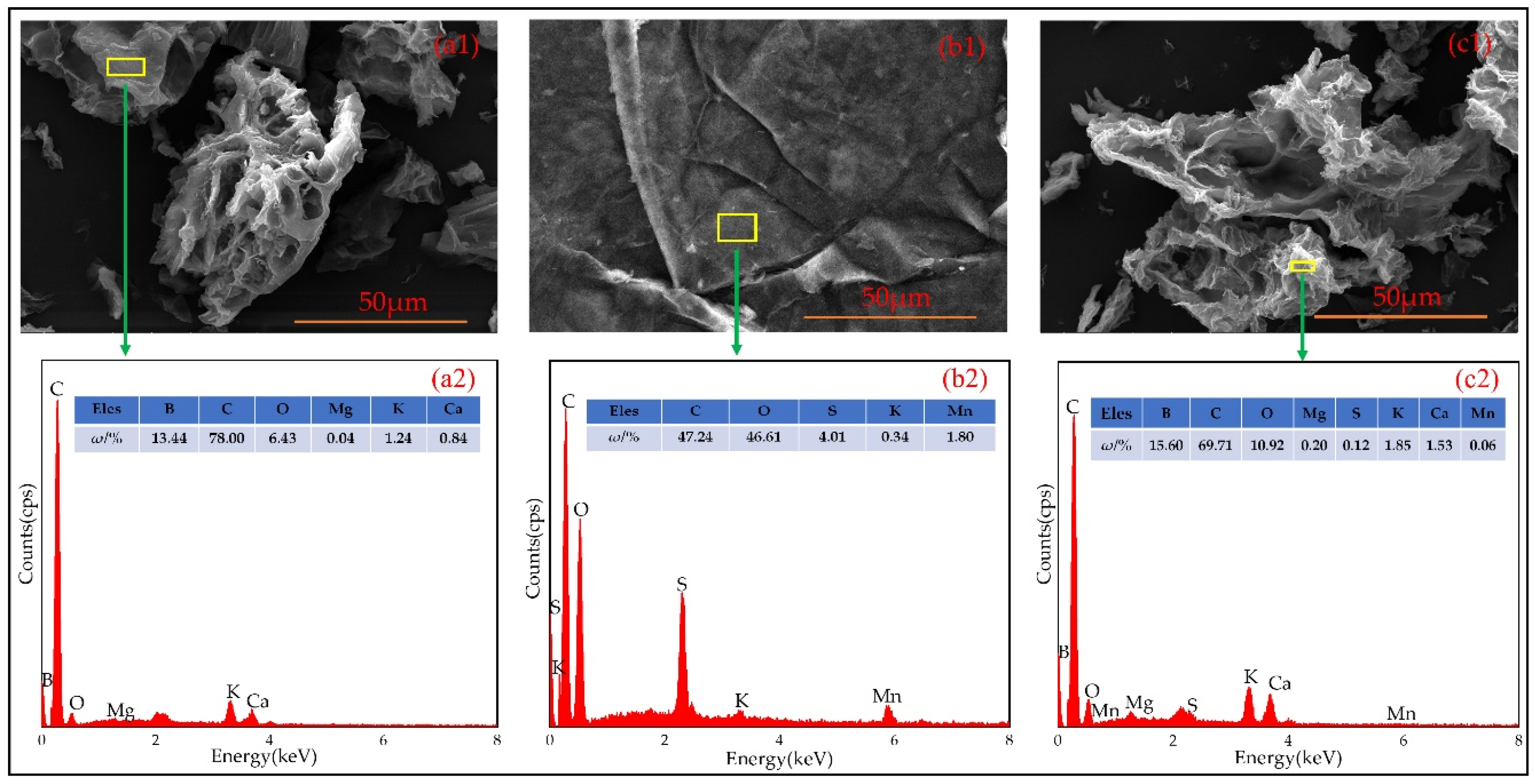
3.1.2. Result of SEM-EDS
3.1.3. Result of FT-IR
3.2. Single-Factor Adsorption Experiment of Material
3.2.1. Adsorption Capacity of the Material
3.2.2. The Effect of Initial pH on Adsorption Capability
3.2.3. The Effect of Rotating Speed on Adsorption Capability
3.2.4. The Effect of Adsorbent Dosage on Adsorption Capability
3.3. Simulation and Optimization of Pb2+ Adsorption Process by Response Surface Methodology
3.4. Adsorption Thermodynamics and Kinetics after Optimization
3.5. Optimized Adsorption Kinetics Fitting
3.6. Adsorption/Desorption Recycling Experiment
3.7. Adsorption Mechanism of Pb2+ on Biochar-Supported Graphene Oxide
3.7.1. SEM-EDS Analysis after Adsorption
3.7.2. XPS Analysis before and after Adsorption
3.7.3. FTIR and XRD Analysis before and after Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.D.; Ouyang, W.; Wang, A.H.; Liu, L.H.; Lin, C.Y.; He, M.C. Synergetic Loss of Heavy Metal and Phosphorus: Evidence from Geochemical Fraction and Estuary Sedimentation. J. Hazard. Mater. 2021, 416, 125710. [Google Scholar] [CrossRef]
- Jia, Z.Z.; Li, S.Y.; Liu, Q.X.; Jiang, F.; Hu, J.T. Distribution and Partitioning of Heavy Metals in Water and Sediments of a Typical Estuary (Modaomen, South China): The Effect of Water Density Stratification Associated with Salinity. Environ. Pollut. 2021, 287, 117277. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, J.P.; Ruder, L.D.; Dawson, R.; Ogden, M.D. Ion Exchange Removal of Cu(II), Fe(II), Pb(II) and Zn(II) from Acid Extracted Sewage Sludge—Resin Screening in Weak Acid Media. Water Res. 2019, 158, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Dai, H.C.; Chen, H.; Lin, Y.; Xie, Y.; Karthikeyan, R. General Equilibrium Analysis of the Cobenefits and Trade-Offs of Carbon Mitigation on Local Industrial Water Use and Pollutants Discharge in China. Environ. Sci. Technol. 2019, 53, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Pateda, S.M.; Sakakibara, M.; Sera, K. Element Rich Area Associated with Human Health Disorders: A Geomedical Science Approach to Potentially Toxic Elements Contamination. Int. J. Environ. Res. Public Health 2021, 18, 12202. [Google Scholar] [CrossRef]
- Liu, Z.W.; Lu, C.B.; Yang, S.; Zeng, J.F.; Yin, S.Y. Release Characteristics of Manganese in Soil under Ion-absorbed Rare Earth Mining Conditions. Soil Sediment Contam. 2020, 29, 703–720. [Google Scholar] [CrossRef]
- Sharma, S.; Nagpal, A.K.; Kaur, I. Appraisal of Heavy Metal Contents in Groundwater and Associated Health Hazards Posed to Human Population of Ropar Wetland, Punjab, India and its Environs. Chemosphere 2019, 227, 179–190. [Google Scholar] [CrossRef]
- Chen, Y.G.; He, X.L.S.; Huang, J.H.; Luo, R.; Ge, H.Z.; Wołowicz, A.; Wawrzkiewicz, M.; Gładysz-Płaska, A.; Li, B.; Yu, Q.X.; et al. Impacts of Heavy Metals and Medicinal Crops on Ecological Systems, Environmental Pollution, Cultivation, and Production Processes in China. Ecotoxicol. Environ. Saf. 2021, 219, 112336. [Google Scholar] [CrossRef]
- Sarma, G.K.; Sen Gupta, S.; Bhattacharyya, K.G. Nanomaterials As Versatile Adsorbents for Heavy Metal Ions in Water: A review. Environ. Sci. Pollut. Res. 2019, 26, 6245–6278. [Google Scholar] [CrossRef] [PubMed]
- Politaeva, N.A.; Smyatskaya, Y.A.; Tatarintseva, E.A. Using Adsorption Material Based on the Residual Biomass of Chlorella Sorokiniana Microalgae for Wastewater Purification to Remove Heavy Metal Ions. Chem. Petrol. Eng. 2020, 55, 907–912. [Google Scholar] [CrossRef]
- Qiao, L.Z.; Li, S.S.; Li, Y.L.; Liu, Y.; Du, K.F. Fabrication of Superporous Cellulose Beads Via Enhanced Inner Cross-linked Linkages for High Efficient Adsorption of Heavy Metal Ions. J. Clean. Prod. 2020, 253, 120017. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular Biochar Compared with Activated Carbon for Wastewater Treatment and Resource Recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Xiao, L.; Muslim, A.; Hojiahmat, M. Improving the Adsorption of Poly(o-phenylenediamine) to Heavy Metal Ions in Aqueous Solution Through its Composite with Carbon Dots. J. Polym. Res. 2021, 28, 404. [Google Scholar] [CrossRef]
- Laysandra, L.; Ondang, I.J.; Ju, Y.H.; Putro, J.N.; Santoso, S.P.; Soetarejo, F.E.; Ismadji, S. An environment-friendly composite as an adsorbent for removal Cu (II) ions. Environ. Sci. Pollut. Res. 2019, 26, 22979–22989. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.B.; Singh, A.; Jha, J.M.; Srivastava, N.; Hashem, A.; Alakeel, M.A.; Allah, E.F.A.; Gupta, V.K. Low-cost Biochar Adsorbents Prepared from Date and Delonix Regia Seeds for Heavy Metal Sorption. Bioresour. Technol. 2021, 339, 125606. [Google Scholar] [CrossRef] [PubMed]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.J.; Li, S.Y.; Gao, Y.Z. Occurrence, Formation, Environmental Fate and Risks of Environmentally Persistent Free Radicals in Biochars. Environ. Int. 2019, 134, 105172. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xie, C.; Wang, J.; Xiong, L. Changes in the Structures and Directions of Heavy Metal-Contaminated Soil Remediation Research from 1999 to 2020: A Bibliometric & Scientometric Study. Int. J. Environ. Res. Public Health 2021, 18, 7358. [Google Scholar] [CrossRef]
- Alam, M.S.; Gorman-Lewis, D.; Chen, N.; Flynn, S.L.; Yong, S.O.; Konhauser, K.O.; Alessi, D.S. Thermodynamic Analysis of Nickel(II) and Zinc(II) Adsorption to Biochar. Environ. Sci. Technol. 2018, 52, 6246–6255. [Google Scholar] [CrossRef]
- Wei, S.Y.; Zhu, M.B.; Fan, X.J.; Song, J.Z.; Peng, P.A.; Li, K.M.; Jia, W.L.; Song, H.Y. Influence of Pyrolysis Temperature and Feedstock on Carbon Fractions of Biochar Produced from Pyrolysis of Rice Straw, Pine Wood, Pig Manure and Sewage Sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.R.; Lu, T.; Huang, H.Y.; Zhao, D.D.; Kobayashi, N.; Chen, Y. Influence of Pyrolysis Temperature on Physical and Chemical Properties of Biochar Made from Sewage Sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of Activation Conditions On the Physicochemical Properties of Activated Biochar: A Review. Biomass Conv. Bioref. 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.J.; Ma, F.; Tankpa, V.; Bai, S.S.; Guo, X.M.; Wang, X. Mechanisms and Reutilization of Modified Biochar Used for Removal of Heavy Metals from Wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Intan, N.N.; Pfaendtner, J. The Composition of Oxygen Functionals Groups at the Surface of Carbon-Based Graphitic Anode. Phys. Chem. 2021. [Google Scholar] [CrossRef]
- Intan, N.N.; Pfaendtner, J. Effect of Fluoroethylene Carbonate Additives on the Initial Formation of the Solid Electrolyte Interphase on an Oxygen-Functionalized Graphitic Anode in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 8169–8180. [Google Scholar] [CrossRef]
- Rustam, S.; Intan, N.N.; Pfaendtner, J. Effect of Graphitic Anode Surface Functionalization on the Structure and Dynamics of Electrolytes at the Interface. J. Chem. Phys. 2021, 155, 134702. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Lee, S.Y.; Choi, J.W.; Lee, Y.J. A Facile One-pot Hydrothermal Synthesis of Sydroxyapatite/Biochar Nanocomposites: Adsorption Behavior and Mechanisms for the Removal of Copper(II) from Aqueous Media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.W.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Yong, S.O.; Cao, X.D. A Review of Biochar as A Low-cost Adsorbent for Aqueous Heavy Metal Removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, B.; Zhao, L.L.; Sun, L.L.; Gao, Y.; Li, J.J.; Yang, F. Biochar-supported Reduced Graphene Oxide Composite for Adsorption and Coadsorption of Atrazine and Lead Ions. Appl. Surf. Sci. 2017, 427, 147–155. [Google Scholar] [CrossRef]
- Zhao, G.X.; Li, J.X.; Ren, X.M.; Chen, C.L.; Wang, X.K. Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Wang, X.; Zhang, C.Z.; Zeng, G.M.; Peng, Z.W.; Zhou, J.C.; Wang, M.; Wang, R.Z.; Hu, Z.X.; Qin, X. Sorptive Removal of Ionizable Antibiotic Sulfamethazine from Aqueous Solution by Graphene Oxide-coated Biochar Nanocomposites: Influencing Factors and Mechanism. Chemosphere 2017, 186, 414–421. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chen, L.B.; Bai, H.; Li, L. Graphene Oxide–chitosan Composite Hydrogels as Broad-spectrum Adsorbents for Water Purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Huang, X.F.; Deng, Q.L.; Wang, X.Z.; Deng, H.Q.; Zhang, T.H.; Liao, H.W.; Jiang, J.L. High-efficient Removal of U(VI) from Aqueous Solution by Self-assembly Pomelo Peel/palygorskite Composite. Environ. Sci. Pollut. Res. 2021, 28, 17290–17305. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1334–1339. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Z.P. A Scalable, Solution-Phase Processing Route to Graphene Oxide and Graphene Ultralarge Sheets. Chem. Comm. 2010, 46, 2611–2613. [Google Scholar] [CrossRef]
- Anyika, C.; Asri, N.A.M.; Majid, Z.A.; Jaafar, J.; Yahya, A. Batch Sorption–desorption of As(III) from Waste Water by Magnetic Palm Kernel Shell Activated Carbon Using Optimized Box–Behnken Design. Appl. Water Sci. 2017, 7, 4573–4591. [Google Scholar] [CrossRef]
- Mohammadnia, E.; Hadavifar, M.; Veisi, H. Kinetics and Thermodynamics of Mercury Adsorption onto Thiolated Graphene Oxide Nanoparticles. Polyhedron 2019, 173, 114139. [Google Scholar] [CrossRef]
- Nigam, M.; Rajoriya, S.; Singh, S.R.; Kumar, P. Adsorption of Cr (VI) ion from tannery wastewater on tea waste: Kinetics, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2019, 7, 103188. [Google Scholar] [CrossRef]
- Peng, W.J.; Li, H.Q.; Liu, Y.Y.; Song, S.X. A Review on Heavy Metal Ions Adsorption from Water by Graphene Oxide and Its Composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.J.; Wang, C.; Hu, Z.H.; Zhou, Y.; Xiao, Y.; Wang, T. Pyrolysis of Sewage Sludge by Electromagnetic Induction: Biochar Properties and Application in Adsorption Removal of Pb(II), Cd(II) from Aqueous Solution. Waste Manag. 2019, 89, 48–56. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Chen, X.X.; Yuan, B.L.; Fu, M.L. A Facile Foaming-polymerization Strategy to Prepare 3D MnO2 Modified Biochar-based Porous Hydrogels for Efficient Removal of Cd(II) and Pb(II). Chemosphere 2020, 239, 124745. [Google Scholar] [CrossRef]
- Wu, C.X.; Li, Y.G.; Chen, M.J.; Luo, X.; Chen, Y.W.; Belzile, N.; Huang, S. Adsorption of Cadmium on Degraded Soils Amended with Maize-Stalk-Derived Biochar. Int. J. Environ. Res. Public Health 2018, 15, 2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, E.; Kumara, A.; Mishr, R.; You, S.; Singh, L.; Kumar, S.; Kumar, S. Pyrolysis of Waste Biomass and Plastics for Production of Biochar and Its Use for Removal of Heavy Metals from Aqueous Solution. Bioresour. Technol. 2021, 320, 124278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Xue, A.L.; Zhao, Y.J.; Wang, Q.W.; Yan, C.; Li, M.Y.; Xing, W.H. Competitive Adsorption of Hg2+, Pb2+ and Co2+ Ions on Polyacrylamide/attapulgite. Desalination 2010, 270, 269–274. [Google Scholar] [CrossRef]
- Deng, X.Y.; Wang, L.X.; Xiu, Q.H.; Wang, Y.; Han, H.; Dai, D.M.; Xu, Y.J.; Gao, H.T.; Liu, X. Adsorption Performance and Physicochemical Mechanism of MnO2-polyethylenimine-tannic Acid Composites for the Removal of Cu(II) and Cr(VI) from Aqueous Solution. Front. Chem. Sci. Eng. 2021, 15, 538–551. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.J.; Wang, X.R.; Li, S.P.; Liu, Y.; Yang, G.H. Influence of Reaction Media on Synthesis of Dialdehyde Cellulose/GO Composites and Their Adsorption Performances on Heavy Metals. Carbohydr. Polym. 2020, 232, 115781. [Google Scholar] [CrossRef] [PubMed]
- Yetilmezsoy, K.; Demirel, S.; Vanderbei, R.J. Response Surface Modeling of Pb(II) Removal from Aqueous Solution by Pistacia Vera, L.: Box–Behnken Experimental Design. J. Hazard. Mater. 2009, 171, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, S.; Prasad, B.S.N.; Nithya, K.; Hosseini-Bandegharaei, A. Back-propagation Neural Network: Box–Behnken Design Modelling for Optimization of Copper Adsorption on Orange Zest Biochar. Int. J. Environ. Sci. Technol. 2021, 1–16. [Google Scholar] [CrossRef]
- Barati, A.; Moghadam, E.A.; Miri, T.; Asgari, M. Rapid Removal of Heavy Metal Cations by Novel Nanocomposite Hydrogels Based on Wheat Bran and Clinoptilolite: Kinetics, Thermodynamics, and Isotherms. Water Air Soil Pollut. 2014, 225, 2096. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Földváry, C.M.; Takács, E. Radiation-induced Grafting of Cellulose for Adsorption of Hazardous Water Pollutants: A Review. Radiat. Phys. Chem. 2010, 79, 848–862. [Google Scholar] [CrossRef]
- Nikkhah, A.A.; Zilouei, H.; Asadinezhad, A.; Keshavarz, A. Removal of Oil from Water Using Polyurethane Foam Modified with Nanoclay. Chem. Eng. J. 2015, 262, 278–285. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Aziz, R.A.; Vidyadharan, B.; Jose, R. Electrochemical Properties of Carbon from Oil Palm Kernel Shell for High Performance Supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef] [Green Version]











| Number | Factors | Variation Range |
|---|---|---|
| A | Solution pH | 2, 3, 4, 5, 6 |
| B | Rotating speed (rpm) | 100, 125, 150, 175, 200 |
| C | Adsorbent dosage (g) | 0.005, 0.01, 0.02, 0.05, 0.1 |
| Samples | pH | Element Content (%) | Atomic Ratio | SSA (m2/g) | PV (cm3/g) | PD (nm) | Yield (%) | Ash (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O a | H/C | O/C | (O + N)/C | |||||||
| B300 | 8.88 | 59.61 | 3.42 | 1.85 | 0.76 | 23.66 | 0.057 | 0.40 | 0.43 | 1.82 | 0.0122 | 1.753 | 43.8 | 10.70 |
| B500 | 9.68 | 67.27 | 2.26 | 1.80 | 0.62 | 17.75 | 0.061 | 0.24 | 0.27 | 5.39 | 0.0151 | 1.773 | 33.5 | 10.30 |
| B700 | 9.98 | 74.09 | 1.77 | 1.01 | 0.38 | 12.40 | 0.024 | 0.17 | 0.18 | 18.50 | 0.0166 | 1.851 | 26.6 | 10.35 |
| BGO300 | 9.43 | 56.63 | 3.28 | 2.02 | 0.49 | 24.39 | 0.058 | 0.43 | 0.47 | 8.67 | 0.0155 | 1.789 | 45.3 | 13.19 |
| BGO500 | 9.64 | 59.42 | 2.28 | 1.75 | 0.62 | 22.45 | 0.038 | 0.38 | 0.41 | 23.86 | 0.0173 | 1.991 | 29.4 | 13.48 |
| BGO700 | 10.1 | 68.23 | 1.37 | 1.38 | 0.74 | 14.94 | 0.020 | 0.22 | 0.24 | 38.23 | 0.0189 | 2.114 | 27.4 | 11.34 |
| Model | Parameter | Temperature (°C) | ||
|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | ||
| Langmuir | (mg/g) | 127.66 | 118.04 | 123.28 |
| (L/mg) | 0.146 | 0.283 | 0.281 | |
| 0.838 | 0.848 | 0.744 | ||
| 65.19 | 15.67 | 62.82 | ||
| Freundlich | 0.108 | 0.079 | 0.091 | |
| (L/mg) | 66.17 | 76.27 | 75.91 | |
| 0.970 | 0.945 | 0.894 | ||
| 12.15 | 5.726 | 26.07 | ||
| Temkin | 57.49 | 72.20 | 71.12 | |
| 11.48 | 8.268 | 9.709 | ||
| 0.959 | 0.935 | 0.875 | ||
| 16.47 | 6.689 | 30.63 | ||
| T(K) | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (KJ/(mol·K) |
|---|---|---|---|
| 298 | −3.934 | 13.35 | 0.058 |
| 308 | −4.514 | ||
| 318 | −5.094 |
| Kinetic Model | Quasi First Order Kinetics | Quasi Second Order Kinetics | |||||||
| Parameter | |||||||||
| BGO700 | 124.42 | 48.21 | 0.00127 | 0.974 | 0.028 | 128.53 | 6.73 | 0.999 | 0.026 |
| B700 | 79.1 | 37.826 | 0.00144 | 0.949 | 0.036 | 81.9 | 9.54 | 0.998 | 0.205 |
| Kinetic Model | Intra-Particle Diffusion Model | ||||||||
| Parameter | |||||||||
| BGO700 | 124.42 | 2.936 | 59.52 | 0.986 | 12.68 | 0.704 | 89.42 | 0.902 | 34.44 |
| B700 | 79.1 | 1.453 | 39.63 | 0.983 | 1.818 | 0.508 | 53.85 | 0.928 | 8.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, S.; Zhang, L.; Zeng, J.; Tian, S.; Lin, Y. The Removal of Pb2+ from Aqueous Solution by Using Navel Orange Peel Biochar Supported Graphene Oxide: Characteristics, Response Surface Methodology, and Mechanism. Int. J. Environ. Res. Public Health 2022, 19, 4790. https://doi.org/10.3390/ijerph19084790
Liu Z, Yang S, Zhang L, Zeng J, Tian S, Lin Y. The Removal of Pb2+ from Aqueous Solution by Using Navel Orange Peel Biochar Supported Graphene Oxide: Characteristics, Response Surface Methodology, and Mechanism. International Journal of Environmental Research and Public Health. 2022; 19(8):4790. https://doi.org/10.3390/ijerph19084790
Chicago/Turabian StyleLiu, Zuwen, Shi Yang, Linan Zhang, Jinfeng Zeng, Shuai Tian, and Yuan Lin. 2022. "The Removal of Pb2+ from Aqueous Solution by Using Navel Orange Peel Biochar Supported Graphene Oxide: Characteristics, Response Surface Methodology, and Mechanism" International Journal of Environmental Research and Public Health 19, no. 8: 4790. https://doi.org/10.3390/ijerph19084790





