A Method for the Analysis of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry
Abstract
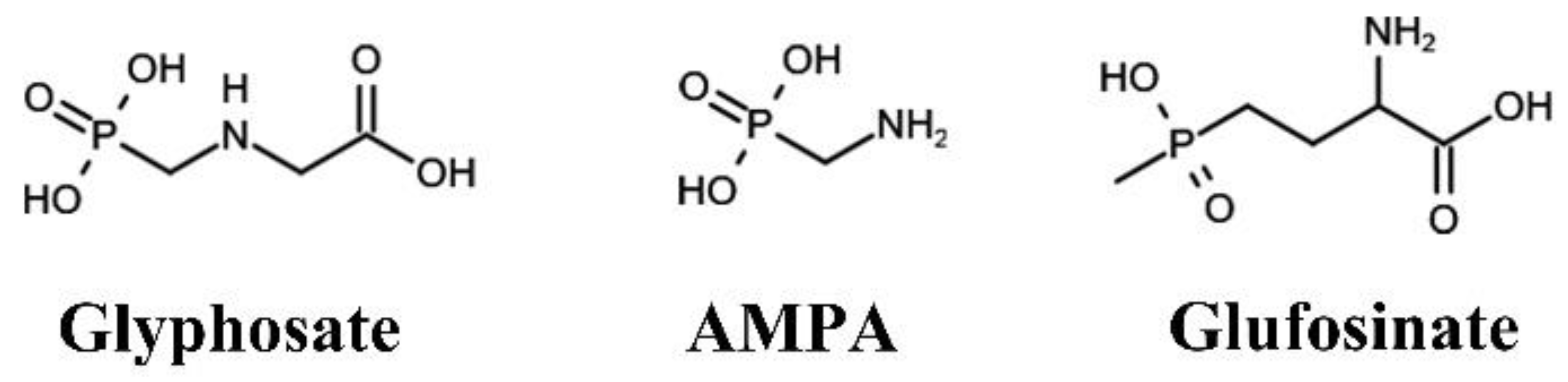
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Sample Preparation
2.3. LC–MS/MS
2.4. Method Validation
3. Results and Discussion
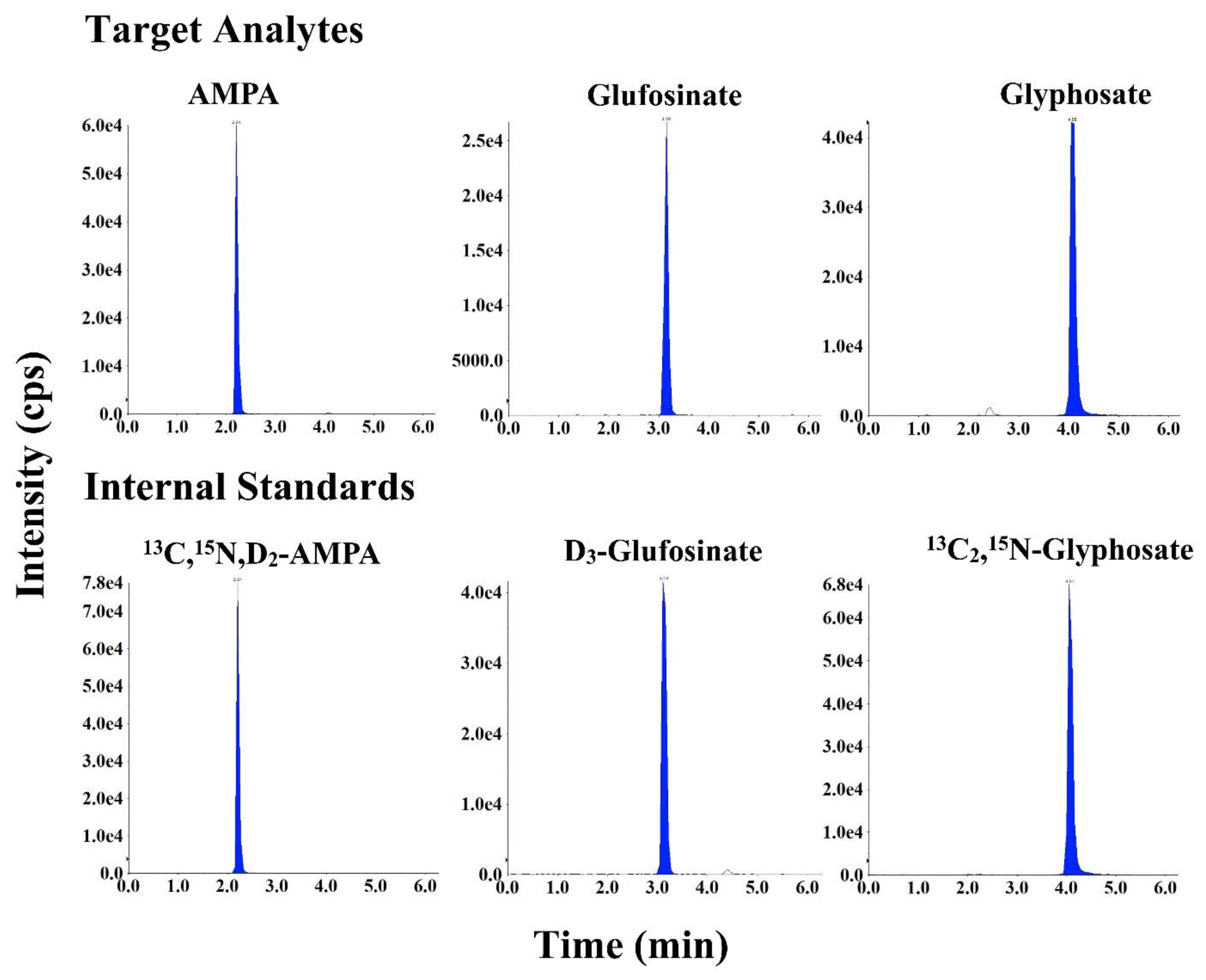
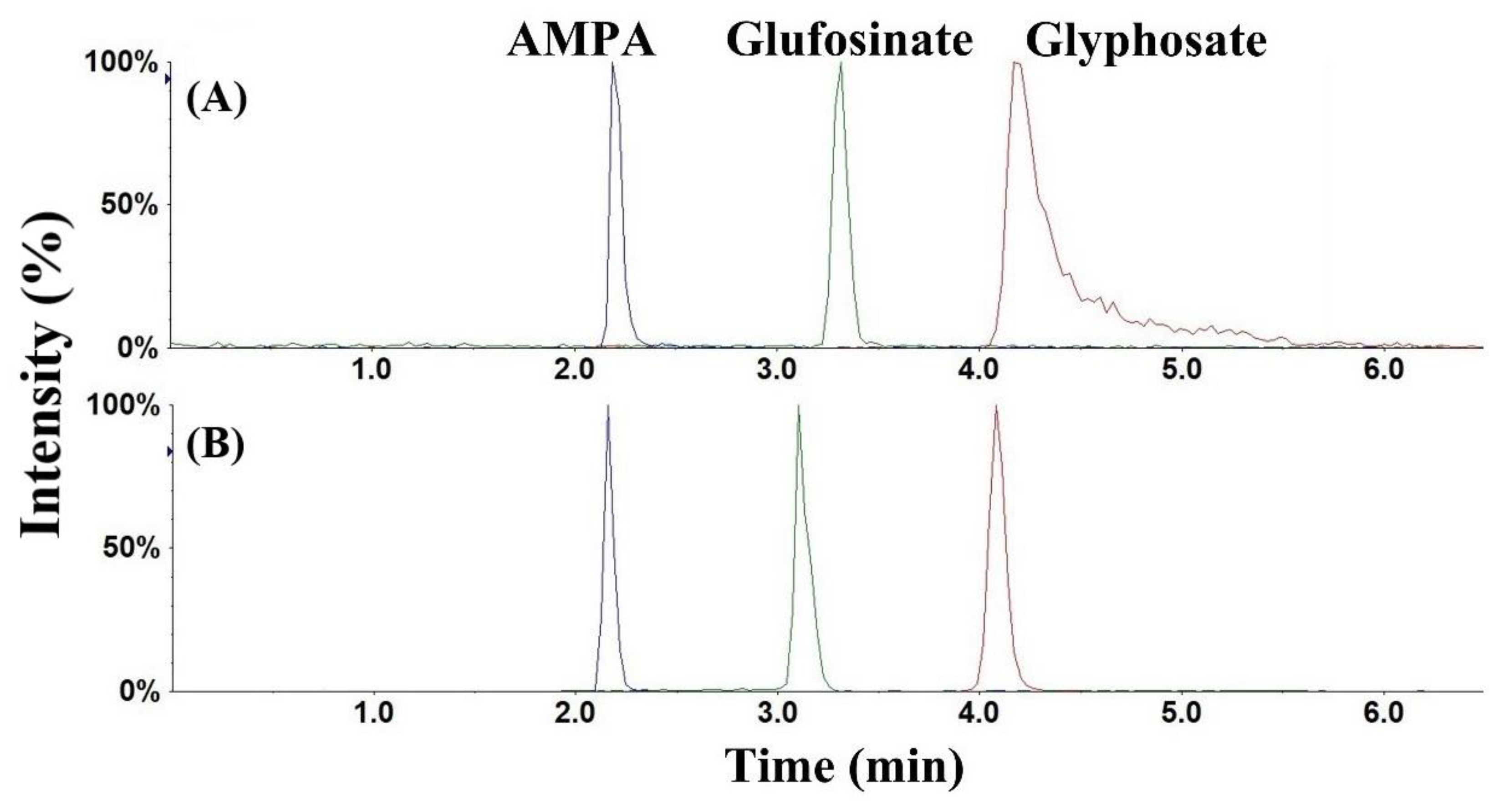
3.1. Chromatography and Mass Spectrometry
3.2. Optimization of Sample Cleanup
3.3. Method Validation
| Sample Type | Analytes | Internal Standards | Sample Cleanup | LC Condition | MS/MS | LODs/LOQs (ng/mL) | Ref(s). |
|---|---|---|---|---|---|---|---|
| Cation-exchange column | |||||||
| Human urine | Glyphosate, AMPA | D2,13C3-Glyphosate; 13C,15N-AMPA | SPE cleanup using Oasis HLB cartridges (3 cc, 60 mg) | Bio-Rad Micro-Guard Cation-H+ column (30 × 4.6 mm, 9 µm); A: water B: 0.2% formic acid in ACN | Glyphosate: 168/63, 168/150; AMPA: 110/79, 110/63; | IDL: 0.02–0.04 IQL: 0.05–0.1 a | [25] |
| Human urine | Glyphosate, AMPA | 13C3,15N-Glyphosate; D2,13C15N-AMPA | Diluted with 0.1% formic acid, shaken and centrifuged | Bio-Rad Micro-guard Cation-H+ column (30 × 4.6 mm, 9 µm); A: 0.1% formic acid in water B: ACN | Glyphosate: 168/63, 168/79 AMPA: 110/63, 110/79 | MDL: 0.023–0.041 MQL: 0.1 | [36,44] |
| Human urine | Glyphosate, AMPA | 13C2,15N-Glyphosate; D2,13C,15N-AMPA | Refer to Jensen et al. [36] | Glyphosate: 168/63, 168/126 AMPA: 110/63, 110/79 | MDL: 0.05–0.09 MQL: 0.20 | [45] | |
| Anion-exchange column | |||||||
| Pet urine (dogs and cats) | Glyphosate, AMPA | 13C2,15N-Glyphosate; D2,13C,15N-AMPA | (1) Sample basified with 1% NH4OH; (2) Cleanup using Oasis MAX SPE cartridge (3 cc, 60 mg) | Dionex IonPac AS21 IC column (250 × 2.0 mm, 7 µm); Isocratic elution: 1% formic acid in ACN/water (5/95) | Glyphosate: 168/63, 168/79; AMPA: 110/63, 110/79; | MDL: 0.15 a MQL: 0.5 | [26] |
| Human urine | Glyphosate | 13C2,15N-Glyphosate | Sample diluted with 1% formic acid, then filtered | Dionex IonPac AS 21 (250 × 2.0 mm, 7 µm); Isocratic elution: 1% formic acid in ACN/water (5:95) | MDL: 0.1 a MQL: 0.33 | [46] | |
| Hybrid-phase column | |||||||
| Human urine | Glyphosate | 13C2-N-Glyphosate | − | Obelisc-N mixed-mode column (100 × 2.1 mm, 5 µm); Isocratic elution: 1% formic acid in water | 168/63, 168/81 | MDL: 0.1 MQL: 0.5 | [47] |
| Reversed-phase column | |||||||
| Human urine | Glyphosate | 13C2,15N-Glyphosate | (1) Sample diluted with water; (2) SPE: Strata SAX (1 cc, 100 mg) | Zorbax SB-C3 column (150 × 4.6 mm, 5 µm), or Zorbax XDB-C8 column (150 × 4.6 mm, 5 µm) A: 1% acetic acid in water B: ACN | 168/63 | MQL: 0.5 | [11,40,41,42] |
| Human urine | Glyphosate | 13C2,15N-Glyphosate | (1) Sample diluted with H2O; (2) SPE: ISOLUTE-96 SCX plate (25 mg), then ISOLUTE-96 NH2 plate (100 mg) | Scherzo SM-C18 MF column (100 × 2 mm, 3 µm) A: MeOH/water (5:95) containing 0.1% formic acid and 5 µM medronic acid B: MeOH and 20 mM ammonium formate (20:80) with 5 µM medronic acid | 170/88, 170/60, 170/42 b | MDL: 0.1 MQL: 0.3 | [39] |
| Human urine | Glyphosate | − | − | SUPELCO Discovery C18 column (50 × 2.1 mm, 5 µm) | − | MDL: 1 MQL: 2 | [43] |
| Reversed-phase column (Ion-pairing chromatography) | |||||||
| Human urine | Glyphosate, Glufosinate | 13C2,15N-Glyphosate; D3-Glufosinate | (1) Dilute with water; (2) Back wash with dichloromethane | Agilent ZORBAX SB-Aq column (100 × 2.1 mm, 1.8 µm) A: 15 mM HFBA; B: ACN | Glyphosate: 170/88, 170/60; Glufosinate: 182/136, 182/119 | MDL: 0.1 | [31] |
| Human urine | Glyphosate, AMPA | 13C3,15N-Glyphosate; 13C,15N-AMPA | Sample diluted with HFBA | Gemini C6-Phenyl column (150 × 4.6 mm, 5 µm) A: 15 mM HFBA in water B: ACN | Glyphosate: 170/88, 170/60; AMPA: 112/30 b | MDL: 2.5 MQL: 5 | [27] |
| Reversed-phase column (derivatization) | |||||||
| Human urine | Glyphosate. AMPA, Glufosinate | 13C3,15N-Glyphosate; D2,13C,15N-AMPA; D3-Glufosinate | (1) EDTA pre-treatment; (2) SPE: Strata-X; (3) Derivatization; (4) SPE: C18 | Kinetex C18 column A: 5 mM AmAc (pH 9):MeOH:ACN (90:5:5) B: MeOH: ACN (50:50) | ESI positive, SIM mode Glyphosate-Fmoc: 392.08937 AMPA-Fmoc: 334.083890 Glufosinate-Fmoc: 404.12575 | MDL: 0.1–0.3 | [32] |
| Glyphosate | AMPA | Glufosinate | |
|---|---|---|---|
| R in solvent a | 0.9995 | 0.9999 | 0.9999 |
| R in matrix b | 0.9982 | 0.9993 | 0.9998 |
| IDL (ng/mL) | 0.01 | 0.01 | 0.01 |
| IQL (ng/mL) | 0.05 | 0.05 | 0.05 |
| MDL (ng/mL) | 0.14 | 0.12 | 0.12 |
| MQL (ng/mL) | 0.48 | 0.40 | 0.41 |
| Spike recovery (%), n = 6 | |||
| 0.5 (ng/mL) | 84.4 ± 9.6 | 109 ± 8 | 110 ± 8 |
| 1 (ng/mL) | 79.1 ± 9.8 | 100 ± 6 | 106 ± 10 |
| 5 (ng/mL) | 81.2 ± 8.4 | 100 ± 4 | 119 ± 5 |
| Matrix effect (%) | –14.4 | 13.2 | 22.2 |
| Intra-day variation (%), n = 6 | |||
| 0.5 (ng/mL) | 8.83 | 10.8 | 10.1 |
| 1 (ng/mL) | 3.13 | 3.19 | 3.46 |
| 5 (ng/mL) | 7.18 | 9.10 | 4.61 |
| Inter-day variation (%), n = 6 | |||
| 0.5 (ng/mL) | 9.09 | 7.51 | 12.9 |
| 1 (ng/mL) | 9.25 | 5.93 | 10.6 |
| 5 (ng/mL) | 7.22 | 7.85 | 6.61 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Maggi, F.; la Cecilia, D.; Tang, F.H.M.; McBratney, A. The global environmental hazard of glyphosate use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Thompson, T.S.; van den Heever, J.P.; Limanowka, R.E. Determination of glyphosate, AMPA, and glufosinate in honey by online solid-phase extraction-liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 434–446. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. Int. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Connolly, A.; Coggins, M.A.; Koch, H.M. Human biomonitoring of glyphosate exposures: State-of-the-art and future research challenges. Toxics 2020, 8, 60. [Google Scholar] [CrossRef]
- Conrad, A.; Schroter-Kermani, C.; Hoppe, H.W.; Ruther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults—Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 4302. [Google Scholar] [CrossRef]
- Connolly, A.; Jones, K.; Basinas, I.; Galea, K.S.; Kenny, L.; McGowan, P.; Coggins, M.A. Exploring the half-life of glyphosate in human urine samples. Int. J. Hyg. Environ. Health 2019, 222, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Uren Webster, T.M.; Santos, E.M. Global transcriptomic profiling demonstrates induction of oxidative stress and of compensatory cellular stress responses in brown trout exposed to glyphosate and Roundup. BMC Genom. 2015, 16, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C.; Seralini, G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Silver, M.K.; Fernandez, J.; Tang, J.; McDade, A.; Sabino, J.; Rosario, Z.; Velez Vega, C.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Prenatal exposure to glyphosate and its environmental degradate, aminomethylphosphonic acid (AMPA), and preterm birth: A nested case-control study in the PROTECT cohort (Puerto Rico). Environ. Health Perspect. 2021, 129, 57011. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Hoerlein, G. Glufosinate (Phosphinothricin), A Natural Amino Acid with Unexpected Herbicidal Properties. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 1994; pp. 73–145. [Google Scholar]
- Schulte-Hermann, R.; Wogan, G.N.; Berry, C.; Brown, N.A.; Czeizel, A.; Giavini, E.; Holmes, L.B.; Kroes, R.; Nau, H.; Neubert, D.; et al. Analysis of reproductive toxicity and classification of glufosinate-ammonium. Regul. Toxicol. Pharmacol. 2006, 44, S1–S76. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for for glufosinate according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2015, 13, 3950–4030. [Google Scholar] [CrossRef]
- IARC. Some Organophosphate Insecticides and Herbicides. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2015; Volume 112, ISBN 978-92-832-0178-6. Available online: https://publications.iarc.fr/549 (accessed on 1 January 2022).
- ECHA. Glyphosate Not Classified as a Carcinogen by ECHA. 2017. Available online: https://echa.europa.eu/-/glyphosate-not-classified-as-a-carcinogen-by-echa (accessed on 1 January 2022).
- EPA. Glyphosate Issue Paper: Evaluation of Carcinogenic Potential; United States Environmental Protection Agency: Washington, DC, USA, 2016. Available online: https://www.epa.gov/sites/production/files/2016-09/documents/glyphosate_issue_paper_evaluation_of_carcincogenic_potential.pdf (accessed on 25 June 2020).
- Hori, Y.; Fujisawa, M.; Shimada, K.; Sato, M.; Kikuchi, M.; Honda, M.; Hirose, Y. Quantitative determination of glufosinate in biological samplesby liquid chromatography with ultraviolet detection after p-nitrobenzoyl derivatization. J. Chromatogr. B 2002, 767, 255–262. [Google Scholar] [CrossRef]
- Hori, Y.; Fujisawa, M.; Shimada, K.; Sato, M.; Honda, M.; Hirose, Y. Enantioselective analysis of glufosinate using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase liquid chromatography. J. Chromatogr. B 2002, 776, 191–198. [Google Scholar] [CrossRef]
- Zoller, O.; Rhyn, P.; Rupp, H.; Zarn, J.A.; Geiser, C. Glyphosate residues in Swiss market foods: Monitoring and risk evaluation. Food Addit. Contam. B Surveill. 2018, 11, 83–91. [Google Scholar] [CrossRef]
- Zoller, O.; Rhyn, P.; Zarn, J.A.; Dudler, V. Urine glyphosate level as a quantitative biomarker of oral exposure. Int. J. Hyg. Environ. Health 2020, 228, 113526. [Google Scholar] [CrossRef] [PubMed]
- Karthikraj, R.; Kannan, K. Widespread occurrence of glyphosate in urine from pet dogs and cats in New York State, USA. Sci. Total Environ. 2019, 659, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Jaikwang, P.; Junkuy, A.; Sapbamrer, R.; Seesen, M.; Khacha-ananda, S.; Mueangkhiao, P.; Wunnapuk, K. A dilute-and-shoot LC–MS/MS method for urinary glyphosate and AMPA. Chromatographia 2020, 83, 467–475. [Google Scholar] [CrossRef]
- Hao, C.; Morse, D.; Morra, F.; Zhao, X.; Yang, P.; Nunn, B. Direct aqueous determination of glyphosate and related compounds by liquid chromatography/tandem mass spectrometry using reversed-phase and weak anion-exchange mixed-mode column. J. Chromatogr. A 2011, 1218, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Granby, K.; Johannesen, S.; Vahl, M. Analysis of glyphosate residues in cereals using liquid chromatography-mass spectrometry (LC-MS/MS). Food Addit. Contam. 2003, 20, 692–698. [Google Scholar] [CrossRef]
- Wang, K.C.; Chen, S.M.; Hsu, J.F.; Cheng, S.G.; Lee, C.K. Simultaneous detection and quantitation of highly water-soluble herbicides in serum using ion-pair liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2008, 876, 211–218. [Google Scholar] [CrossRef]
- Tsao, Y.C.; Lai, Y.C.; Liu, H.C.; Liu, R.H.; Lin, D.L. Simultaneous determination and quantitation of paraquat, diquat, glufosinate and glyphosate in postmortem blood and urine by LC-MS-MS. J. Anal. Toxicol. 2016, 40, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.A.; Li, X.; Lai, J.F. Analysis of glyphosate, aminomethylphosphonic acid, and glufosinate from human urine by HRAM LC-MS. Anal. Bioanal. Chem. 2020, 412, 8313–8324. [Google Scholar] [CrossRef]
- Martinez-Moral, M.P.; Kannan, K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ. Int. 2019, 123, 382–389. [Google Scholar] [CrossRef]
- Myint, K.T.; Uehara, T.; Aoshima, K.; Oda, Y. Polar anionic metabolome analysis by nano-LC/MS with a metal chelating agent. Anal. Chem. 2009, 81, 7766–7772. [Google Scholar] [CrossRef]
- Guo, H.; Riter, L.S.; Wujcik, C.E.; Armstrong, D.W. Direct and sensitive determination of glyphosate and aminomethylphosphonic acid in environmental water samples by high performance liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. A 2016, 1443, 93–100. [Google Scholar] [CrossRef]
- Jensen, P.K.; Wujcik, C.E.; McGuire, M.K.; McGuire, M.A. Validation of reliable and selective methods for direct determination of glyphosate and aminomethylphosphonic acid in milk and urine using LC-MS/MS. J. Environ. Sci. Health B 2016, 51, 254–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, J.J.; Potter, O.G.; Chu, T.W.; Yin, H. Improved LC/MS methods for the analysis of metal-sensitive analytes using medronic acid as a mobile phase additive. Anal. Chem. 2018, 90, 9457–9464. [Google Scholar] [CrossRef] [PubMed]
- Nagatomi, Y.; Yoshioka, T.; Yanagisawa, M.; Uyama, A.; Mochizuki, N. Simultaneous LC-MS/MS analysis of glyphosate, glufosinate, and their metabolic products in beer, barley tea, and their ingredients. Biosci. Biotechnol. Biochem. 2013, 77, 2218–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, H.; Hamada, R.; Saito, I.; Nakane, K.; Sawa, R.; Ukai, M.; Shibata, E.; Sato, M.; Kamijima, M.; Ueyama, J. Optimization and validation of a highly sensitive method for determining glyphosate in human urine by solid-phase extraction and liquid chromatography with tandem mass spectrometry: A methodological study. Environ. Health Prev. Med. 2020, 25, 83. [Google Scholar] [CrossRef]
- Connolly, A.; Jones, K.; Galea, K.S.; Basinas, I.; Kenny, L.; McGowan, P.; Coggins, M. Exposure assessment using human biomonitoring for glyphosate and fluroxypyr users in amenity horticulture. Int. J. Hyg. Environ. Health 2017, 220, 1064–1073. [Google Scholar] [CrossRef]
- Connolly, A.; Basinas, I.; Jones, K.; Galea, K.S.; Kenny, L.; McGowan, P.; Coggins, M.A. Characterising glyphosate exposures among amenity horticulturists using multiple spot urine samples. Int. J. Hyg. Environ. Health 2018, 221, 1012–1022. [Google Scholar] [CrossRef]
- Connolly, A.; Leahy, M.; Jones, K.; Kenny, L.; Coggins, M.A. Glyphosate in Irish adults—A pilot study in 2017. Environ. Res. 2018, 165, 235–236. [Google Scholar] [CrossRef]
- Mesnage, R.; Moesch, C.; Grand, R.L.G.; Lauthier, G.; Vendômois, J.S.d.; Gress, S.; Séralini, G.-E. Glyphosate exposure in a farmer’s family. J. Environ. Prot. 2012, 3, 1001–1003. [Google Scholar] [CrossRef] [Green Version]
- Fagan, J.; Bohlen, L.; Patton, S.; Klein, K. Organic diet intervention significantly reduces urinary glyphosate levels in U.S. children and adults. Environ. Res. 2020, 189, 109898. [Google Scholar] [CrossRef]
- Soukup, S.T.; Merz, B.; Bub, A.; Hoffmann, I.; Watzl, B.; Steinberg, P.; Kulling, S.E. Glyphosate and AMPA levels in human urine samples and their correlation with food consumption: Results of the cross-sectional KarMeN study in Germany. Arch. Toxicol. 2020, 94, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasande, L.; Aldana, S.I.; Trachtman, H.; Kannan, K.; Morrison, D.; Christakis, D.A.; Whitlock, K.; Messito, M.J.; Gross, R.S.; Karthikraj, R.; et al. Glyphosate exposures and kidney injury biomarkers in infants and young children. Environ. Pollut. 2020, 256, 113334. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Gerona, R.R.; Proctor, C.; Friesen, M.; Ashby, J.L.; Reiter, J.L.; Lui, Z.; Winchester, P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health 2018, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Van Eeckhaut, A.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC-MS/MS assays: Evaluation of matrix effects. J. Chromatogr. B 2009, 877, 2198–2207. [Google Scholar] [CrossRef]

| OSEQAS Round 2021-01 | ||||||
| ID | Glyphosate (ng/mL) | AMPA (ng/mL) | ||||
| Assigned value | Acceptable range | Our results | Assigned value | Acceptable range | Our results | |
| OS-U-E2101 | 1.24 | 0.713–1.77 | 1.55 | 1.65 | 0.954–2.35 | 1.65 |
| OS-U-E2102 | 1.67 | 0.949–2.39 | 2.18 | 6.62 | 3.72–9.52 | 6.94 |
| OS-U-E2103 | 2.23 | 1.24–3.22 | 2.80 | 2.2 | 1.31–3.09 | 2.15 |
| G-EQUAS Round 66/2020 | ||||||
| ID | Glyphosate (ng/mL) | |||||
| Assigned value | Acceptable range | Our results | ||||
| 9A | 0.64 | 0.49–0.79 | 0.78 | |||
| 9B | 1.2 | 0.93–1.47 | 1.37 | |||
| ID | Location | Glyphosate (ng/mL) | AMPA (ng/mL) | Glufosinate (ng/mL) |
|---|---|---|---|---|
| 1 | Iowa, USA | 0.54 | 0.50 | <MDL |
| 2 | Iowa, USA | <MDL | <MDL | <MDL |
| 3 | Iowa, USA | 0.91 | 0.39 (<MQL) | <MDL |
| 4 | Iowa, USA | 3.04 | 1.21 | <MDL |
| 5 | Iowa, USA | 0.36 (<MQL) | 0.44 | <MDL |
| 6 | Iowa, USA | <MDL | <MDL | <MDL |
| 7 | Iowa, USA | 0.70 | 0.85 | <MDL |
| 8 | Iowa, USA | 1.40 | 1.42 | <MDL |
| 9 | Iowa, USA | 0.49 | 0.19 (<MQL) | <MDL |
| 10 | Iowa, USA | 0.27 (<MQL) | 0.20 (<MQL) | <MDL |
| 11 | New York, USA | <MDL | <MDL | <MDL |
| 12 | New York, USA | <MDL | <MDL | <MDL |
| 13 | New York, USA | <MDL | <MDL | <MDL |
| 14 | New York, USA | <MDL | <MDL | <MDL |
| 15 | New York, USA | <MDL | <MDL | <MDL |
| 16 | New York, USA | <MDL | <MDL | <MDL |
| 17 | New York, USA | <MDL | <MDL | <MDL |
| 18 | New York, USA | <MDL | <MDL | <MDL |
| 19 | New York, USA | <MDL | <MDL | <MDL |
| 20 | New York, USA | 0.53 | 0.39 (<MQL) | <MDL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-M.; Kannan, K. A Method for the Analysis of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. Int. J. Environ. Res. Public Health 2022, 19, 4966. https://doi.org/10.3390/ijerph19094966
Li Z-M, Kannan K. A Method for the Analysis of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. International Journal of Environmental Research and Public Health. 2022; 19(9):4966. https://doi.org/10.3390/ijerph19094966
Chicago/Turabian StyleLi, Zhong-Min, and Kurunthachalam Kannan. 2022. "A Method for the Analysis of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry" International Journal of Environmental Research and Public Health 19, no. 9: 4966. https://doi.org/10.3390/ijerph19094966
APA StyleLi, Z.-M., & Kannan, K. (2022). A Method for the Analysis of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. International Journal of Environmental Research and Public Health, 19(9), 4966. https://doi.org/10.3390/ijerph19094966







