A Successful New Case of Twin Pregnancy in a Patient with Swyer Syndrome—An Up-to-Date Review on the Incidence and Outcome of Twin/Multiple Gestations in the Pure 46,XY Gonadal Dysgenesis
Abstract
:1. Introduction
2. A Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swyer, G.I. Male pseudohermaphroditism: A hitherto undescribed form. Br. Med. J. 1955, 2, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Michala, L.; Goswami, D.; Creighton, S.; Conway, G. Swyer syndrome: Presentation and outcomes. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.J.; Anvret, M.; Hall, K.; Scherer, G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature 1990, 348, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.uptodate.com/contents/causes-of-primary-amenorrhea (accessed on 21 October 2021).
- Ayres-de-Campos, D.; Spong, C.Y.; Chandraharan, E. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int. J. Gynecol. Obstet. 2015, 131, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Nicolaides, K.H.; Wright, D.; Syngelaki, A.; Wright, A.; Akolekar, R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound. Obstet. Gynecol. 2021, 52, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Fetal Medicine Foundation. The Fetal Medicine Foundation. Accredit 11-13+6 Week Scan. 2010. Available online: https://fetalmedicine.org/research/assess/growth (accessed on 21 October 2021).
- Arboleda, V.A.; Sandberg, D.E.; Vilain, E. DSDs: Genetics, underlying pathologies and psychosexual differentiation. Nat. Rev. Endocrinol. 2014, 10, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, L.; Holterhus, P.M.; Wessel, L.; Hiort, O. Patients with disorders of sex development (DSD) at risk of gonadal tumour development: Management based on laparoscopic biopsy and molecular diagnosis. BJU Int. 2012, 110, E958–E965. [Google Scholar] [CrossRef]
- Rocha, V.B.; Guerra-Júnior, G.; Marques-de-Faria, A.P.; de Mello, M.P.; Maciel-Guerra, A.T. Complete gonadal dysgenesis in clinical practice: The 46,XY karyotype accounts for more than one third of cases. Fertil. Steril. 2011, 96, 1431–1434. [Google Scholar] [CrossRef]
- Huang, H.; Wang, C.; Tian, Q. Gonadal tumour risk in 292 phenotypic female patients with disorders of sex development containing Y chromosome or Y-derived sequence. Clin. Endocrinol. 2017, 86, 621–627. [Google Scholar] [CrossRef]
- Massanyi, E.Z.; Dicarlo, H.N.; Migeon, C.J.; Gearhart, J.P. Review and management of 46,XY disorders of sex development. J. Pediatr. Urol. 2013, 9, 368–379. [Google Scholar] [CrossRef]
- Available online: https://www.eshre.eu/-/media/sitecore-files/Guidelines/POI/ESHRE-guideline_POI-2015_summary_01122015.pdf?la=en&hash=04AFBA15E89FAAD0D81BB41DD64403BE6B04678E (accessed on 21 October 2021).
- Dural, O.; Evruke, I.; Can, S.; Yasa, C.; Ugurlucan, F.G.; Akhan, S.E. Atypical Presentation of Swyer Syndrome. J. Pediatr. Adolesc. Gynecol. 2019, 32, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Coutin, A.S.; Hamy, A.; Fondevilla, M.; Sauvigny, B.; Paineau, J.; Visset, J. La dysgenesie gonadidique pure a 46 XY. J. Gynecol. Obstet. Biol. Reprod. 1996, 25, 792–796. [Google Scholar]
- Chen, M.J.; Yang, J.H.; Mao, T.L.; Ho, H.N.; Yang, Y.S. Successful pregnancy in a gonadectomised woman with 46, XY gonadal dysgenesis and gonadoblastoma. Fertil. Steril. 2005, 84, 217. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hoorn, M.L.; Lashley, E.E.; Bianchi, D.W.; Claas, F.H.; Schonkeren, C.M.; Scherjon, S.A. Clinicaland immunologic aspects of oocyte donation pregnancies: A systematic review. Hum. Reprod. Update 2010, 16, 704–712. [Google Scholar] [CrossRef]
- Savasi, V.M.; Mandia, L.; Laoreti, A.; Cetin, I. Maternal and fetal outcomes in oocyte donation pregnancies. Hum. Reprod. Update 2016, 22, 620–633. [Google Scholar] [CrossRef]
- Taneja, J.; Ogutu, D.; Ah-Moye, M. Rare successful pregnancy in a patient with Swyer Syndrome. Case Rep. Women’s Health 2014, 12, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Sauer, M.V.; Lobo, R.A.; Paulson, R.J. Successful twin pregnancy after embryo donation to a patient with XY gonadal dysgenesis. Am. J. Obstet. Gynecol. 1989, 161, 380–381. [Google Scholar] [CrossRef]
- Kan, A.K.; Abdalla, H.I.; Oskarsson, T. Two successful pregnancies in a 46, XY patient. Hum. Reprod. 1997, 12, 1434–1435. [Google Scholar] [CrossRef] [Green Version]
- Ko, P.C.; Peng, H.H.; Soong, Y.K.; Chang, S.D. Triplet pregnancy complicated with one hydatidiform mole and preeclampsia in a 46, XY female with gonadal dysgenesis. Taiwan J. Obstet. Gynecol. 2007, 46, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Tulic, I.; Tulic, L.; Micic, J. Pregnancy in patient with Swyer syndrome. Fertil. Steril. 2011, 95, 1789. [Google Scholar] [CrossRef]
- Creatsas, G.; Deligeoroglou, E.; Tsimaris, P.; Pantos, K.; Kreatsa, M. Successful pregnancy in a Swyer syndrome patient with preexisting hypertension. Fertil. Steril. 2011, 96, e83–e85. [Google Scholar] [CrossRef]
- Selvaraj, K.; Ganesh, V.; Selvaraj, P. Successful pregnancy in a patient with a 46,XY karyotype. Fertil. Steril. 2002, 78, 419–420. [Google Scholar] [CrossRef]
- Lutjen, P.; Trounson, A.; Leeton, J.; Findlay, J.; Wood, C.; Renou, P. The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature 1984, 307, 174–175. [Google Scholar] [CrossRef]
- Cornet, D.; Alvarez, S.; Antoine, J.; Tibi, C.; Mandelbaum, J.; Plachot, M.; Salat-Baroux, J. Pregnancies following ovum donation in gonadal dysgenesis. Hum. Reprod. 1990, 5, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Dirnfeld, M.; Bider, D.; Abramovicia, H.; Calderon, I.; Blumenfeld, Z. Subsequent successful pregnancy and delivery after intracytoplasmic sperm injection in a patient with XY gonadal dysgenesisms. Eur. J Obstet. Gynecol. Reprod. Biol. 2000, 88, 101–102.1789. [Google Scholar] [CrossRef]
- Plante, B.J.; Fritz, M.A. A case report of successful pregnancy in a patient with pure 46, XY gonadal dysgenesis. Fertil. Steril. 2008, 90, 2015.e1–2015.e2. [Google Scholar] [CrossRef]
- Bardeguez, A.D.; De Ziegler, D.; Weiss, G. Multifetal pregnancy in a gonadal dysgenesis mosaic. Obstet. Gynecol. 1990, 76, 502–504. [Google Scholar]
- Bianco, S.; Agrifoglio, V.; Mannino, F.; Cefalù, E.; Cittadini, E. Successful pregnancy in a pure gonadal dysgenesis with karyotype 46,XY patient (Swyer’s syndrome) following oocyte donation and hormonal treatment. Acta Eur. Fertil. 1992, 23, 37–38. [Google Scholar] [PubMed]
- Frydman, R.; Parneix, I.; Fries, N.; Testart, J.; Raymond, J.P.; Bouchard, P. Pregnancy in a 46, XY patient. Fertil. Steril. 1988, 50, 813–814. [Google Scholar] [CrossRef]
- De Santis, M.; Spagnuolo, T.; Barone, D.; Licameli, A. Successful twin pregnancy in a 46,XY pure gonadal dysgenesis. J. Obstet. Gynaecol. 2013, 33, 737–738. [Google Scholar] [CrossRef]
- Gao, S.S.; Sheng, Y.; Li, Y.; Li, M.; Yang, H.J.; Chen, Z.J. Twin delivery of a 46,XY gonadal dysgenetic woman following vitrified oocytes donation. Chin. Med. J. Engl. 2011, 124, 1109–1110. [Google Scholar] [PubMed]
- Murtinger, M.; Hradecký, L.; Spitzer, D.; Zech, N.H. Unexpected labor and successful twin birth to a pure gonadal dysgenetic woman. Arch. Gynecol. Obstet. 2013, 288, 1425–1426. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Nadkarni, P.K.; Singh, P.P.; Nadkarni, A.A. Successful pregnancy outcome in a case of Swyer Syndrome with hypertension and morbid obesity. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 2061–2064. [Google Scholar] [CrossRef]
- Shah, J.S.; Viteri, O.A.; Longo, M.; Abdallah, M.; Sibai, B. Twin gestation in a Swyer syndrome patient with superimposed pre-eclampsia. J. Obstet. Gynecol. 2018, 38, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.; Knap-Wielgus, W.; Grymowicz, M.; Smolarczyk, R. Two successful pregnancies after in vitro fertilisation with oocyte donation in a patient with Swyer syndrome—A case report. Prz. Menopauzalny 2021, 20, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bajaj, R.; Jindal, U.N. A rare case of Swyer syndrome in two sisters with successful pregnancy outcome in both. J. Hum. Reprod. Sci. 2019, 12, 267–269. [Google Scholar] [PubMed]

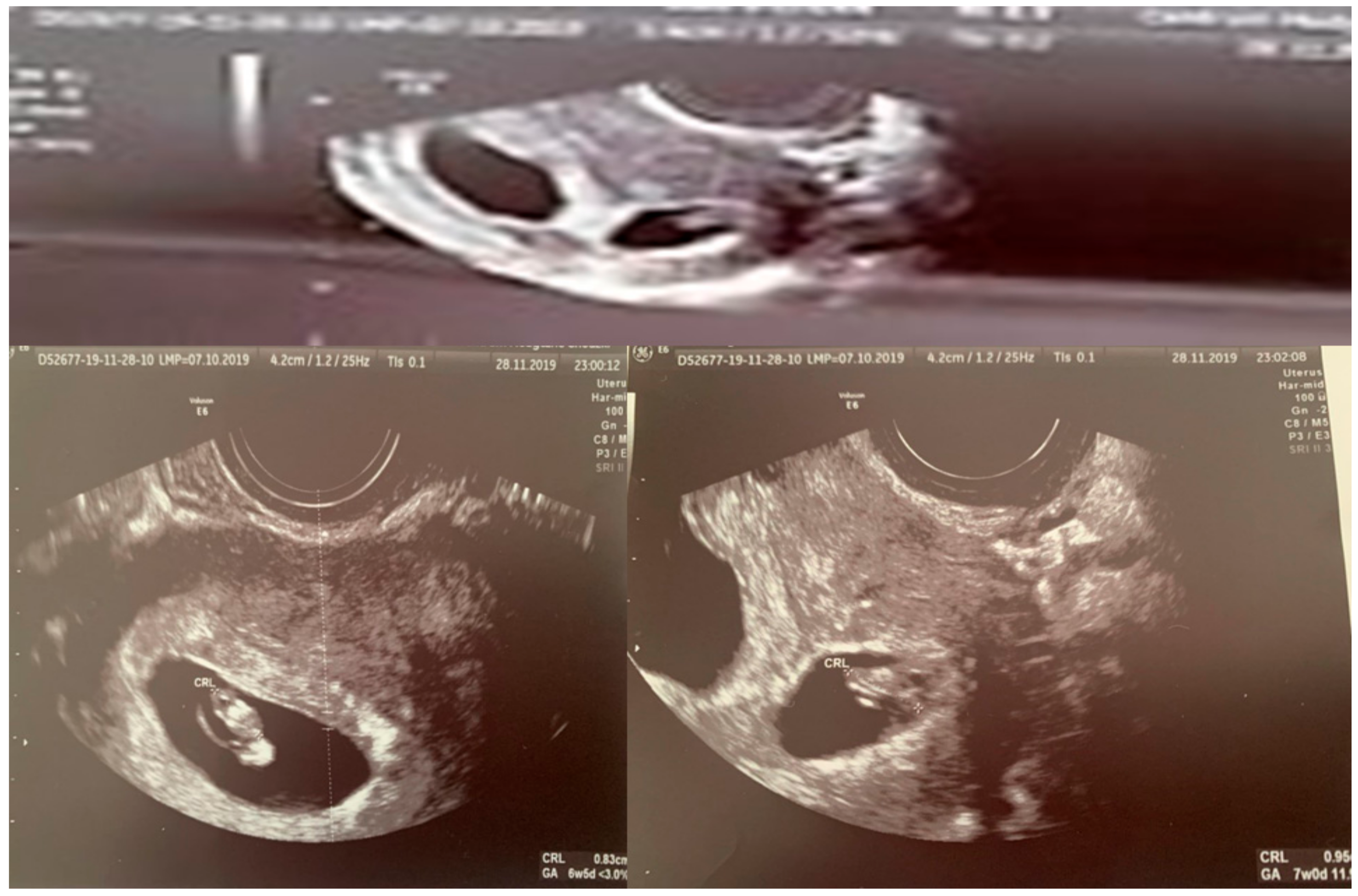
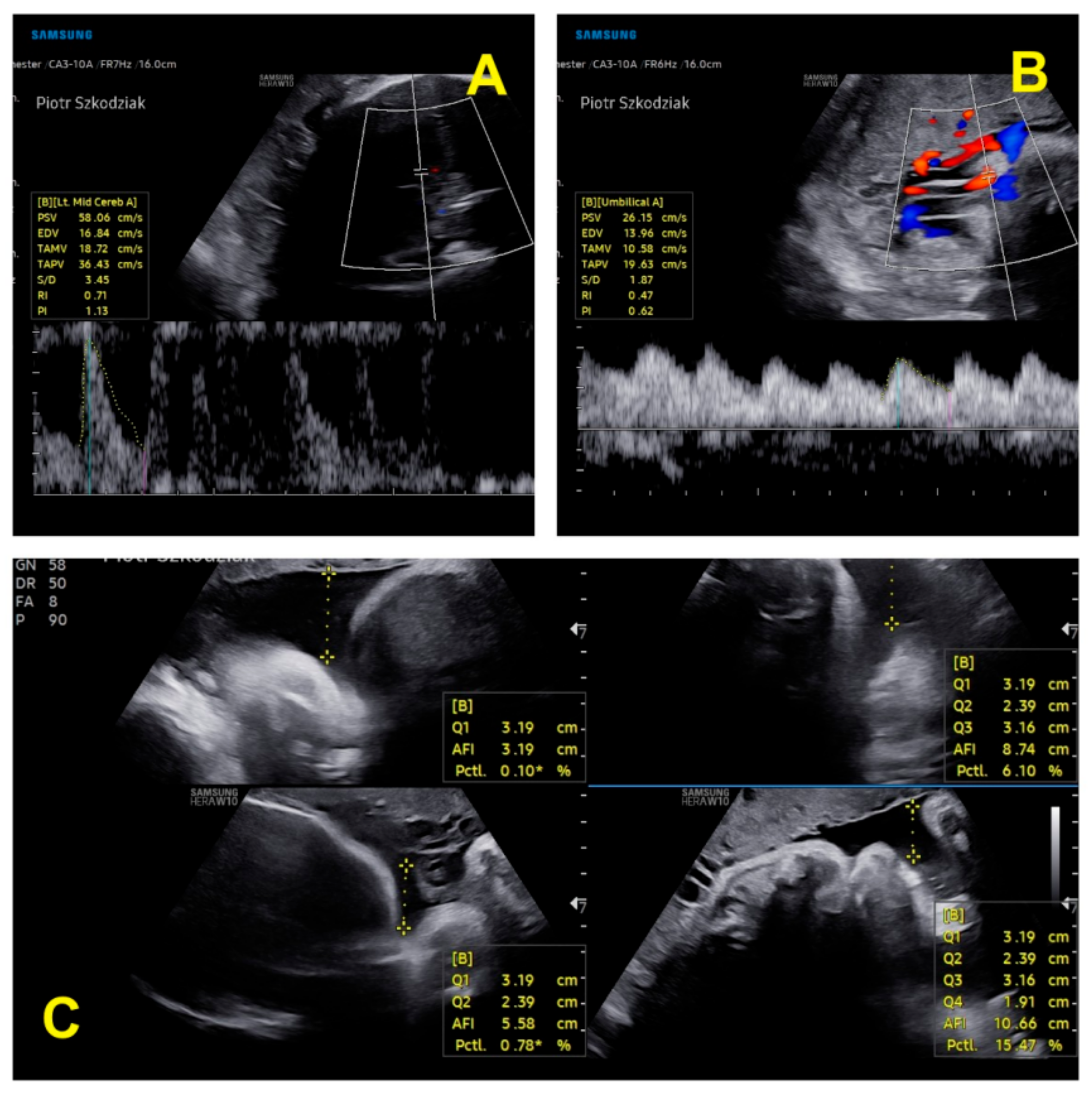


| Parameter | I Trimester | II Trimester | III Trimester |
|---|---|---|---|
| Estrofem (estradiol) orally | 2 mg + 2 mg + 2 mg | 2 mg + 0 + 2 mg | 1 mg + 0 + 1 mg |
Progesterone
| 1 × 25 mg i.m./s.c. for every third day 200 mg + 200 mg + 200 mg | 1 × 25 mg i.m./s.c. for every fifth day 200 mg + 0 + 200 mg | 1 × 25 mg i.m./s.c. for one in week 0 + 0 + 200 mg |
| Encorton (prednisone) | 5 mg | 5 mg | 2.5 mg |
| Aspirin (acetylsalicylic acid) | 150 mg | 150 mg | 150 mg until its discontinuation at week 32 |
| Other drugs | Pimafucin (Natamycin 100 mg) 1 × 1 tablet intravaginally for 6 days | Monural (fosfomycin 3 g) orally | Essentiale forte (phospholipids 300 mg − 1 tablet) 3 × 2 tablets |
| Parameter | Twin 1 | Twin 2 |
|---|---|---|
| Chorionicity | Dichorionic Diamniotic | |
| CRL(crown–rump length) (mm) | 49.2–11w5d | 51.30–11w6 |
| BPD (biparietal diameter) (mm) | 14.5–12w0 | 17.2–12w4d |
| FHR/min (fetal heart rate) | 160 | 161 |
| NT (mm) (nuchal translucency) | 1.42 | 1.2 |
| Parameter | Twin 1 in Trimester II | Twin 2 in Trimester II | Twin 1 in Trimester III | Twin 2 in Trimester III |
|---|---|---|---|---|
| BPD (mm) (biparietal diameter) | 58.7–23w1d | 57–22w5d | 76.4–29w3d | 75.3–29w0d |
| HC (mm) (head circumference) | 221–24w1d | 207–22w6d | 275.3–29w3d | 261–27w6d |
| FL (femur length) (mm) | 40.8 mm–23w1d | 37.6–22w0 | 54.9–29w2d | 51.6–27w5 |
| AC (abdominal circumference) (mm) | 180 | 174 | 240 | 221.5 |
| AFI (amniotic fluid) | normal | normal | normal | normal |
| FHR/min (fetal heart rate) | 153 | 160 | 150 | 148 |
| EFW (g) (estimated fetal weight) | 570 | 493 | 1298 | 1057 |
| EFW discrepancy | 13.5% (assessment at 22 weeks 6 days) | 18.6% (assessment at 28 weeks 0 days) | ||
| Authors | Individuals | Age at Delivery | Pregnancy Single/Multiple | Mode of Delivery | Week at Delivery | Complications |
|---|---|---|---|---|---|---|
| Taneja et al., 2014 [19] | 1 | 39 | single | cesarean delivery | 39 | pre-eclampsia |
| Selvaraj et al., 2002 [25] | 1 | 27 | single | cesarean delivery | not known | none |
| Lutjen P. 1984 [26] | 1 | 25 | single | cesarean delivery | 38 | none |
| Cornet et al., 1990 [27] | 1 | 25 | single | cesarean delivery | 36-41 | none |
| Bardeguez et al., 1990 [30] | 1 | unknown | triplet | cesarean delivery | Unknown | unknown |
| Bianco et al., 1992 [31] | 1 | unknown | single | not known | Unknown | unknown |
| Kan et al., 1997 [21] | 2 | 30, 32 ( the same woman, two attempts) | 1. single 2. twin | 1. vaginal delivery 2. cesarean delivery | 1. >37 2. 36 weeks | 1. pre-eclampsia 2. blood pressure elevated |
| Sauer et al., 1989 [20] | 1 | 28 | twin | cesarean delivery | 35 | pregnancy-induced hypertension |
| Ko et al., 2007 [22] | 1 | 35 | triplet (twins alive, boy and girl) | cesarean delivery | 33 | pre-eclampsia proteinuria, mola hydatidosa during pregnancy |
| Dirnfeld et al., 2000 [28] | 2 | 30 (the same woman, two attempts) | 1. single 2. twin | cesarean delivery (both) | 1. 41 2. term delivery | 1. spontaneous demise of one fetus occurred at 19 weeks of pregnancy 2. none |
| Plante and Fritz et al., 2008 [29] | 1 | 27 | single | cesarean delivery | 38 | none |
| Tulic et al., 2011 [23] | 1 | 30 | single | cesarean delivery | 39 | reduced amniotic fluid |
| Chen et al., 2005 [15] | 1 | 31 | twin | cesarean delivery | 36 | none |
| Frydman et al., 1988 [34] | 1 | 37 | single | cesarean delivery | 41 | none |
| Michala et al., 2008 [2] | 3 | unknown | single | 1 and 2 vaginal deliveries 3. cesarean delivery | 1, 2. unknown 3. 36 | 1, 2. none 3. pre-eclampsia |
| Creatsas et al., 2011 [24] | 1 | 35 | single | cesarean delivery | 38 | oligohydramnios and abdominal circumference below the 5th percentile |
| De Santis et al., 2013 [33] | 1 | 27 | twin | cesarean delivery | 35 | none |
| Gao et al., 2011 [34] | 1 | 32 | twin | cesarean delivery | Unknown | none |
| Murtinger et al., 2013 [35] | 1 | 30 | twin | cesarean delivery | 36 | none |
| Kalra et al., 2016 [36] | 1 | 27 | single | cesarean delivery | 34 | severe pre-eclampsia |
| Shah et al., 2018 [37] | 1 | 32 | twin | cesarean delivery | 34 | hypertension |
| Urban et al. [38] | 2 | 32,34 | single | cesarean delivery | 40, 39 | no medical evidence of uterine dilatation efficacy |
| Gupta et al. [39] | 2 | 25, unknown(younger)—two sisters | Single, single | Details not known | Details not known | Details not known |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, I.; Jaszczuk, I.; Gogacz, M.; Szkodziak, P.; Paszkowski, T.; Skorupska, K.; Ciebiera, M.; Skrzypczak, M. A Successful New Case of Twin Pregnancy in a Patient with Swyer Syndrome—An Up-to-Date Review on the Incidence and Outcome of Twin/Multiple Gestations in the Pure 46,XY Gonadal Dysgenesis. Int. J. Environ. Res. Public Health 2022, 19, 5027. https://doi.org/10.3390/ijerph19095027
Winkler I, Jaszczuk I, Gogacz M, Szkodziak P, Paszkowski T, Skorupska K, Ciebiera M, Skrzypczak M. A Successful New Case of Twin Pregnancy in a Patient with Swyer Syndrome—An Up-to-Date Review on the Incidence and Outcome of Twin/Multiple Gestations in the Pure 46,XY Gonadal Dysgenesis. International Journal of Environmental Research and Public Health. 2022; 19(9):5027. https://doi.org/10.3390/ijerph19095027
Chicago/Turabian StyleWinkler, Izabela, Ilona Jaszczuk, Marek Gogacz, Piotr Szkodziak, Tomasz Paszkowski, Katarzyna Skorupska, Michał Ciebiera, and Maciej Skrzypczak. 2022. "A Successful New Case of Twin Pregnancy in a Patient with Swyer Syndrome—An Up-to-Date Review on the Incidence and Outcome of Twin/Multiple Gestations in the Pure 46,XY Gonadal Dysgenesis" International Journal of Environmental Research and Public Health 19, no. 9: 5027. https://doi.org/10.3390/ijerph19095027
APA StyleWinkler, I., Jaszczuk, I., Gogacz, M., Szkodziak, P., Paszkowski, T., Skorupska, K., Ciebiera, M., & Skrzypczak, M. (2022). A Successful New Case of Twin Pregnancy in a Patient with Swyer Syndrome—An Up-to-Date Review on the Incidence and Outcome of Twin/Multiple Gestations in the Pure 46,XY Gonadal Dysgenesis. International Journal of Environmental Research and Public Health, 19(9), 5027. https://doi.org/10.3390/ijerph19095027






