A Retrospective, Single-Centre Study on the Learning Curve for Liver Tumor Open Resection in Patients with Hepatocellular Cancers and Intrahepatic Cholagangiocarcinomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Procedures Characteristics
2.2. Basic Characteristics of Hepatobiliary Surgical Training
2.3. Statistical Analysis
3. Results
3.1. Patients and Procedure
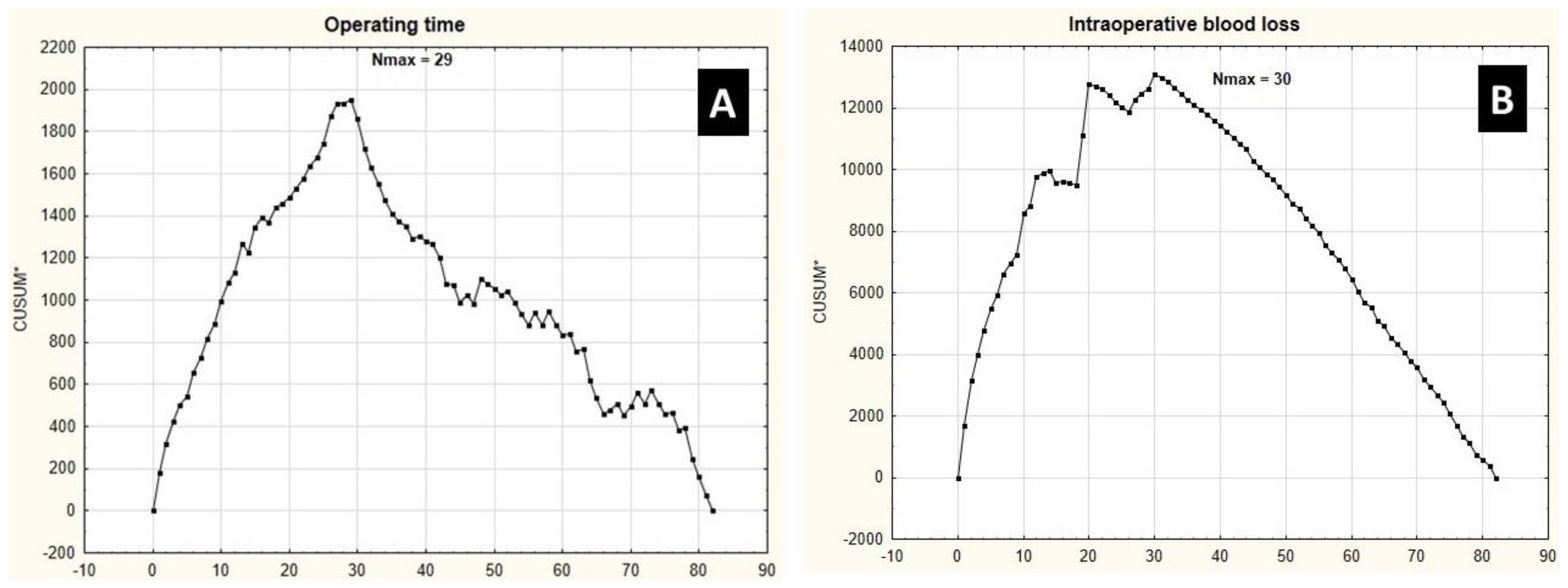
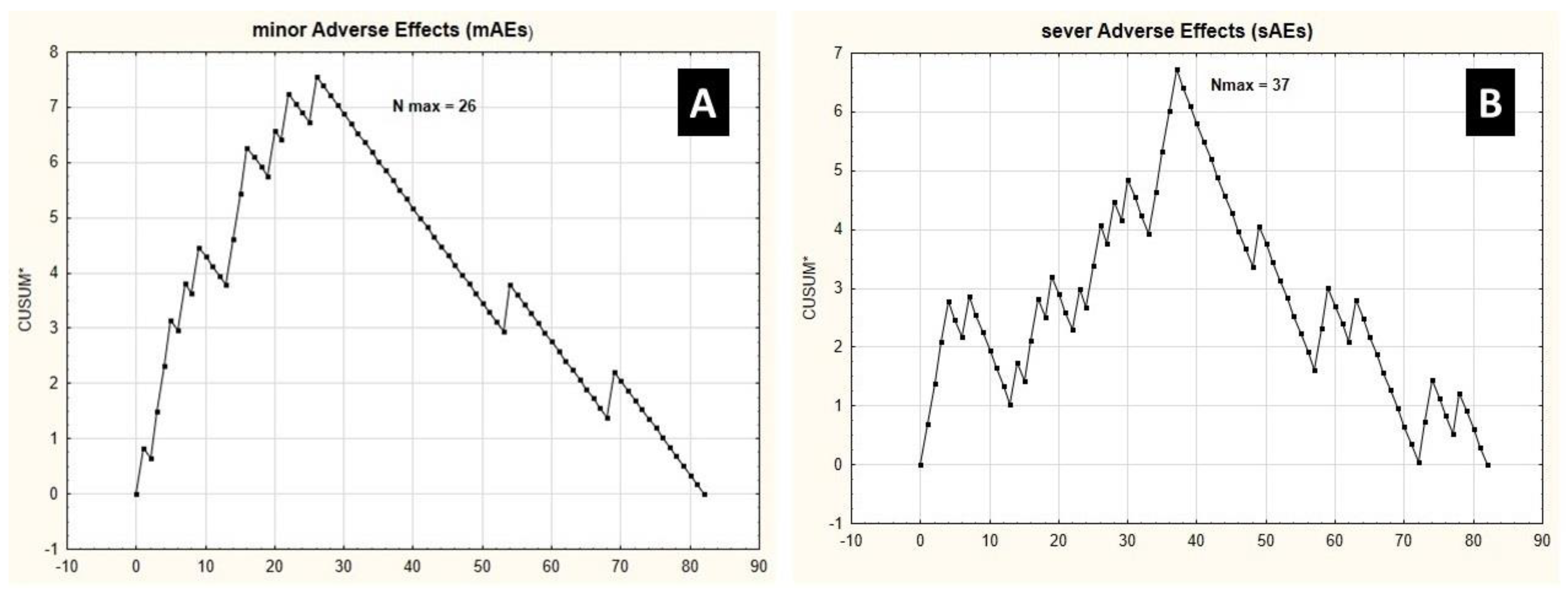
3.2. Learning Curve Endpoints
3.3. Patient Safety
4. Discussion
5. Conclusions
- Hemihepatectomy procedures present similar learning curves to achieve stabilization in operating time and intraoperative blood loss.
- Operative time and intraoperative blood loss cannot be, however, considered as surrogates for the risk of grade IIIb-IVb complications, according to the Clavien–Dindo classification, as they present significantly different learning curves [19].
- Learning curves for major liver resections due to metastatic tumors are similar to our findings; therefore, the type and character of a liver tumor (primary or metastatic) does not seem to influence the learning curve.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Yang, T.; Wu, M.; Shen, F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016, 379, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Banas, B.; Bereza, K. Incidence and mortality rates in malignant neoplasm of liver and intrahepatic bile ducts in Polish population: An analysis of population-based data (1999–2016). Przegląd Lek. 2019, 76, 101–104. [Google Scholar]
- Yang, J.D.; Heimbach, J.K. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 2020, 371, m3544. [Google Scholar] [CrossRef]
- Sirica, A.E.; Gores, G.J.; Groopman, J.D.; Selaru, F.M.; Strazzabosco, M.; Wei Wang, X.; Zhu, A.X. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology 2019, 69, 1803–1815. [Google Scholar] [CrossRef]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Hartke, J.; Johnson, M.; Ghabril, M. The diagnosis and treatment of hepatocellular carcinoma. Semin. Diagn. Pathol. 2017, 34, 153–159. [Google Scholar] [CrossRef]
- Grandhi, M.S.; Kim, A.K.; Ronnekleiv-Kelly, S.M.; Kamel, I.R.; Ghasebeh, M.A.; Pawlik, T.M. Hepatocellular carcinoma: From diagnosis to treatment. Surg. Oncol. 2016, 25, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.M.; Cai, H.; Shrubsole, M.J.; Bailey, C.E.; Idrees, K.; Shu, X.O. Outcomes of robotic-assisted liver surgery versus laparoscopic liver surgery for treatment of stage I hepatocellular carcinoma. Cancer 2022, 128, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fei, Y.; Liu, J.; Yu, T.; Tang, J.; Wei, F. Laparoscopic surgery and robotic surgery for hilar cholangiocarcinoma: An updated systematic review. ANZ J. Surg. 2021, 91, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Banas, B.; Gwizdak, P.; Zabielska, P.; Kolodziejczyk, P.; Richter, P. Learning Curve for Metastatic Liver Tumor Open Resection in Patients with Primary Colorectal Cancer: Use of the Cumulative Sum Method. Int. J. Environ. Res. Public Health 2022, 19, 1068. [Google Scholar] [CrossRef]
- Guilbaud, T.; Birnbaum, D.J.; Berdah, S.; Farges, O.; Beyer Berjot, L. Learning Curve in Laparoscopic Liver Resection, Educational Value of Simulation and Training Programmes: A Systematic Review. World J. Surg. 2019, 43, 2710–2719. [Google Scholar] [CrossRef]
- Luft, H.S.; Bunker, J.P.; Enthoven, A.C. Should operations be regionalized? The empirical relation between surgical volume and mortality. N. Engl. J. Med. 1979, 301, 1364–1369. [Google Scholar] [CrossRef]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Belghiti, J.; Clavien, P.A.; Gadzijev, E.; Garden, J.O.; Lau, W.Y.; Makuuchi, M.; Strong, R.W. Terminology Committee of the International Hepato-Pancreato-Biliary Association. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB 2000, 2, 333–339. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, H.; Wang, T.; Xia, S.; Chen, W.; Li, B. Learning Curve Analysis of Open Kasai Portoenterostomy for Biliary Atresia. J. Surg. Res. 2019, 239, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jinhuan, Y.; Yi, W.; Yuanwen, Z.; Delin, M.; Xiaotian, C.; Yan, W.; Liming, D.; Haitao, Y.; Lijun, W.; Tuo, D.; et al. Laparoscopic Versus Open Surgery for Early-Stage Intrahepatic Cholangiocarcinoma After Mastering the Learning Curve: A Multicenter Data-Based Matched Study. Front. Oncol. 2022, 11, 742544. [Google Scholar] [CrossRef] [PubMed]
- Šubrt, Z.; Ferko, A.; Vošmik, M.; Linter-Kapišinská, M.; Oliverius, M.; Gürlich, R. Laparoscopic versus open liver resections for colorectal cancer liver metastases: Short-term results. Rozhl. Chir. 2019, 98, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.G.; Kang, I.; Rho, S.Y.; Choi, G.H.; Han, D.H.; Kim, K.S.; Choi, J.S. Major Laparoscopic Versus Open Resection for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis Based on Surgeons’ Learning Curve. Ann. Surg. Oncol. 2021, 28, 447–458. [Google Scholar] [CrossRef]
- Van Gulik, T. Open versus laparoscopic resection for liver tumours. HPB 2009, 11, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Chan, F.K.; Cheng, K.C.; Yeung, Y.P.; Ho, K.M. Learning Curve for Laparoscopic Major Hepatectomy: Use of the Cumulative Sum Method. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, e41–e45. [Google Scholar] [CrossRef]
- Morino, M.; Morra, I.; Rosso, E.; Miglietta, C.; Garrone, C. Laparoscopic vs open hepatic resection: A comparative study. Surg. Endosc. 2003, 17, 1914–1918. [Google Scholar] [CrossRef]
- Nishikimi, K.; Tate, S.; Matsuoka, A.; Shozu, M. Learning curve of high-complexity surgery for advanced ovarian cancer. Gynecol. Oncol. 2020, 156, 54–61. [Google Scholar] [CrossRef] [Green Version]
| Total (N = 82) | HCC % (N = 61) | ICC %% (N = 21) | p * | |
|---|---|---|---|---|
| Mean age (±SD $) [years] | 55.07 (±11.54) | 60.95 ± 7.32 | 53.05 ± 12.21 | 0.006 ** |
| Mean BMI $$ (±SD $) [kg/m2] | 23.94 (±3.39). | 24.52 ± 3.44 | 22.26 ± 42.64 | 0.008 ** |
| Male (%)/Female (%) | 47 (57.32%)/ 35 (42.68%) | 35 (57.38%)/ 26 (45.62%) | 13 (59.10%)/ 8(40.90%) | 0.716 |
| Median operating time (IQR $$$) [min] | 255 (IQR: 110) | 250 (IQR: 105) | 290 (IQR: 60) | 0.245 |
| Median intraoperative blood loss (IQR $$$) [mL] | 342 (IQR: 185) | 355 (IQR: 310) | 340 (IQR: 315) | 0.232 |
| Median post-operative hospital stay (IQR $$$) [days] | 10 (IQR: 9) | 11 (IQR: 9) | 6 (IQR: 7) | 0.060 |
Number of grade I-IIIa complications according to the Clavien–Dindo classification (%)
| 6 (9.84%) 25 (30.86%) 9 (14.75%) | 4 (9.84%) 18 (42.62%) 7 (14.75%) | 2 (9.84%) 8 (42.62%) 2 (14.75%) | 0.467 |
Number of grade IIIb-V complications according to the Clavien–Dindo classification (%)
| 0 (0.00%) 14 (17.07%) & 4 (4.88%) 5 (6.10%) 2 (2.44%) 3 (3.66%) & | 0 (0.00%) 10 (16.39%) 3 (4.92%) 3 (4.92%) 2 (3.29%) 2 (3.29%) | 0 (0.00%) 4 (19.05%) 1 (4.76%) 2 (9.52%) 0 (0.00%) 1 (4.76%) | 0.677 |
| Incidence of Pinard’s maneuver (%) | 39 (47.56%) | 29 (47.54%) | 10 (47.62%) | 0.997 |
| Median time of Pringle’s maneuver (IQR $$$) [min] | 15 (IQR: 30) | 15 (IQR: 30) | 15 (IQR: 15) | 0.687 |
| Prevalence of preoperative anemia (defined as hemoglobin level < 12.0 and or hematocrit level < 35.0) | 28 (34.15%) | 20 (32.79%) | 8 (38.10%) | 0.878 |
| Prevalence of right/left hemihepatectomy && (%) | 52 (63.41%)/ 30 (36.59%) | 41 (67,21%)/ 20 (32,79%) | 11 (52.38%) 10 (47.62%) | 0.224 |
| Prevalence of liver cirrhosis cirrhosis (%) | 59 (62.19%) | 50 (81,97%) | 9 (42.86%) | 0.001 ** |
| Median preoperative ASA &&& score IQR $$$) | 3 (IQR: 1) | 3 (IQR:1) | 2 (IQR: 1) | 0.754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banas, B.; Kolodziejczyk, P.; Czerw, A.; Banas, T.; Kotwas, A.; Richter, P. A Retrospective, Single-Centre Study on the Learning Curve for Liver Tumor Open Resection in Patients with Hepatocellular Cancers and Intrahepatic Cholagangiocarcinomas. Int. J. Environ. Res. Public Health 2022, 19, 4872. https://doi.org/10.3390/ijerph19084872
Banas B, Kolodziejczyk P, Czerw A, Banas T, Kotwas A, Richter P. A Retrospective, Single-Centre Study on the Learning Curve for Liver Tumor Open Resection in Patients with Hepatocellular Cancers and Intrahepatic Cholagangiocarcinomas. International Journal of Environmental Research and Public Health. 2022; 19(8):4872. https://doi.org/10.3390/ijerph19084872
Chicago/Turabian StyleBanas, Bartlomiej, Piotr Kolodziejczyk, Aleksandra Czerw, Tomasz Banas, Artur Kotwas, and Piotr Richter. 2022. "A Retrospective, Single-Centre Study on the Learning Curve for Liver Tumor Open Resection in Patients with Hepatocellular Cancers and Intrahepatic Cholagangiocarcinomas" International Journal of Environmental Research and Public Health 19, no. 8: 4872. https://doi.org/10.3390/ijerph19084872
APA StyleBanas, B., Kolodziejczyk, P., Czerw, A., Banas, T., Kotwas, A., & Richter, P. (2022). A Retrospective, Single-Centre Study on the Learning Curve for Liver Tumor Open Resection in Patients with Hepatocellular Cancers and Intrahepatic Cholagangiocarcinomas. International Journal of Environmental Research and Public Health, 19(8), 4872. https://doi.org/10.3390/ijerph19084872






