Arbuscular Mycorrhizal Fungi and Glomalin Play a Crucial Role in Soil Aggregate Stability in Pb-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Substrate Soil, Plant and AMF Inoculum
2.2. Experimental Design
2.3. Sampling, Harvest, and Chemical Analysis
2.4. Statistical Analysis
3. Results
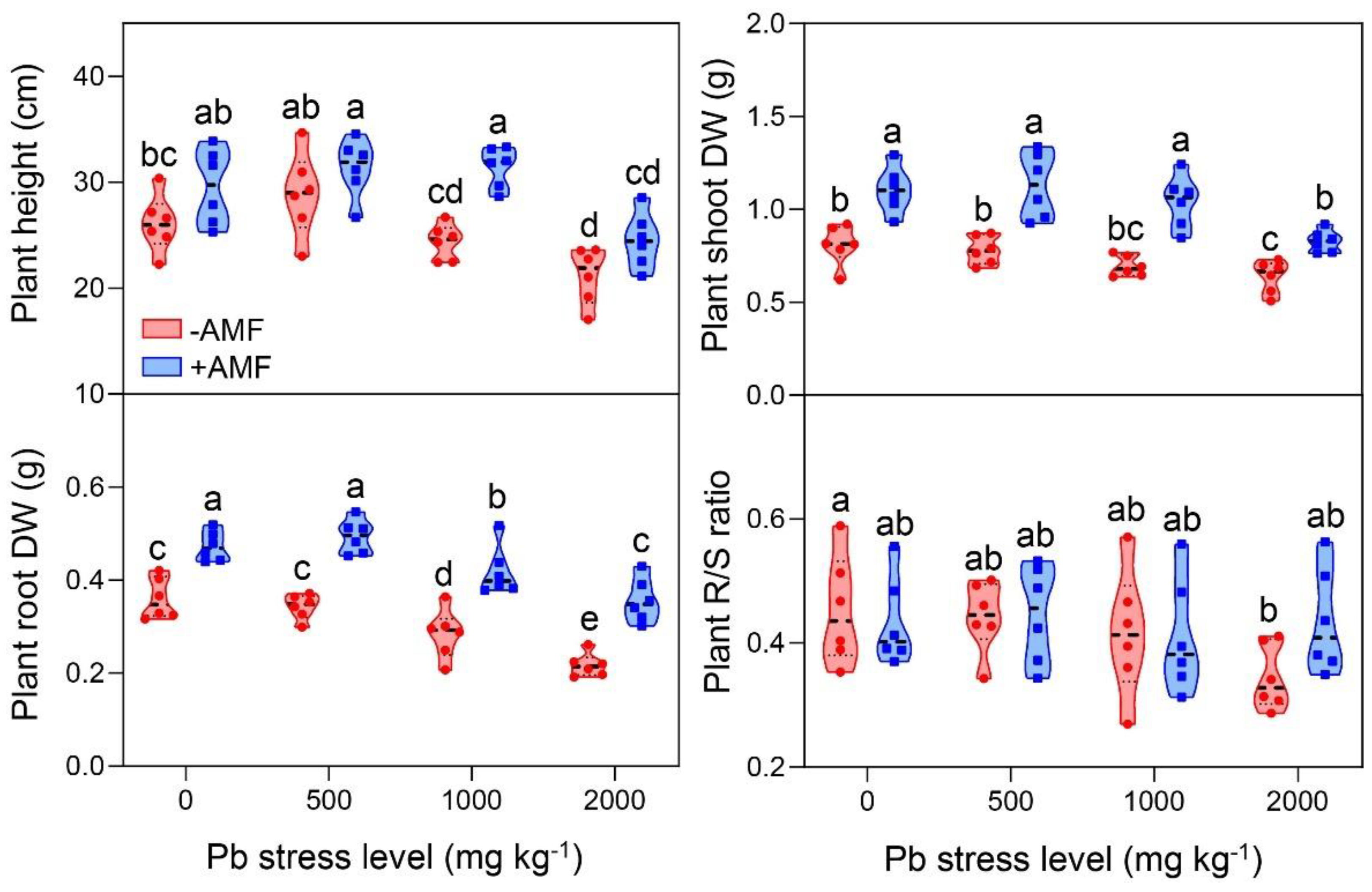
3.1. Effects of Pb Stress and AMF Inoculation on Plant Growth
3.2. Effects of Pb Stress on AMF Growth Parameters
3.3. Effects of Pb Stress and AMF Inoculation on Glomalin-Related Soil Protein Content
3.4. Effect of Pb Stress and AMF Inoculation on Soil Aggregate Distribution
3.5. Effects of Pb Stress and AMF Inoculation on Soil Aggregate Stability
3.6. Correlations between AMF Status, Glomalin-Related Soil Protein, and Stability of Soil Aggregates
4. Discussion
4.1. Effect of Pb Stress on AMF Growth Parameters and Glomalin-Related Soil Proteins
4.2. Effects of Pb Stress on Soil-Aggregate Stability
4.3. Pathways of Pb Affecting Soil-Aggregate Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.E.; David, J.R. Mycorrhizal Symbiosis; Academic Press: London, UK, 2010. [Google Scholar]
- Caruso, T.; Rillig, M.C. Direct, positive feedbacks produce instability in models of interrelationships among soil structure, plants and arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2011, 43, 1198–1206. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, K.; Wang, J. Effect of different inoculation treatments on AM fungal communities and the sustainability of soil remediation in Daliuta coal mining subsidence area in northwest China. Appl. Soil Ecol. 2018, 132, 107–113. [Google Scholar] [CrossRef]
- Lendenmann, M.; Thonar, C.; Barnard, R.L.; Salmon, Y.; Werner, R.A.; Frossard, E.; Jansa, J. Symbiont identity matters: Carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 2011, 21, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Kyriazopoulos, A.P.; Orfanoudakis, M.; Abraham, E.M.; Parissi, Z.M.; Serafidou, N. Effects of arbuscular mycorrhiza fungi on growth characteristics of Dactylis glomerata L. under drought stress conditions. Not. Bot. Horti. Agrobot. 2014, 42, 132–137. [Google Scholar] [CrossRef]
- Weissenhorn, I.; Leyval, C.; Belgy, G.; Berthelin, J. Arbuscular mycorrhizal contribution to heavy metal uptake by maize (Zea mays L.) in pot culture with contaminated soil. Mycorrhiza 1995, 5, 245–251. [Google Scholar]
- Walder, F.; Niemann, H.; Natarajan, M.; Lehmann, M.F.; Boller, T.; Wiemken, A. Mycorrhizal networks: Common goods of plants shared under unequal terms of trade. Plant Physiol. 2012, 159, 789–797. [Google Scholar] [CrossRef]
- Janoušková, M.; Rydlová, J.; Püschel, D.; Száková, J.; Vosátka, M. Extraradical mycelium of arbuscular mycorrhizal fungi radiating from large plants depresses the growth of nearby seedlings in a nutrient deficient substrate. Mycorrhiza 2011, 21, 641–650. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal fungi influence soil structure. In Arbuscular Mycorrhizas: Physiology and Function; Kluwer Academic Publishers: London, UK, 2000; pp. 3–18. [Google Scholar]
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106. [Google Scholar] [CrossRef]
- Gillespie, A.W.; Farrell, R.E.; Walley, F.L.; Ross, A.R.S.; Leinweber, P.; Eckhardt, K.U.; Regier, T.Z.; Blyth, R.I.R. Glomalin-related soil protein contains non-mycorrhizal-related heat-stable proteins, lipids and humic materials. Soil Biol. Biochem. 2011, 43, 766–777. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev. Environ. Sci. Biotechnol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Van Oort, F.; Jongmans, A.G.; Citeau, L.; Lamy, I.; Chevallier, P. Microscale Zn and Pb distribution patterns in subsurface soil horizons: An indication for metal transport dynamics. Eur. J. Soil Sci. 2006, 57, 154–166. [Google Scholar] [CrossRef]
- Ashraf, U.; Tang, X. Yield and quality responses, plant metabolism and metal distribution pattern in aromatic rice under lead (Pb) toxicity. Chemosphere 2017, 176, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Obiora, S.C.; Chukwu, A.; Davies, T.C. Heavy metals and health risk assessment of arable soils and food crops around Pb-Zn mining localities in Enyigba, southeastern Nigeria. J. Afr. Earth Sci. 2016, 116, 182–189. [Google Scholar] [CrossRef]
- Sun, J.; Hu, G.; Yu, R.; Lin, C.; Wang, X.; Huang, Y. Human health risk assessment and source analysis of metals in soils along the G324 Roadside, China, by Pb and Sr isotopic tracing. Geoderma 2017, 305, 293–304. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.W.; Tsang, D.C.W.; Yang, J.; Hou, D.; Baek, K.; Kua, H.W.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef]
- Santos, M.; Melo, V.F.; Monte Serrat, B.; Bonfleur, E.; Araújo, E.M.; Cherobim, V.F. Hybrid technologies for remediation of highly Pb contaminated soil: Sewage sludge application and phytoremediation. Int. J. Phytoremediation 2021, 23, 328–335. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R.G. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Jakobsen, I.; Abbott, L.K.; Robson, A.D. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Karlen, D.L.; Cambardella, C.A.; Kovar, J.L.; Colvin, T.S. Soil quality response to long-term tillage and crop rotation practices. Soil Tillage Res. 2013, 133, 54–64. [Google Scholar] [CrossRef]
- Lal, R.; Shukla, M.K. Principles of Soil Physics; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Mikhajlov, V.F.; Mazurik, V.K.; Burlakova, E.B. Signal function of the reactive oxygen species in regulatory networks of the cell reaction to damaging effects: Contribution of radiosensitivity and genome instability. Radiatsionnaia Biol. Radioecol. 2003, 43, 5–18. [Google Scholar]
- Finzgar, N.; Kos, B.; Lestan, D. Bioavailability and mobility of Pb after soil treatment with different remediation methods. Plant Soil Environ. 2006, 1, 25–34. [Google Scholar] [CrossRef]
- Wieczorek, J.; Baran, A.; Urbański, K.; Mazurek, R.; Klimowicz-Pawlas, A. Assessment of the pollution and ecological risk of lead and cadmium in soils. Environ. Geochem. Health 2018, 40, 2325–2342. [Google Scholar] [CrossRef]
- Ma, J.; Hao, Z.; Sun, Y.; Liu, B.; Jing, W.; Du, J.; Li, J. Heavy metal concentrations differ along wetland-to-grassland soils: A case study in an ecological transition zone in Hulunbuir, Inner Mongolia. J. Soils Sediments 2022, 22, 1176–1187. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Huang, L.; Ban, Y.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef]
- Cui, H.; Ma, K.; Fan, Y.; Peng, X.; Mao, J.; Zhou, D.; Zhang, Z.; Zhou, J. Stability and heavy metal distribution of soil aggregates affected by application of apatite, lime, and charcoal. Environ. Sci. Pollut. Res. Int. 2016, 23, 10808–10817. [Google Scholar] [CrossRef]
- Li, Q.; Du, H.; Chen, W.; Hao, J.; Huang, Q.; Cai, P.; Feng, X. Aging shapes the distribution of copper in soil aggregate size fractions. Environ. Pollut. 2018, 233, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.X.; Huang, D.Y.; Wu, J.S.; Zhu, Q.H.; Zhu, H.H.; Xu, C.; Xiong, J.; Wang, H.; Duan, M.M. Distribution and availability of cadmium in profile and aggregates of a paddy soil with 30-year fertilization and its impact on Cd accumulation in rice plant. Environ. Pollut. 2018, 239, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil. Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 238, 325–333. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, J.; Hu, J.; Zhang, T.; Wu, X.; Yang, Y. Arbuscular Mycorrhizal Fungi and Glomalin Play a Crucial Role in Soil Aggregate Stability in Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 5029. https://doi.org/10.3390/ijerph19095029
Li Y, Xu J, Hu J, Zhang T, Wu X, Yang Y. Arbuscular Mycorrhizal Fungi and Glomalin Play a Crucial Role in Soil Aggregate Stability in Pb-Contaminated Soil. International Journal of Environmental Research and Public Health. 2022; 19(9):5029. https://doi.org/10.3390/ijerph19095029
Chicago/Turabian StyleLi, Yinong, Jiazheng Xu, Jin Hu, Tianyu Zhang, Xuefeng Wu, and Yurong Yang. 2022. "Arbuscular Mycorrhizal Fungi and Glomalin Play a Crucial Role in Soil Aggregate Stability in Pb-Contaminated Soil" International Journal of Environmental Research and Public Health 19, no. 9: 5029. https://doi.org/10.3390/ijerph19095029
APA StyleLi, Y., Xu, J., Hu, J., Zhang, T., Wu, X., & Yang, Y. (2022). Arbuscular Mycorrhizal Fungi and Glomalin Play a Crucial Role in Soil Aggregate Stability in Pb-Contaminated Soil. International Journal of Environmental Research and Public Health, 19(9), 5029. https://doi.org/10.3390/ijerph19095029




