Anti-Inflammatory and Anti-Bacterial Potential of Mulberry Leaf Extract on Oral Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts
2.2. Used Microorganisms and Culture
2.3. Cell Culture
2.4. Cell Viability Test
2.5. Flow Cytometry
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Bacterial Growth
2.8. Statistical Analysis
3. Results
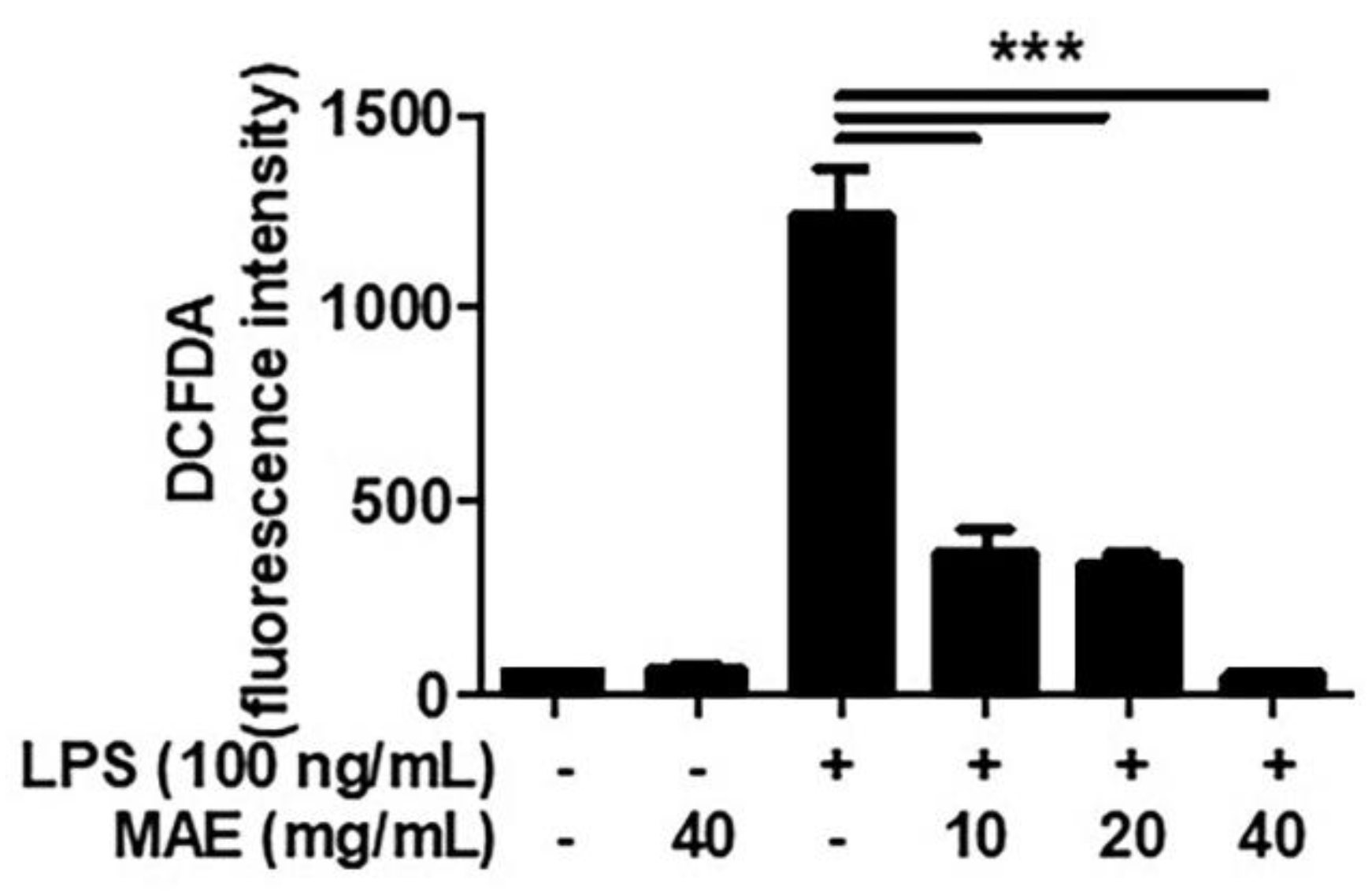
3.1. MAE Decreased the Production of ROS in LPS-Stimulated THP-1 and Oral Cells
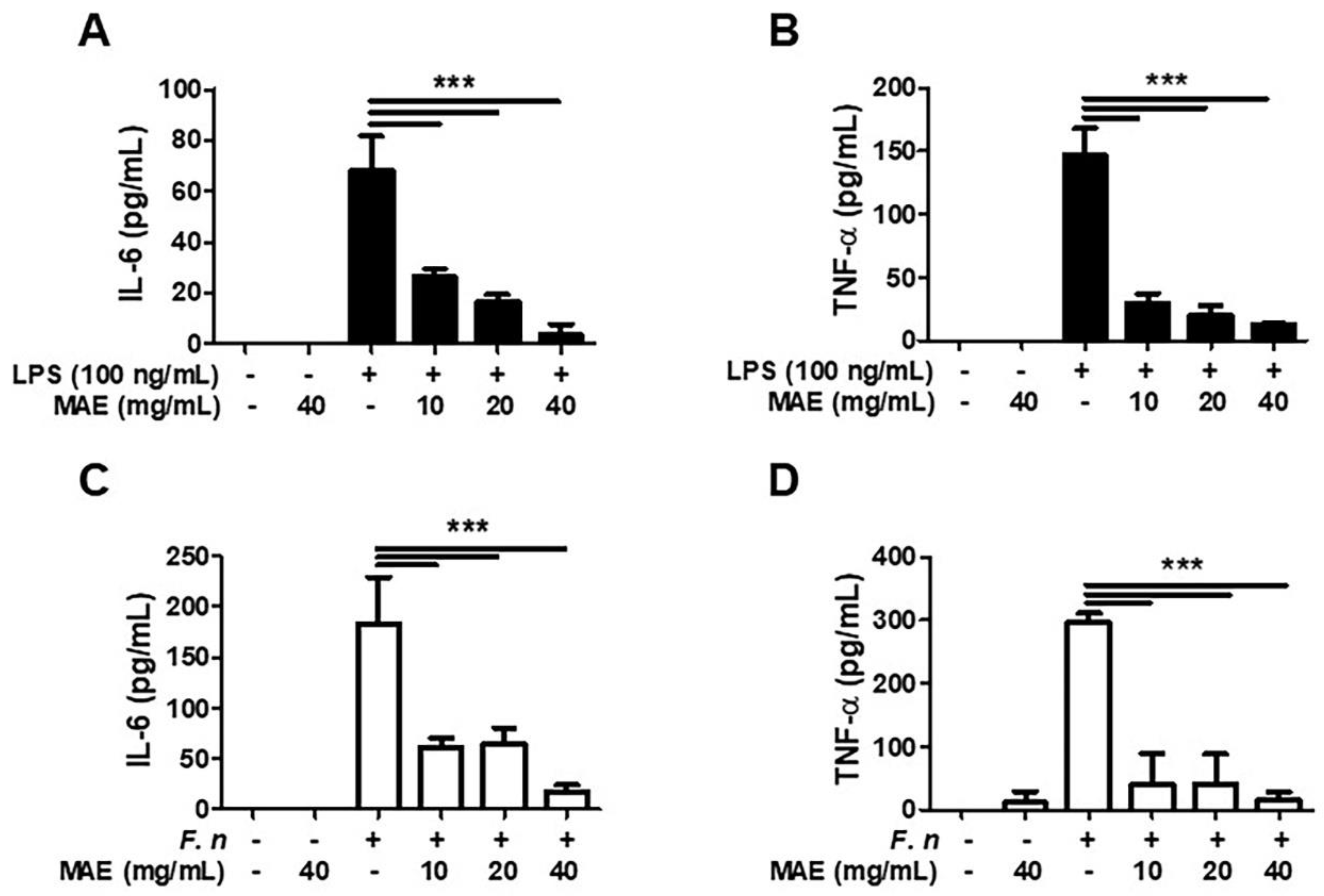
3.2. MAE Decreased the Generation of IL-6 and TNF-α in LPS/F. nucleatum-Stimulated THP-1 Cells
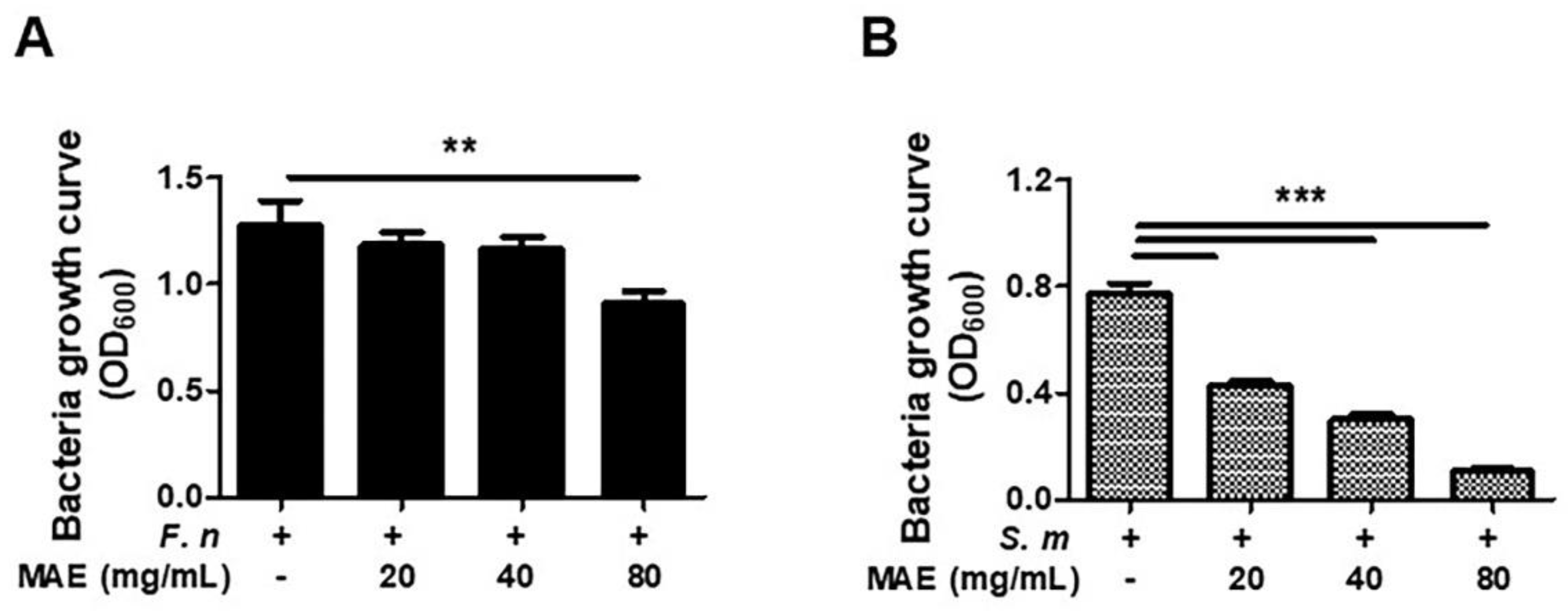
3.3. MAE Has Antibiotic Effects against F. nucleatum and S. mutans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Smith, E.G.; Spatafora, G.A. Gene regulation in s. Mutans: Complex control in a complex environment. J. Dent. Res. 2012, 91, 133–141. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Z.; Liu, Q.H.; Liu, Z.; Tang, J.W.; Chua, E.G.; Li, F.; Xiong, X.S.; Wang, M.M.; Wen, P.B.; Shi, X.Y.; et al. Ethanol extract of mulberry leaves partially restores the composition of intestinal microbiota and strengthens liver glycogen fragility in type 2 diabetic rats. BMC Complement. Med. Ther. 2021, 21, 172. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [CrossRef]
- Islam, B.; Khan, S.N.; Haque, I.; Alam, M.; Mushfiq, M.; Khan, A.U. Novel anti-adherence activity of mulberry leaves: Inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba. J. Antimicrob. Chemother. 2008, 62, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Gunjal, S.; Ankola, A.V.; Bhat, K. In vitro antibacterial activity of ethanolic extract of Morus alba leaf against periodontal pathogens. Indian J. Dent. Res. 2015, 26, 533–536. [Google Scholar] [CrossRef]
- Lee, H.-J.; Guo, H.-Y.; Lee, S.-K.; Jeon, B.-H.; Jun, C.-D.; Lee, S.-K.; Park, M.-H.; Kim, E.-C. Effects of nicotine on proliferation, cell cycle, and differentiation in immortalized and malignant oral keratinocytes. J. Oral Pathol. Med. 2005, 34, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Illeperuma, R.P.; Park, Y.J.; Kim, J.M.; Bae, J.Y.; Che, Z.M.; Son, H.K.; Han, M.R.; Kim, K.-M.; Kim, J. Immortalized gingival fibroblasts as a cytotoxicity test model for dental materials. J. Mater. Sci. Mater. Med. 2012, 23, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Poppenga, R.H. Herbal medicine: Potential for intoxication and interactions with conventional drugs. Clin. Tech. Small Anim. Pract. 2002, 17, 6–18. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liang, C.; Hu, J.; Yu, Z. Safety evaluation of mulberry leaf extract: Acute, subacute toxicity and genotoxicity studies. Regul. Toxicol. Pharmacol. 2018, 95, 220–226. [Google Scholar] [CrossRef]
- Hong, M.; Lu, M.; Qian, Y.; Wei, L.; Zhang, Y.; Pan, X.; Li, H.; Chen, H.; Tang, N. A 90-day Sub-chronic Oral Toxicity Assessment of Mulberry Extract in Sprague Dawley Rats. Inq. J. Health Care Organ. Provis. Financ. 2021, 58, 469580211056044. [Google Scholar] [CrossRef]
- Sohn, H.-Y.; Son, K.; Kwon, C.-S.; Kang, S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The mulberry (Morus alba L.) fruit—A review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Chan, K.-C.; Yang, M.-Y.; Lin, M.-C.; Lee, Y.-J.; Chang, W.-C.; Wang, C.-J. Mulberry Leaf Extract Inhibits the Development of Atherosclerosis in Cholesterol-Fed Rabbits and in Cultured Aortic Vascular Smooth Muscle Cells. J. Agric. Food Chem. 2013, 61, 2780–2788. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.N.H.; Nam, N.H.; Elhady, M.T.; Tran, L.; Hassan, O.G.; Sadik, M.; Tien, P.T.M.; Elshafei, G.A.; Huy, N.T. Effects of Mulberry on The Central Nervous System: A Literature Review. Curr. Neuropharmacol. 2020, 19, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sakamoto, Y.; Mizowaki, Y.; Iwagaki, Y.; Kimura, T.; Nakagawa, K.; Miyazawa, T.; Tsuduki, T. Intake of mulberry 1-deoxynojirimycin prevents colorectal cancer in mice. J. Clin. Biochem. Nutr. 2017, 61, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-J.; Min, T.-R.; Chi, G.-Y.; Choi, Y.-H.; Park, S.-H. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018, 505, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Proietto, A.I.; O’Keeffe, M.; Gartlan, K.; Wright, M.D.; Shortman, K.; Wu, L.; Lahoud, M.H. Differential production of inflammatory chemokines by murine dendritic cell subsets. Immunobiology 2004, 209, 163–172. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.; Savelkoul, H.F.J.; Wichers, H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin inhibits lps-induced macrophage m1 polarization via nf-small ka, cyrillicb and mapk pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Lee, J.S.; Lee, K.R.; Kim, Y.E.; Baek, N.I.; Hong, E.K. Effects of mulberry ethanol extracts on hydrogen peroxide-induced oxidative stress in pancreatic beta-cells. Int. J. Mol. Med. 2014, 33, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.-H.; Hoang, T.-H.; Jung, E.-S.; Jung, S.-J.; Chae, S.-W.; Chae, H.-J. Mulberry Extract Attenuates Endothelial Dysfunction through the Regulation of Uncoupling Endothelial Nitric Oxide Synthase in High Fat Diet Rats. Nutrients 2019, 11, 978. [Google Scholar] [CrossRef] [Green Version]
- Yiemwattana, I.; Chaisomboon, N.; Jamdee, K. Antibacterial and Anti-inflammatory Potential of Morus alba Stem Extract. Open Dent. J. 2018, 12, 265–274. [Google Scholar] [CrossRef]
- Özan, F.; Tepe, B.; Polat, Z.A.; Er, K. Evaluation of in vitro effect of Morus rubra (red mulberry) on survival of periodontal ligament cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, e66–e69. [Google Scholar] [CrossRef]
- Manji, F.; Dahlén, G.; Fejerskov, O. Caries and Periodontitis: Contesting the Conventional Wisdom on Their Aetiology. Caries Res. 2018, 52, 548–564. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, B.E.C.; Filho, A.P.R.; Burne, R.A.; Zeng, L. The Route of Sucrose Utilization by Streptococcus mutans Affects Intracellular Polysaccharide Metabolism. Front. Microbiol. 2021, 12, 33. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Nativel, B.; Couret, D.; Giraud, P.; Meilhac, O.; D’Hellencourt, C.L.; Viranaïcken, W.; Da Silva, C.R. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci. Rep. 2017, 7, 15789. [Google Scholar] [CrossRef] [PubMed]
- Yiemwattana, I.; Kaomongkolgit, R.; Wirojchanasak, S.; Chaisomboon, N. Morus alba stem extract suppresses matrix metalloproteinases (mmp)-1, mmp-9, and tissue inhibitors of metalloproteinase (timp)-1 expression via inhibition of ikappabalpha degradation induced by porphyromonas gingivalis lps signal in thp-1 cells. Eur. J. Dent. 2019, 13, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, F.M.; Liu, H.L. Fusobacterium nucleatum and colorectal cancer: A review. World J. Gastrointest. Oncol. 2018, 10, 71–81. [Google Scholar] [CrossRef]
- Strauss, J.; Kaplan, G.; Beck, P.L.; Rioux, K.; Panaccione, R.; DeVinney, R.; Lynch, T.; Allen-Vercoe, E. Invasive potential of gut mucosa-derived fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm. Bowel Dis. 2011, 17, 1971–1978. [Google Scholar] [CrossRef]
- Thé, J.; Ebersole, J.L. Rheumatoid factor (RF) distribution in periodontal disease. J. Clin. Immunol. 1991, 11, 132–142. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kwon, J.-S.; Choi, E.-H.; Kim, K.-M. The Effects of Non-Thermal Atmospheric Pressure Plasma treated Titanium Surface on Behaviors of Oral Soft Tissue Cells. Sci. Rep. 2018, 8, 15963. [Google Scholar] [CrossRef] [Green Version]
- Marton, A.; Kolozsi, C.; Kusz, E.; Olah, Z.; Letoha, T.; Vizler, C.; Pecze, L. Propylene-glycol aggravates LPS-induced sepsis through production of TNF-α and IL-6. Iran. J. Immunol. IJI 2014, 11, 113–122. [Google Scholar]
- Song, R.; Kim, J.; Yu, D.; Park, C.; Park, J. Kinetics of IL-6 and TNF-α changes in a canine model of sepsis induced by endotoxin. Veter Immunol. Immunopathol. 2012, 146, 143–149. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Bowers, W.J. Tumor Necrosis Factor-alpha and the Roles it Plays in Homeostatic and Degenerative Processes Within the Central Nervous System. J. Neuroimmune Pharmacol. 2011, 7, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and nf-kappab signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kang, K.-H. Anti-Inflammatory and Anti-Bacterial Potential of Mulberry Leaf Extract on Oral Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 4984. https://doi.org/10.3390/ijerph19094984
Kim D, Kang K-H. Anti-Inflammatory and Anti-Bacterial Potential of Mulberry Leaf Extract on Oral Microorganisms. International Journal of Environmental Research and Public Health. 2022; 19(9):4984. https://doi.org/10.3390/ijerph19094984
Chicago/Turabian StyleKim, Dokyeong, and Kyung-Hee Kang. 2022. "Anti-Inflammatory and Anti-Bacterial Potential of Mulberry Leaf Extract on Oral Microorganisms" International Journal of Environmental Research and Public Health 19, no. 9: 4984. https://doi.org/10.3390/ijerph19094984





