Comparison of Two Diet and Exercise Approaches on Weight Loss and Health Outcomes in Obese Women
Highlights
- Overweight sedentary women perform more physical activity when engaged in a supervised exercise programs.
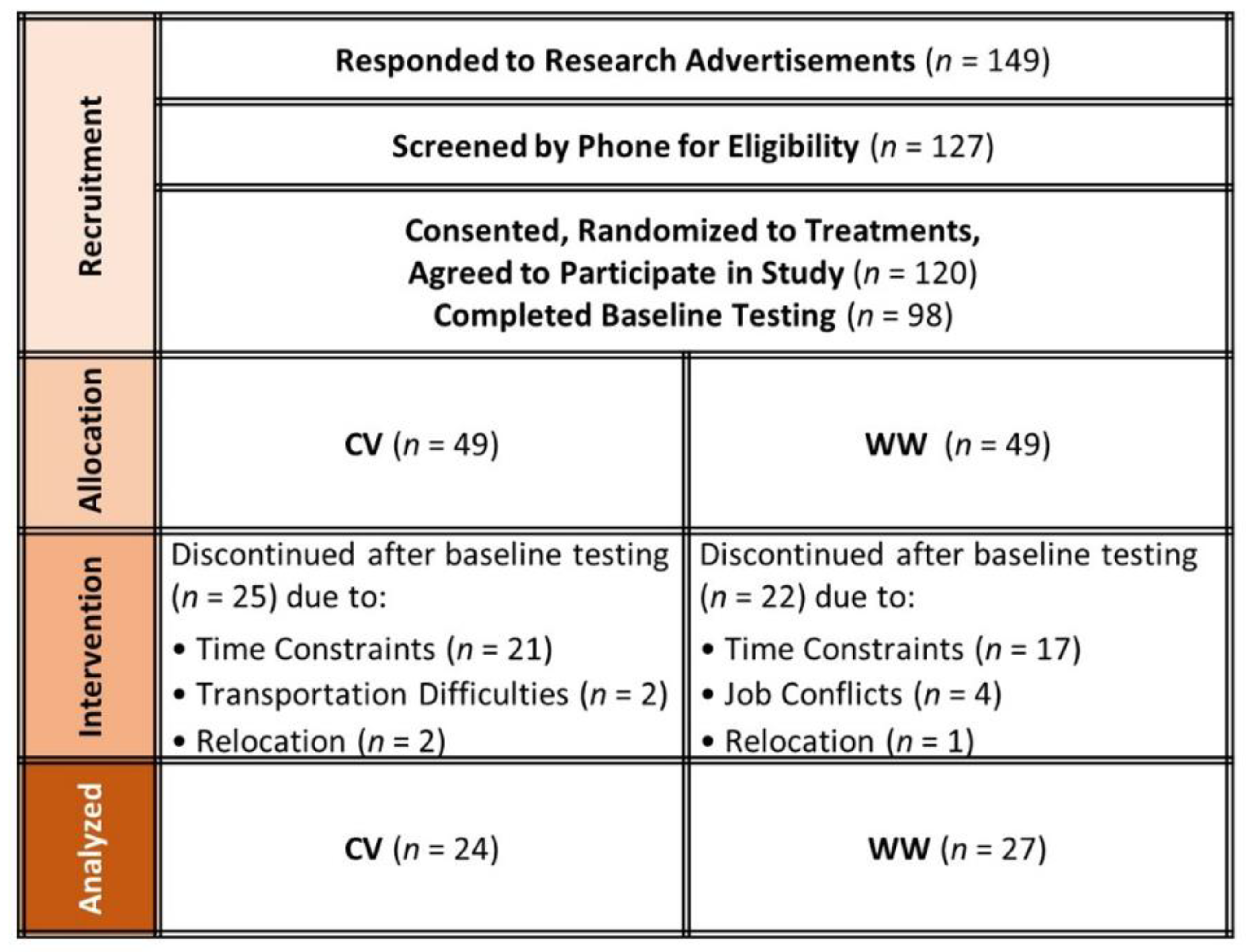
- Adherence to WW and CV programs for 16 weeks promoted similar weight loss, but those in the CV program lost more fat and gained more lean mass.
- Both programs led to a decrease in total and LDL cholesterol; however, CV also led to an improvement in HDL and CHL:HDL ratio.
- A hypo-energetic high-protein diet plus circuit-style resistance program leads to better improvement in body composition.
- A diet-based intervention with an unsupervised physical activity recommendation does not preserve lean body mass.
- Sixteen weeks of either CV or WW weight management program can improve blood lipid profiles, but CV may show greater improvement.
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Participants
2.3. Diet Intervention
2.4. Exercise Intervention
3. Procedures
3.1. Dietary Assessment
3.2. Physical Activity Assessment
3.3. Resting Energy Expenditure and Metabolism
3.4. Body Composition and Anthropometrics
3.5. Resting Hemodynamics and Exercise Capacity
3.6. Blood Collection and Analysis
3.7. Statistical Analysis
4. Results
4.1. Baseline Characteristics
4.2. Energy Intake and Resting Energy Expenditure
4.3. Physical Activity
4.4. Weight and Body Composition
4.5. Fitness and Health-Related Variables
4.6. Metabolic and Hormonal Profiles
5. Discussion and Conclusions
5.1. Primary Outcome—Weight Loss and Body Composition
5.2. Secondary Outcomes—Markers of Fitness and Health
5.3. Limitations
5.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, C.; Herrick, K.; Sarafrazi, N.; Ogden, C. Attempts to Lose Weight among Adults in the United States, 2013–2016; NCHS Data Brief, No 313; National Center for Health Statistics: Hyattsville, MD, USA, 2018.
- Varkevisser, R.D.M.; van Stralen, M.M.; Kroeze, W.; Ket, J.C.F.; Steenhuis, I.H.M. Determinants of weight loss maintenance: A systematic review. Obes. Rev. 2019, 20, 171–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B. Exercise and nutritional strategies to promote weight loss: A narrative review. OBM Integ. Comp. Med. 2021, 6, 19. [Google Scholar] [CrossRef]
- Cleo, G.; Beller, E.; Glasziou, P.; Isenring, E.; Thomas, R. Efficacy of habit-based weight loss interventions: A systematic review and meta-analysis. J. Behav. Med. 2020, 43, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Istfan, N.W.; Apovian, C.M. Obesity: Overview of Weight Management. Endocr. Pract. 2021, 27, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle Modification for Obesity. Circulation 2012, 125, 1157–1170. [Google Scholar] [CrossRef] [Green Version]
- Soeliman, F.A.; Azadbakht, L. Weight loss maintenance: A review on dietary related strategies. J. Res. Med. Sci. 2014, 19, 268–275. [Google Scholar]
- Schneider, J.; Peterli, R.; Gass, M.; Slawik, M.; Peters, T.; Wolnerhanssen, B.K. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: A prospective randomized trial. Surg. Obes. Relat. Dis. 2016, 12, 563–570. [Google Scholar] [CrossRef]
- Heshka, S.; Lemos, T.; Astbury, N.M.; Widen, E.; Davidson, L.; Goodpaster, B.H.; DeLany, J.P.; Strain, G.W.; Pomp, A.; Courcoulas, A.P.; et al. Resting Energy Expenditure and Organ-Tissue Body Composition 5 Years After Bariatric Surgery. Obes. Surg. 2020, 30, 587–594. [Google Scholar] [CrossRef]
- Baetge, C.; Earnest, C.P.; Lockard, B.; Coletta, A.M.; Galvan, E.; Rasmussen, C.; Levers, K.; Simbo, S.Y.; Jung, Y.P.; Koozehchian, M.; et al. Efficacy of a randomized trial examining commercial weight loss programs and exercise on metabolic syndrome in overweight and obese women. Appl. Physiol. Nutr. Metab. 2017, 42, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Magrans, T.; Wilborn, C.; Wismann, J.; Beckham, J.; Campbell, B.; Galbreath, M.; Kerksick, C.; Rasmussen, C.; Ounpraseuth, S.; Casey, P.; et al. Long-term effects o f the Curves fitness and weight loss program: Body composition and resting energy expenditure. FASEB J. 2005, LBA56. [Google Scholar]
- Kerksick, C.M.; Thomas, A.; Campbell, B.; Taylor, L.; Wilborn, C.; Marcello, B.; Roberts, M.D.; Pfau, E.; Grimstvedt, M.; Opusunju, J.; et al. Effects of a popular exercise and weight loss program on weight loss, body composition, energy expenditure and health in obese women. Nutr. Metabol. 2009, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerksick, C.M.; Wismann-Bunn, J.; Fogt, D.; Thomas, A.R.; Taylor, L.; Campbell, B.I.; Wilborn, C.D.; Harvey, T.; Roberts, M.D.; La Bounty, P.; et al. Changes in weight loss, body composition and cardiovascular disease risk after altering macronutrient distributions during a regular exercise program in obese women. Nutr. J. 2010, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canon, C.; Kresta, J.Y.; Byrd, M.; Oliver, J.M.; Mardock, M.; Simbo, S.; Jung, Y.; Koozehchian, M.; Khanna, D.; Lockard, B.; et al. Long-term efficacy of women participating in the curves fitness and weight loss program. J. Int. Soc. Sports Nutr. 2010, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Magrans-Courtney, T.; Wilborn, C.; Rasmussen, C.; Ferreira, M.; Greenwood, L.; Campbell, B.; Kerksick, C.M.; Nassar, E.; Li, R.; Iosia, M.; et al. Effects of diet type and supplementation of glucosamine, chondroitin, and MSM on body composition, functional status, and markers of health in women with knee osteoarthritis initiating a resistance-based exercise and weight loss program. J. Int. Soc. Sports Nutr. 2011, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B.; Rasmussen, C.; Kerksick, C.M.; Wilborn, C.; Taylor, L.t.; Campbell, B.; Magrans-Courtney, T.; Fogt, D.; Ferreira, M.; Li, R.; et al. A carbohydrate-restricted diet during resistance training promotes more favorable changes in body composition and markers of health in obese women with and without insulin resistance. Phys. Sportsmed. 2011, 39, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Serra, M.; Beavers, K.M.; Moreillon, J.; Kresta, J.Y.; Byrd, M.; Oliver, J.M.; Gutierrez, J.; Hudson, G.; Deike, E.; et al. A Structured Diet and Exercise Program Promotes Favorable Changes in Weight Loss, Body Composition, and Weight Maintenance. J. Am. Diet. Assoc. 2011, 111, 828–843. [Google Scholar] [CrossRef]
- Coletta, A.M.; Sanchez, B.; O’Connor, A.; Dalton, R.; Springer, S.; Koozehchian, M.S.; Murano, P.S.; Woodman, C.R.; Rasmussen, C.; Kreider, R.B. Alignment of diet prescription to genotype does not promote greater weight loss success in women with obesity participating in an exercise and weight loss program. Obes. Sci. Pract. 2018, 4, 554–574. [Google Scholar] [CrossRef]
- Galbreath, M.; Campbell, B.; La Bounty, P.; Bunn, J.; Dove, J.; Harvey, T.; Hudson, G.; Gutierrez, J.; Levers, K.; Galvan, E.; et al. Effects of Adherence to a Higher Protein Diet on Weight Loss, Markers of Health, and Functional Capacity in Older Women Participating in a Resistance-Based Exercise Program. Nutrients 2018, 10, 1070. [Google Scholar] [CrossRef] [Green Version]
- Kerksick, C.M.; Roberts, M.D.; Campbell, B.I.; Galbreath, M.M.; Taylor, L.W.; Wilborn, C.D.; Lee, A.; Dove, J.; Bunn, J.W.; Rasmussen, C.J.; et al. Differential Impact of Calcium and Vitamin D on Body Composition Changes in Post-Menopausal Women Following a Restricted Energy Diet and Exercise Program. Nutrients 2020, 12, 713. [Google Scholar] [CrossRef] [Green Version]
- Kresta, J.; Byrd, M.; Oliver, J.; Baetge, C.; Mardock, M.; Simbo, S.; Jung, Y.; Koozehchian, M.; Khanna, D.; Lockard, B.; et al. Effects of Energy and Macronutrient Cycling on Weight Loss, Body Composition, and Markers of Health in Obese Women Participating in a Resistance-Based Exercise Program. Med. Res. Arch. 2020, 8. [Google Scholar] [CrossRef]
- Lockard, B.; Earnest, C.P.; Oliver, J.; Goodenough, C.; Rasmussen, C.; Greenwood, M.; Kreider, R.B. Retrospective Analysis of Protein- and Carbohydrate-Focused Diets Combined with Exercise on Metabolic Syndrome Prevalence in Overweight and Obese Women. Metab. Syndr. Relat. Disord. 2016, 14, 228–237. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Heavin, G.; Rodman, N.; Findley, C. Curves Fitness & Weight Management Program; Curves International: Waco, TX, USA, 2008. [Google Scholar]
- Gagliardi, N.; Runyan, E.; Mintcheff, D.; Maas, R.; Smith, W.; Cook, C. WeightWatchers New Complete Cookbook: Momentum Program Edition; Wiley Publishing Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Farris, G.; Wismann, J.; Farris, R.; Gandy, N.; Long, L.; Pfau, E.; Kreider, R. Exercise intensity and energy expenditure analysis of women participating in the Curves® exercise program. FASEB J. 2006, 20, LB93–LB94. [Google Scholar] [CrossRef]
- La Bounty, P.; Wilborn, C.; Marcello, B.; Campbell, B.; Faries, M.; Shim, J.; Kreider, R. Analysis of exercise intensities of women using the Curves® hydraulic training equipment. FASEB J. 2006, 20, LB93. [Google Scholar] [CrossRef]
- Kreider, R.; Parker, A.; Moreillon, J.; Rasmussen, C.; Greenwood, M. Energy expenditure analysis of women participating in a computerized hydraulic circuit training program. J. Strength Cond. Res. 2008, 22, A69–A70. [Google Scholar]
- Ainsworth, B.E.; Bassett, D.R., Jr.; Strath, S.J.; Swartz, A.M.; O’Brien, W.L.; Thompson, R.W.; Jones, D.A.; Macera, C.A.; Kimsey, C.D. Comparison of three methods for measuring the time spent in physical activity. Med. Sci. Sports Exerc. 2000, 32, S457–S464. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Macera, C.A.; Jones, D.A.; Reis, J.P.; Addy, C.L.; Bowles, H.R.; Kohl, H.W., 3rd. Comparison of the 2001 BRFSS and the IPAQ Physical Activity Questionnaires. Med. Sci. Sports Exerc. 2006, 38, 1584–1592. [Google Scholar] [CrossRef]
- Booth, M. Assessment of physical activity: An international perspective. Res. Q. Exerc. Sport 2000, 71, S114–S120. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Liguori, G. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2021. [Google Scholar]
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef]
- McAuley, K.A.; Williams, S.M.; Mann, J.I.; Walker, R.J.; Lewis-Barned, N.J.; Temple, L.A.; Duncan, A.W. Diagnosing insulin resistance in the general population. Diabetes Care 2001, 24, 460–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726–736. [Google Scholar] [PubMed]
- Layman, D.K.; Evans, E.; Baum, J.I.; Seyler, J.; Erickson, D.J.; Boileau, R.A. Dietary Protein and Exercise Have Additive Effects on Body Composition during Weight Loss in Adult Women. J. Nutri. 2005, 135, 1903–1910. [Google Scholar] [CrossRef] [Green Version]
- Layman, D.K.; Shiue, H.; Sather, C.; Erickson, D.J.; Baum, J. Increased Dietary Protein Modifies Glucose and Insulin Homeostasis in Adult Women during Weight Loss. J. Nutr. 2003, 133, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Meckling, K.A.; Sherfey, R. A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the Metabolic Syndrome in overweight and obese women. Appl. Physiol. Nutr. Metabol. 2007, 32, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone Diets for Weight Loss and Heart Disease Risk Reduction. JAMA 2005, 293, 43. [Google Scholar] [CrossRef] [PubMed]
- Truby, H.; Baic, S.; Delooy, A.; Fox, K.R.; Livingstone, M.B.E.; Logan, C.M.; Macdonald, I.A.; Morgan, L.M.; Taylor, M.A.; Millward, D.J. Randomised controlled trial of four commercial weight loss programmes in the UK: Initial findings from the BBC “diet trials”. BMJ 2006, 332, 1309–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahern, A.L.; Olson, A.D.; Aston, L.M.; Jebb, S.A. Weight Watchers on prescription: An observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health 2011, 11, 434. [Google Scholar] [CrossRef] [Green Version]
- Bellisle, F.; Dalix, A.M.; De Assis, M.A.; Kupek, E.; Gerwig, U.; Slama, G.; Oppert, J.M. Motivational effects of 12-week moderately restrictive diets with or without special attention to the Glycaemic Index of foods. Br. J. Nutr. 2007, 97, 790–798. [Google Scholar] [CrossRef]
- Dixon, K.J.L.; Shcherba, S.; Kipping, R.R. Weight loss from three commercial providers of NHS primary care slimming on referral in North Somerset: Service evaluation. J. Pub. Health 2012, 34, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Heshka, S.; Anderson, J.W.; Atkinson, R.L.; Greenway, F.L.; Hill, J.O.; Phinney, S.D.; Kolotkin, R.L.; Miller-Kovach, K.; Pi-Sunyer, F.X. Weight Loss With Self-help Compared With a Structured Commercial Program. JAMA 2003, 289, 1792. [Google Scholar] [CrossRef] [Green Version]
- Jebb, S.A.; Ahern, A.L.; Olson, A.D.; Aston, L.M.; Holzapfel, C.; Stoll, J.; Amann-Gassner, U.; Simpson, A.E.; Fuller, N.R.; Pearson, S.; et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: A randomised controlled trial. Lancet 2011, 378, 1485–1492. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.; Maiberger, M.; Donegan, S.; Kaplan, N.C.; Kinner, P. Cost Effectiveness of Two Lifestyle Interventions in the Vermont WISEWOMAN Program. Prev. Chronic Dis. 2019, 16, 180417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, K.; Lewis, A.; Beach, J.; Denley, J.; Adab, P.; Deeks, J.J.; Daley, A.; Aveyard, P. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011, 343, d6500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luszczynska, A.; Sobczyk, A.; Abraham, C. Planning to lose weight: Randomized controlled trial of an implementation intention prompt to enhance weight reduction among overweight and obese women. Health Psychol. 2007, 26, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Marrero, D.G.; Palmer, K.N.; Phillips, E.O.; Miller-Kovach, K.; Foster, G.D.; Saha, C.K. Comparison of Commercial and Self-Initiated Weight Loss Programs in People With Prediabetes: A Randomized Control Trial. Am. J. Public Health 2016, 106, 949–956. [Google Scholar] [CrossRef]
- Pinto, A.M.; Fava, J.L.; Hoffmann, D.A.; Wing, R.R. Combining behavioral weight loss treatment and a commercial program: A randomized clinical trial. Obesity 2013, 21, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.A.; Bray, G.A.; Ryan, D.H. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 2015, 23, 2319–2320. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.D.; Ryan, D.H.; Donato, K.A.; Apovian, C.M.; Ard, J.D.; Yanovski, S.Z. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity 2014, 22 (Suppl. S2), S5–S39. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 2, 187–194. [Google Scholar] [CrossRef]
- Witjaksono, F.; Jutamulia, J.; Annisa, N.G.; Prasetya, S.I.; Nurwidya, F. Comparison of low calorie high protein and low calorie standard protein diet on waist circumference of adults with visceral obesity and weight cycling. BMC Res. Notes 2018, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramie, J.J.; Barber, J.L.; Sarzynski, M.A. Effects of exercise on HDL functionality. Curr. Opin. Lipidol. 2019, 30, 16–23. [Google Scholar] [CrossRef]
- Layman, D.K.; Boileau, R.A.; Erickson, D.J.; Painter, J.E.; Shiue, H.; Sather, C.; Christou, D.D. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003, 133, 411–417. [Google Scholar] [CrossRef]
- Hu, T.; Mills, K.T.; Yao, L.; Demanelis, K.; Eloustaz, M.; Yancy, W.S., Jr.; Kelly, T.N.; He, J.; Bazzano, L.A. Effects of Low-Carbohydrate Diets Versus Low-Fat Diets on Metabolic Risk Factors: A Meta-Analysis of Randomized Controlled Clinical Trials. Am. J. Epidemiol. 2012, 176, S44–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifton, P.M.; Keogh, J. Metabolic effects of high-protein diets. Curr. Atheroscler. Rep. 2007, 9, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.; Griffin, B.; Millward, D.; Delooy, A.; Fox, K.; Baic, S.; Bonham, M.; Wallace, J.; Macdonald, I.; Taylor, M.; et al. Comparison of the effects of four commercially available weight-loss programmes on lipid-based cardiovascular risk factors. Pub. Health Nutr. 2009, 12, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Mardock, M.; Kresta, J.; Byrd, M.; Oliver, J.; Baetge, C.; Simbo, S.; Jung, Y.; Koozehchian, M.; Khanna, D.; Lockard, B.; et al. Effects of intermittent dieting during resistance training in women II: Health markers. Med. Sci. Sports Exerc. 2011, 43 (Suppl. S1), 471–472. [Google Scholar] [CrossRef]
- Dalton, R.; Baetge, C.; Lockard, B.; Levers, K.; Galvan, E.; Jagim, A.; Simbo, S.; Byrd, M.; Jung, Y.; Oliver, J.; et al. Analysis of efficacy and cost effectiveness of popular weight loss and fitness programs. J. Inter. Soc. Sports Nutr. 2013, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, E.A.; Kruger, E. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity 2014, 22, 1942–1951. [Google Scholar] [CrossRef]
- Lymer, S.; Schofield, D.; Cunich, M.; Lee, C.M.Y.; Fuller, N.; Caterson, I.; Colagiuri, S. The Population Cost-Effectiveness of Weight Watchers with General Practitioner Referral Compared with Standard Care. Obesity 2018, 26, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Vaid, I.; Wigington, C.; Borbely, D.; Ferry, P.; Manheim, D. WISEWOMAN: Addressing the Needs of Women at High Risk for Cardiovascular Disease. J. Womens Health 2011, 20, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Gudzune, K.A.; Doshi, R.S.; Mehta, A.K.; Chaudhry, Z.W.; Jacobs, D.K.; Vakil, R.M.; Lee, C.J.; Bleich, S.N.; Clark, J.M. Efficacy of commercial weight-loss programs: An updated systematic review. Ann. Intern. Med. 2015, 162, 501–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| WW | CV | Mean | p-Level | |
|---|---|---|---|---|
| (n = 27) | (n = 24) | (n = 51) | ||
| Age (years) | 34.0 ± 6.1 | 35.1 ± 9.2 | 34.5 ± 7.7 | 0.633 |
| Weight (kg) | 89.5 ± 13.6 | 90.8 ± 15.6 | 90.1 ± 14.5 | 0.770 |
| Height (cm) | 162.6 ± 6.7 | 163.0 ± 7.4 | 162.8 ± 7.0 | 0.843 |
| BMI (kg/m2) | 33.9 ± 5.2 | 34.1 ± 5.1 | 34.0 ± 5.1 | 0.899 |
| Body fat (%) | 45.1 ± 7.3 | 48.1 ± 6.5 | 46.5 ± 7.1 | 0.138 |
| Weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 6 | 10 | 12 | 16 | Effect | p-Level | ηp2 | ||
| Glucose (mg/dL) | WW | 89.1 ± 9 | 89.2 ± 8 | 85.3 ± 8 | 87.4 ± 6 | 88.1 ± 5 | 89.0 ± 7 | Group | 0.658 | 0.004 |
| CV | 91.5 ± 15 | 88.7 ± 14 | 86.9 ± 9 | 90.7 ± 19 | 88.4 ± 8 | 87.9 ± 16 | Time | 0.237 | 0.028 | |
| G × T | 0.628 | 0.012 | ||||||||
| Insulin (IU/mL) | WW | 11.0 ± 5 | 10.7 ± 4 | 10.7 ± 8 | 11.2 ± 6 | 11.1 ± 5 | 10.6 ± 5 | Group | 0.000 | 0.307 |
| CV | 20.5 ± 12 | 15.9 ± 7 † | 16.8 ± 8 | 14.5 ± 7 † | 17.4 ± 7 | 16.1 ± 8 † | Time | 0.214 | 0.029 | |
| G × T | 0.240 | 0.028 | ||||||||
| HOMA | WW | 2.40 ± 1 | 2.35 ± 1 | 2.27 ± 2 | 2.39 ± 1 | 2.42 ± 1 | 2.35 ± 1 | Group | 0.000 | 0.310 |
| CV | 4.71 ± 3 | 3.44 ± 1 † | 3.66 ± 2 | 3.23 ± 2 † | 3.77 ± 1 † | 3.56 ± 2 † | Time | 0.134 | 0.036 | |
| G × T | 0.174 | 0.032 | ||||||||
| Leptin (ng/mL) | WW | 51.3 ± 22 | 46.8 ± 27 † | 43.2 ± 32 | 45.5 ± 35 † | 45.5 ± 30 | 42.3 ± 29 † | Group | 0.837 | 0.001 |
| CV | 66.3 ± 27 b | 53.0 ± 26 | 50.0 ± 22 | 42.1 ± 27 | 48.3 ± 23 | 52.7 ± 25 | Time | 0.001 | 0.094 | |
| G × T | 0.123 | 0.036 | ||||||||
| Total Cholesterol (mg/dL) | WW | 186 ± 30 | 172 ± 27 † | 172 ± 24 † | 175 ± 31 † | 177 ± 29 | 173 ± 30 † | Group | 0.328 | 0.020 |
| CV | 175 ± 30 | 164 ± 31 † | 168 ± 33 | 167 ± 31 | 173 ± 30 | 171 ± 33 | Time | 0.001 | 0.091 | |
| G × T | 0.589 | 0.015 | ||||||||
| LDLc (mg/dL) | WW | 110 ± 26 b | 103 ± 24 † | 102 ± 23 † | 105 ± 25 | 101 ± 24 † | 96 ± 22 † | Group | 0.837 | 0.001 |
| CV | 97.7 ± 26 | 90.5 ± 29 | 93.3 ± 28 | 94.0 ± 25 | 97.0 ± 27 | 92.9 ± 28 | Time | 0.010 | 0.062 | |
| G × T | 0.260 | 0.026 | ||||||||
| HDLc (mg/dL) | WW | 52.1 ± 14 | 46.8 ± 13 † | 46.3 ± 10 † | 46.4 ± 12 † | 48.0 ± 11 † | 50.8 ± 14 | Group | 0.699 | 0.003 |
| CV | 51.7 ± 15 | 49.7 ± 12 | 51.7 ± 13 | 52.7 ± 14 b | 52.3 ± 14 | 54.3 ± 16 | Time | 0.000 | 0.115 | |
| G × T | 0.017 | 0.059 | ||||||||
| CHL:HDL Ratio | WW | 3.77 ± 1.0 | 3.87 ± 1.0 | 3.88 ± 1.0 b | 3.96 ± 1.1 † | 3.94 ± 1.3 | 3.64 ± 1.2 | Group | 0.959 | 0.000 |
| CV | 3.60 ± 1.1 | 3.48 ± 0.9 | 3.42 ± 1.0 | 3.42 ± 0.9 b | 3.55 ± 1.0 | 3.45 ± 1.0 | Time | 0.214 | 0.029 | |
| G × T | 0.139 | 0.034 | ||||||||
| Triglycerides (mg/dL) | WW | 120 ± 44 | 122 ± 49 | 115 ± 35 | 122 ± 53 | 123 ± 49 | 131 ± 99 | Group | 0.505 | 0.009 |
| CV | 126 ± 59 | 113 ± 58 | 115 ± 50 | 109 ± 49 † | 119 ± 54 | 124 ± 63 | Time | 0.363 | 0.021 | |
| G × T | 0.678 | 0.010 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lockard, B.; Mardock, M.; Oliver, J.M.; Byrd, M.; Simbo, S.; Jagim, A.R.; Kresta, J.; Baetge, C.C.; Jung, Y.P.; Koozehchian, M.S.; et al. Comparison of Two Diet and Exercise Approaches on Weight Loss and Health Outcomes in Obese Women. Int. J. Environ. Res. Public Health 2022, 19, 4877. https://doi.org/10.3390/ijerph19084877
Lockard B, Mardock M, Oliver JM, Byrd M, Simbo S, Jagim AR, Kresta J, Baetge CC, Jung YP, Koozehchian MS, et al. Comparison of Two Diet and Exercise Approaches on Weight Loss and Health Outcomes in Obese Women. International Journal of Environmental Research and Public Health. 2022; 19(8):4877. https://doi.org/10.3390/ijerph19084877
Chicago/Turabian StyleLockard, Brittanie, Michelle Mardock, Jonathan M. Oliver, Mike Byrd, Sunday Simbo, Andrew R. Jagim, Julie Kresta, Claire C. Baetge, Yanghoon Peter Jung, Majid S. Koozehchian, and et al. 2022. "Comparison of Two Diet and Exercise Approaches on Weight Loss and Health Outcomes in Obese Women" International Journal of Environmental Research and Public Health 19, no. 8: 4877. https://doi.org/10.3390/ijerph19084877









