Sport Activity Load and Skeletomuscular Robustness in Elite Youth Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Data Collection
- Had severe degenerative lesions or fractures/deformations in the measurement area;
- Were unable to reach the correct position and/or remain immobile during measurement;
- Had a very high or low body mass index (i.e., a BMI that could adversely affect the accuracy of measurement process);
- Were exposed to an enhanced X-ray/CT scan several days prior to the study;
- Were pregnant.
2.4. Statistical Analyses
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yingling, V.R.; Ferrari-Church, B.; Strickland, A. Tibia functionality and Division II female and male collegiate athletes from multiple sports. PeerJ 2018, 6, e5550. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.; Monteleone, M.; Van Loan, M.; Promenzio, L.; Tarantino, U.; De Lorenzo, A. Effects of different sports on bone density and muscle mass in highly trained athletes. Med. Sci. Sports Exerc. 2001, 33, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1094–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente-Rodriguez, G. How does exercise affect bone development during growth? Sports Med. 2006, 36, 561–569. [Google Scholar] [CrossRef]
- Moreno, L.A.; Valtuena, J.; Perez-Lopez, F.; González-Gross, M. Health effects related to low vitamin D concentrations: Beyond bone metabolism. Ann. Nutr. Metab. 2011, 59, 22–27. [Google Scholar] [CrossRef]
- Stagi, S.; Cavalli, L.; Iurato, C.; Seminara, S.; Brandi, M.L.; de Martino, M. Bone metabolism in children and adolescents: Main characteristics of the determinants of peak bone mass. Clin. Cases Miner. Bone Metabol. 2013, 10, 172. [Google Scholar]
- Gomez-Bruton, A.; Montero-Marin, J.; González-Agüero, A.; Garcia-Campayo, J.; Moreno, L.A.; Casajus, J.A.; Vicente-Rodriguez, G. The effect of swimming during childhood and adolescence on bone mineral density: A systematic review and meta-analysis. Sports Med. 2016, 46, 365–379. [Google Scholar] [CrossRef]
- Herbert, A.J.; Williams, A.G.; Hennis, P.J.; Erskine, R.M.; Sale, C.; Day, S.H.; Stebbings, G.K. The interactions of physical activity, exercise and genetics and their associations with bone mineral density: Implications for injury risk in elite athletes. Eur. J. Appl. Physiol. 2019, 119, 29–47. [Google Scholar] [CrossRef] [Green Version]
- Moraes, M.S.; Martins, P.C.; Silva, D.A.S. Comparison of bone parameters by body region in university athletes: Systematic review. Rev. Brasileira Med. Esporte 2021, 27, 627–636. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Brahm, H.; Strom, H.; Piehl-Aulin, K.; Mallmin, H.; Ljunghall, S. Bone metabolism in endurance trained athletes: A comparison to population-based controls based on DXA, SXA, quantitative ultrasound, and biochemical markers. Calcif. Tissue Int. 1997, 61, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. The effect of long-distance running on bone strength and bone biochemical markers. J. Exerc. Rehabil. 2019, 15, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bruton, A.; Gonzalez-Aguero, A.; Gomez-Cabello, A.; Casajus, J.A.; Vicente-Rodríguez, G. Is Bone Tissue Really Affected by Swimming? A Systematic Review. PLoS ONE 2013, 8, e70119. [Google Scholar] [CrossRef] [Green Version]
- Bellver, M.; Del Rio, L.; Jovell, E.; Drobnic, F.; Trilla, A. Bone mineral density and bone mineral content among female elite athletes. Bone 2019, 127, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Multani, N.K.; Kaur, H.; Chahal, A. Impact of sporting activities on bone mineral density. J. Exerc. Sci. Physiother. 2011, 7, 103–109. [Google Scholar] [CrossRef]
- Shenoy, S.; Shadagopan, S.P.; Sandhu, J.S. Radial and tibial bone ultrasound densitometry Multani in weight bearing and non-weight bearing activity: A comparative analysis. Serb. J. Sports Sci. 2012, 1, 29–35. [Google Scholar]
- Madsen, K.L.; Adams, W.C.; Van Loan, M.D. Effects of physical activity, body weight and composition, and muscular strength on bone density in young women. Med. Sci. Sports Exerc. 1998, 30, 114–120. [Google Scholar] [CrossRef]
- Malina, R.M. Secular trends in growth, maturation and physical performance: A review. Anthrop. Rev. 2004, 67, 3–31. [Google Scholar]
- Bodzsar, E.B.; Zsakai, A.; Mascie-Taylor, N. Secular growth and maturation changes in Hungary in relation to socioeconomic and demographic changes. J. Biosoc. Sci. 2016, 48, 158–173. [Google Scholar] [CrossRef]
- Kalabiska, I.; Zsakai, A.; Malina, R.M.; Szabo, T. Bone mineral reference values for athletes 11 to 20 years of age. Int. J. Environ. Res. Public Health 2020, 17, 4930. [Google Scholar] [CrossRef]
- Malina, R.M.; e Silva, M.J.C. Physical Activity, Growth, and Maturation of Youth. In Body Composition; CRC Press: Boca Raton, FL, USA, 2017; pp. 69–88. [Google Scholar]
- Krzykala, M.; Leszczynski, P. Asymmetry in body composition in female hockey players. Homo 2015, 66, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Zemkova, E.; Poor, O.; Jelen, M. Between-side differences in trunk rotational power in athletes trained in asymmetric sports. J. Back Musculoskelt. Rehabil. 2019, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Lijewski, M.; Burdukiewicz, A.; Pietraszewska, J.; Andrzejewska, J.; Stachon, A. Asymmetry of Muscle Mass Distribution and Grip Strength in Professional Handball Players. Int. J. Environ. Res. Pub. Health. 2021, 18, 1913. [Google Scholar] [CrossRef] [PubMed]
- Veis, A.; Kanasova, J.; Halmova, N. The level of body posture, the flexibility of backbone and flat feet in competition fitness in 8–11 year old girls. Trends Sport Sci. 2022, 29, 5–11. [Google Scholar]
- Esco, M.R.; Snarr, R.L.; Leatherwood, M.D.; Chamberlain, N.A.; Redding, M.L.; Flatt, A.A.; Williford, H.N. Comparison of total and segmental body composition using DXA and multifrequency bioimpedance in collegiate female athletes. J. Strength Cond. Res. 2015, 29, 918–925. [Google Scholar] [CrossRef]
- Haapasalo, H.; Kontulainen, S.; Sievänen, H.; Kannus, P.; Järvinen, M.; Vuori, I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: A peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 2000, 27, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Greene, D.A.; Naughton, G.A.; Bradshaw, E.; Moresi, M.; Ducher, G. Mechanical loading with or without weight-bearing activity: Influence on bone strength index in elite female adolescent athletes engaged in water polo, gymnastics, and track-and-field. J. Bone Miner. Metab. 2012, 30, 580–587. [Google Scholar] [CrossRef]
- Kontulainen, S.; Sievänen, H.; Kannus, P.; Pasanen, M.; Vuori, I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: A peripheral quantitative computed tomography study between young and old starters and controls. J. Bone Miner. Res. 2003, 18, 352–359. [Google Scholar] [CrossRef]
- Hind, K.; Gannon, L.; Whatley, E.; Cooke, C.; Truscott, J. Bone cross-sectional geometry in male runners, gymnasts, swimmers and non-athletic controls: A hip-structural analysis study. Eur. J. Appl. Physiol. 2012, 112, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Warden, S.J.; Roosa, S.M.M. Physical activity completed when young has residual bone benefits at 94 years of age: A within-subject controlled case study. J. Musculoskelt. Neuranal. Interact. 2014, 14, 239–243. [Google Scholar]
- Jackowski, S.A.; Kontulainen, S.A.; Cooper, D.M.L.; Lanovaz, J.L.; Beck, T.J.; Baxter-Jones, A.D.G. Adolescent physical activity and bone strength at the proximal femur in adulthood. Med. Sci. Sports Exerc. 2014, 46, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, I. Effects of exercises on bone mineral density of proximal femour region among athletes of different branches. Int. J. Phys. Sci. 2010, 5, 2705–2714. [Google Scholar] [CrossRef]
- Kutseryb, T.; Vovkanych, L.; Hrynkiv, M.; Majevska, S.; Muzyka, F. Peculiarities of the somatotype of athletes with different directions of the training process. J. Phys. Educ. Sport 2017, 17, 431–433. [Google Scholar] [CrossRef]
- Blake, T.E. Relationship of Energy Balance and Body Composition in Elite Female Gymnasts. Master’s Thesis, Georgia State University, Atlanta, GA, USA, 2015. Available online: https://scholarworks.gsu.edu/nutrition_theses/76 (accessed on 10 January 2022).
- Moss, S.L.; McWhannell, N.; Michalsik, L.B.; Twist, C. Anthropometric and physical per-formance characteristics of top-elite, elite and non-elite youth female team handball players. J. Sports Sci. 2015, 33, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, A.; Górski, M.; Gajewski, J.; Buśko, K. Anthropometric profile of female handball players is related to bone mineral density. Anthr. Rev. 2018, 81, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Stojanovic, E.; Radovanovic, D.; Dalbo, V.J.; Jakovljevic, V.; Ponorac, N.; Agostinete, R.R.; Scanlan, A.T. Basketball players possess a higher bone mineral density than matched non-athletes, swimming, soccer, and volleyball athletes: A systematic review and meta-analysis. Arch. Osteoporos. 2020, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Krzykala, M.; Leszczyński, P.; Grzeskowiak, M.; Podgorski, T.; Wozniewicz-Dobrzynska, M.; Konarska, A.; Konarski, J.M. Does field hockey increase morphofunctional asymmetry? A pilot study. Homo 2018, 69, 43–49. [Google Scholar] [CrossRef]
- Sanchis-Moysi, J.; Dorado, C.; Olmedillas, H.; Serrano-Sanchez, J.A.; Calbet, J.A. Bone and lean mass inter-arm asymmetries in young male tennis players depend on training frequency. Eur. J. Appl. Physiol. 2010, 110, 83–90. [Google Scholar] [CrossRef]
- Strong, W.B.; Malina, R.M.; Blimkie, C.J.R.; Daniels, S.R.; Dishman, R.K.; Gutin, B.; Hergenroeder, A.C.; Must, A.; Nixon, P.A.; Pivarnik, J.M.; et al. Evidence based physical activity for school youth. J. Pediatrics 2005, 146, 732–737. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Committee. Physical Activity Guidelines Advisory Committee Report 2008; Part G, Section 9: Youth, G-1-G-9-33; Department of Health and Human Services: Washington, DC, USA, 2008.
- Institute of Medicine. Fitness Measures and Health Outcomes in Youth; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Nikander, R.; Sievänen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2020, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- O’Donovan, G.; Blazevich, A.J.; Boreham, C.; Cooper, A.R.; Crank, H.; Ekelund, U.; Stamatakis, E. The ABC of Physical Activity for Health: A consensus statement from the British Association of Sport and Exercise Sciences. J. Sport Sci. 2020, 28, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Beneke, R.; Bloch, W.; Bucksch, J.; Dordel, S.; Eiser, S.; Ferrari, N.; Koch, B.; Krug, S.; Lawrenz, W.; et al. Recommendations for promoting physical activity for children and adolescents in Germany. A consensus statement. Obes. Facts 2014, 7, 178–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reaburn, P. The Masters Athlete: IMPROVE Your Performance, Improve Your Fitness, Improve Your Life; Central Queensland University, Institute for Health and Social Science Research (IHSSR); Info Publishing: Mackay, QLD, Australia, 2009. [Google Scholar]
- Szulc, P. Bone density, geometry, and fracture in elderly men. Curr. Osteoporos. Rep. 2006, 4, 57–63. [Google Scholar] [CrossRef] [PubMed]
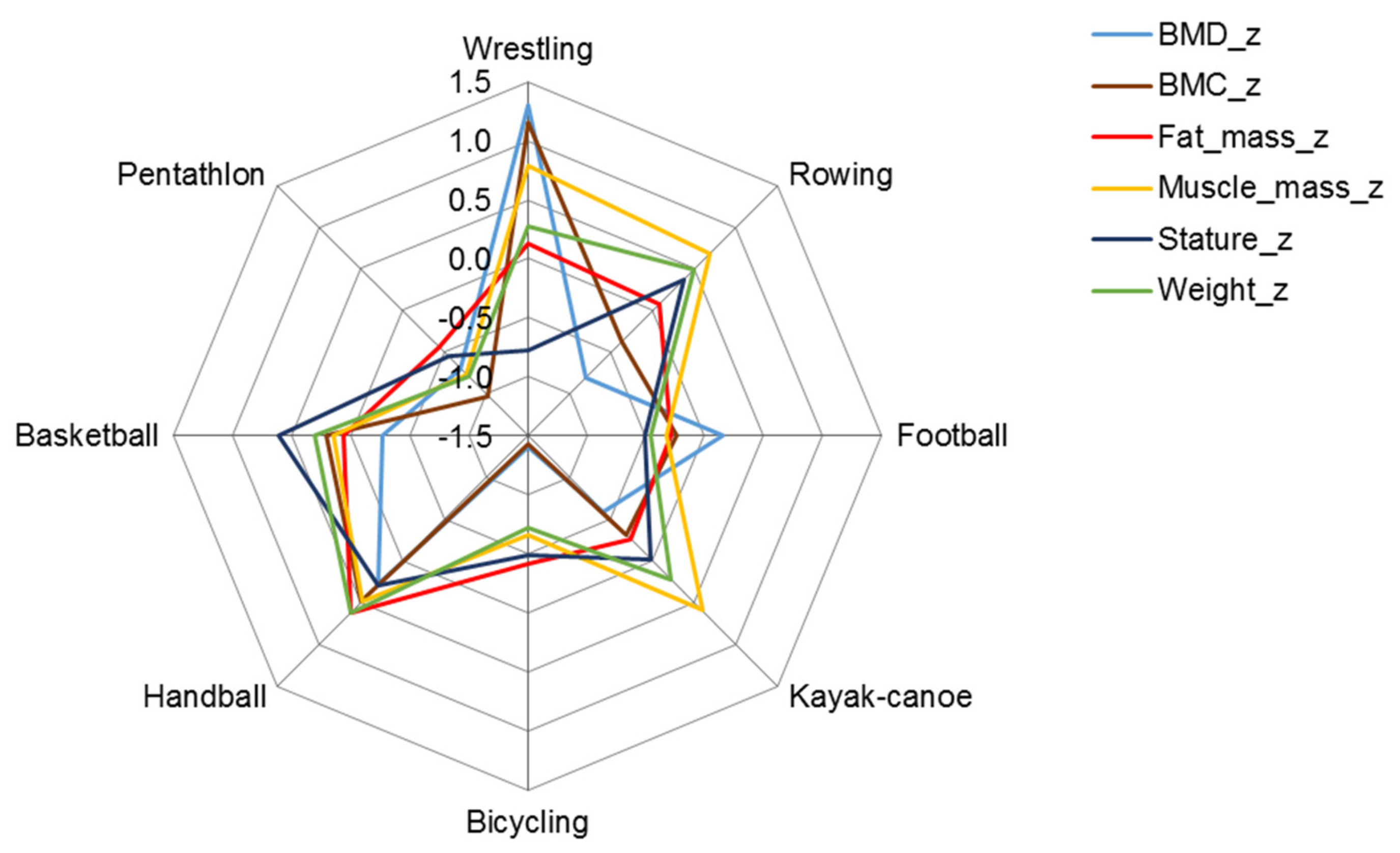

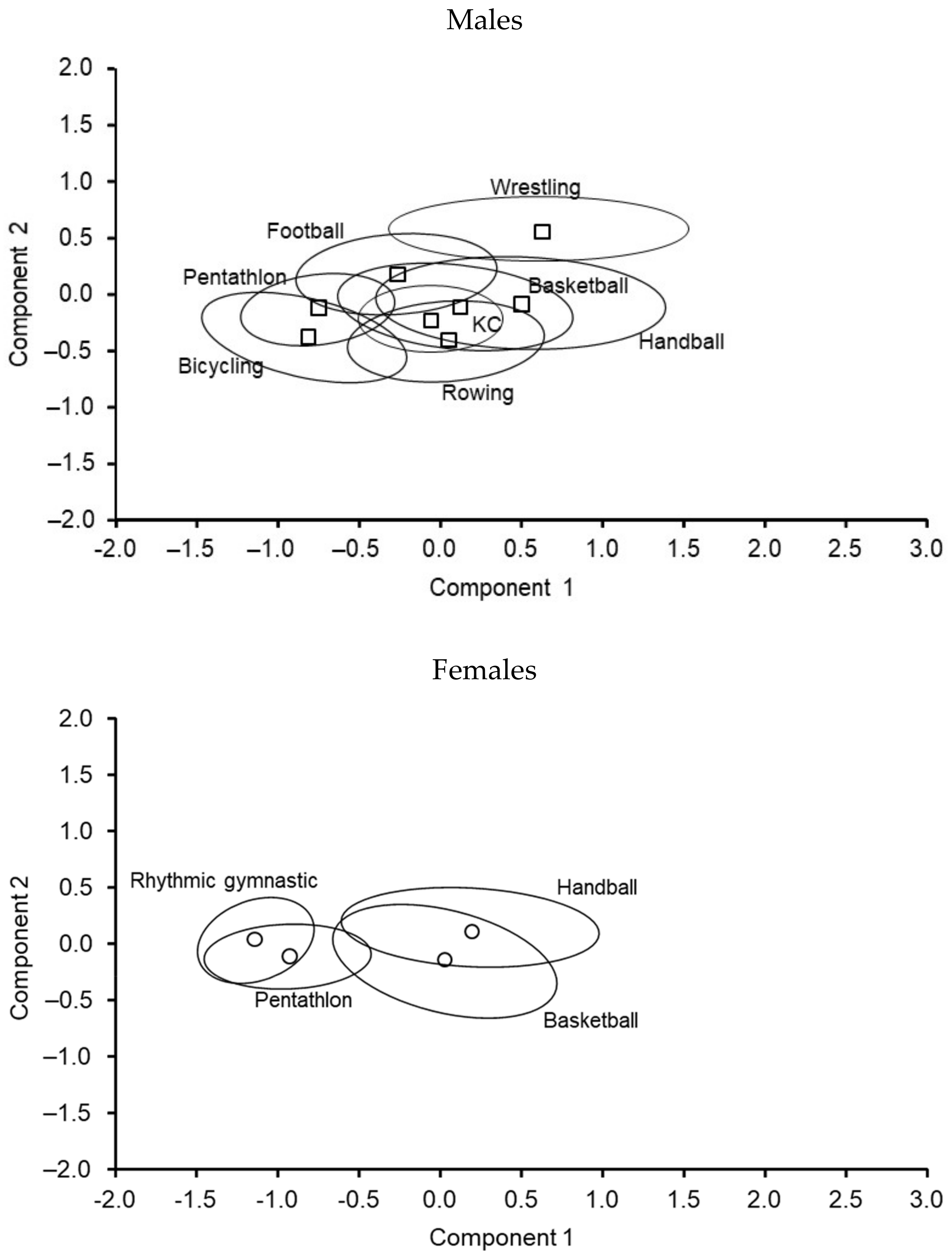





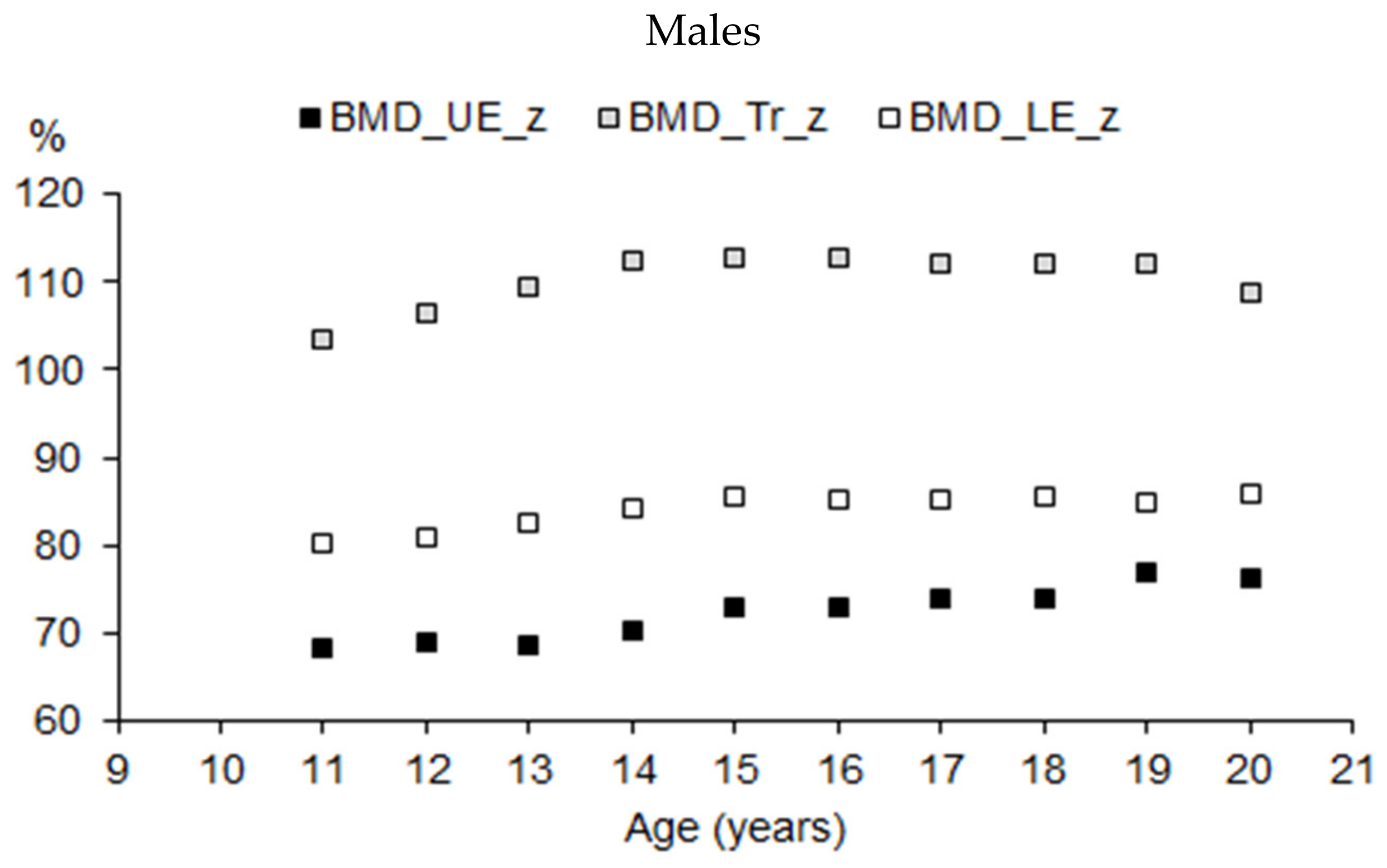


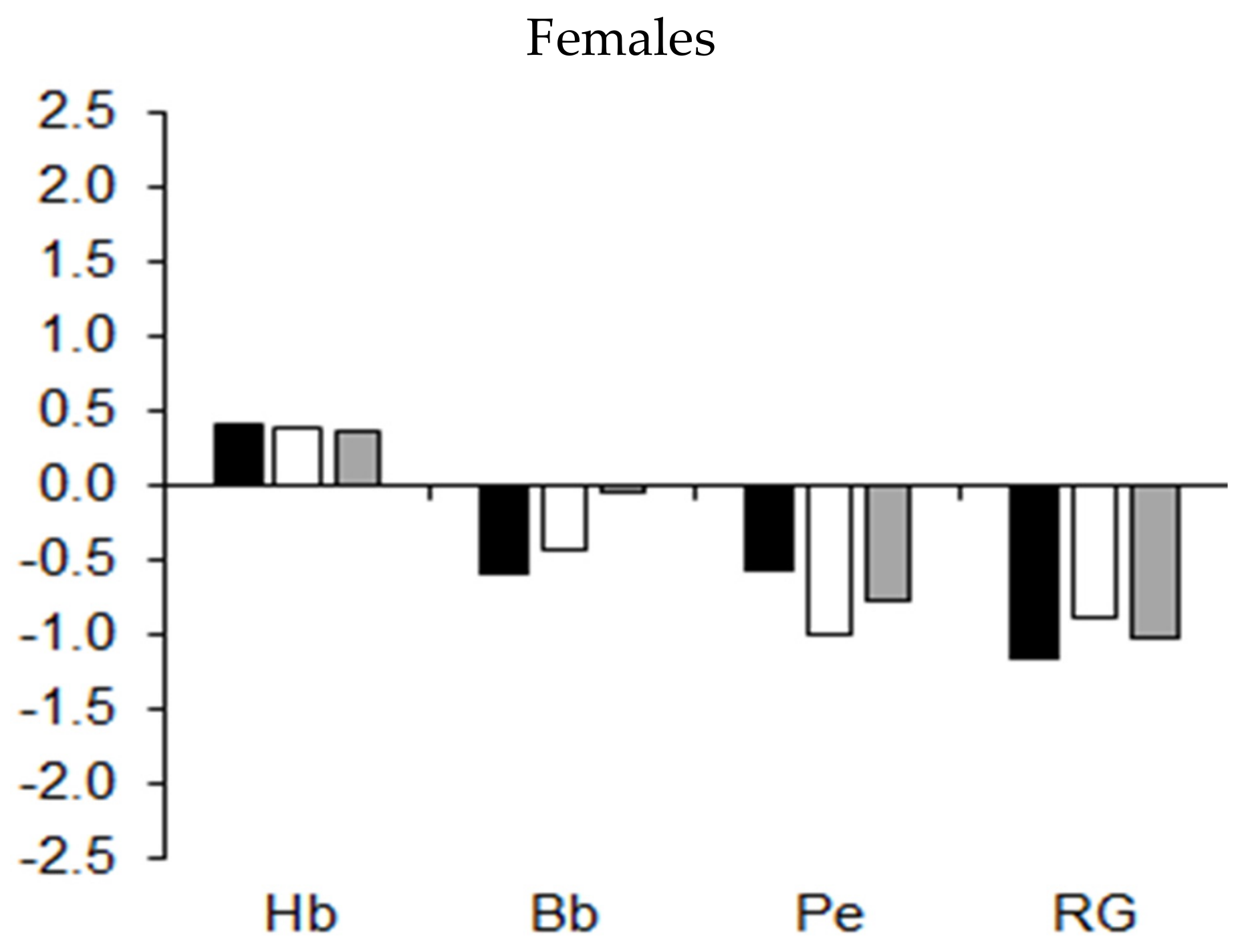

| Age (Years) | Males | Females | Sports | Males | Females |
|---|---|---|---|---|---|
| 11 | 25 | - | Wrestling | 19 | - |
| 12 | 36 | - | Rowing | 16 | - |
| 13 | 89 | 32 | Football | 531 | - |
| 14 | 138 | 97 | Kayak/canoe | 19 | - |
| 15 | 240 | 103 | Bicycling | 33 | - |
| 16 | 308 | 57 | Handball | 225 | 251 |
| 17 | 226 | 71 | Basketball | 428 | 139 |
| 18 | 154 | 31 | Pentathlon | 28 | 22 |
| 19 | 50 | 25 | Rhythmic gymnastics | - | 23 |
| 20 | 33 | 19 | |||
| Total | 1299 | 435 | 1299 | 435 |
| Age (Years) | Height (cm) | Weight (kg) | Fat Mass (kg) | Muscle Mass (kg) | BMC (g) | BMD (g/cm2) |
|---|---|---|---|---|---|---|
| Males | ||||||
| 11 | 155.8 | 44.5 | 3.3 | 31.6 | 1689.0 | 0.962 |
| 12 | 163.2 | 43.7 | 3.4 | 33.0 | 1878.0 | 1.007 |
| 13 | 169.8 | 50.9 | 3.3 | 38.1 | 2123.0 | 1.055 |
| 14 | 176.4 | 61.2 | 3.7 | 48.4 | 2573.0 | 1.159 |
| 15 | 179.3 | 67.9 | 4.1 | 53.4 | 2901.0 | 1.246 |
| 16 | 179.8 | 70.0 | 4.1 | 55.8 | 3082.5 | 1.298 |
| 17 | 182.6 | 73.5 | 4.4 | 59.3 | 3230.0 | 1.349 |
| 18 | 183.2 | 75.0 | 4.4 | 60.3 | 3272.0 | 1.367 |
| 19 | 180.6 | 73.8 | 4.2 | 60.5 | 3377.5 | 1.378 |
| 20 | 178.0 | 74.2 | 4.3 | 60.3 | 3455.5 | 1.443 |
| Females | ||||||
| 13 | 168.5 | 54.9 | 5.2 | 39.0 | 2193.5 | 1.111 |
| 14 | 172.5 | 63.4 | 6.7 | 44.1 | 2573.0 | 1.241 |
| 15 | 171.4 | 62.5 | 6.6 | 44.4 | 2584.0 | 1.226 |
| 16 | 172.3 | 64.8 | 6.6 | 46.3 | 2699.0 | 1.284 |
| 17 | 172.6 | 67.7 | 7.0 | 47.1 | 2753.0 | 1.295 |
| 18 | 172.7 | 65.9 | 7.2 | 47.6 | 2840.0 | 1.307 |
| 19 | 174.4 | 68.2 | 6.1 | 48.6 | 2949.5 | 1.356 |
| 20 | 174.1 | 71.6 | 6.4 | 51.7 | 2983.0 | 1.376 |
| Males | Wr | Ro | Fo | KC | Bi | Hb | Bb | Pe |
|---|---|---|---|---|---|---|---|---|
| Weight | 0.629 | 0.003 | p < 0.001 | 0.184 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Height | 0.001 | 0.007 | p < 0.001 | 0.629 | 0.002 | p < 0.001 | p < 0.001 | p < 0.001 |
| Muscle mass | 0.006 | 0.002 | p < 0.001 | 0.001 | 0.003 | p < 0.001 | 0.009 | p < 0.001 |
| Fat mass | 0.444 | 0.836 | p < 0.001 | 0.049 | 0.001 | p < 0.001 | 0.116 | p < 0.001 |
| BMC | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| BMD total | p < 0.001 | 0.006 | p < 0.001 | 0.003 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| BMD UE | p < 0.001 | 0.379 | p < 0.001 | 0.004 | 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| BMD Tr | 0.004 | 0.002 | p < 0.001 | p < 0.001 | p < 0.001 | 0.049 | p < 0.001 | p < 0.001 |
| BMD Le | p < 0.001 | 0.003 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Females | Hb | Bb | Pe | RG | ||||
| Weight | 0.245 | 0.008 | p < 0.001 | p < 0.001 | ||||
| Height | 0.044 | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| Muscle mass | 0.075 | 0.908 | p < 0.001 | p < 0.001 | ||||
| Fat mass | 0.069 | 0.735 | p < 0.001 | p < 0.001 | ||||
| BMC | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| BMD total | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| BMD UE | p < 0.001 | p < 0.001 | 0.017 | p < 0.001 | ||||
| BMD Tr | p < 0.001 | 0.315 | p < 0.001 | p < 0.001 | ||||
| BMD Le | p < 0.001 | p < 0.001 | p < 0.001 | 0.002 | ||||
| Component 1 | Component 2 | |
|---|---|---|
| Eigenvalue | 3.172 | 1.074 |
| % of variance | 63.43 | 19.87 |
| BMD_z | 0.742 | 1.693 |
| BMC_z | 1.101 | 0.708 |
| Fat mass_z | 0.865 | −0.980 |
| Muscle mass_z | 1.090 | −0.272 |
| Weight_z | 1.141 | −0.797 |
| Males | Wr | Ro | Fo | KC | Bi | Hb | Bb | Pe |
|---|---|---|---|---|---|---|---|---|
| BMC–UE | p < 0.001 | 0.569 | p < 0.001 | 0.013 | p < 0.001 | p < 0.001 | 0.070 | p < 0.001 |
| BMC–Tr | p < 0.001 | 0.045 | p < 0.001 | 0.107 | p < 0.001 | p < 0.001 | 0.002 | p < 0.001 |
| BMC–LE | 0.398 | 0.034 | 0.002 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Muscle mass–UE | p < 0.001 | 0.001 | p < 0.001 | p < 0.001 | 0.001 | p < 0.001 | 0.034 | 0.002 |
| Muscle mass–Tr | 0.022 | 0.002 | p < 0.001 | 0.001 | 0.003 | p < 0.001 | 0.042 | 0.001 |
| Muscle mass–LE | 0.872 | 0.023 | p < 0.001 | 0.070 | 0.003 | p < 0.001 | 0.001 | p < 0.001 |
| Fat mass–UE | 0.354 | 0.234 | p < 0.001 | 0.295 | 0.002 | p < 0.001 | 0.112 | 0.001 |
| Fat mass–Tr | 0.351 | 0.877 | p < 0.001 | 0.004 | 0.002 | p < 0.001 | p < 0.001 | p < 0.001 |
| Fat mass–LE | 0.184 | 0.569 | p < 0.001 | 0.334 | 0.001 | p < 0.001 | 0.345 | 0.010 |
| Females | Hb | Bb | Pe | RG | ||||
| BMC–UE | p < 0.001 | 0.027 | p < 0.001 | p < 0.001 | ||||
| BMC–Tr | p < 0.001 | 0.683 | p < 0.001 | p < 0.001 | ||||
| BMC–LE | 0.049 | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| Muscle mass–UE | 0.008 | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| Muscle mass–Tr | 0.115 | 0.740 | 0.008 | 0.001 | ||||
| Muscle mass–LE | 0.204 | 0.027 | 0.026 | p < 0.001 | ||||
| Fat mass–UE | 0.073 | 0.240 | p < 0.001 | p < 0.001 | ||||
| Fat mass–Tr | 0.210 | 0.445 | p < 0.001 | p < 0.001 | ||||
| Fat mass–LE | 0.208 | 0.449 | p < 0.001 | p < 0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalabiska, I.; Zsakai, A.; Annar, D.; Malina, R.M.; Szabo, T. Sport Activity Load and Skeletomuscular Robustness in Elite Youth Athletes. Int. J. Environ. Res. Public Health 2022, 19, 5083. https://doi.org/10.3390/ijerph19095083
Kalabiska I, Zsakai A, Annar D, Malina RM, Szabo T. Sport Activity Load and Skeletomuscular Robustness in Elite Youth Athletes. International Journal of Environmental Research and Public Health. 2022; 19(9):5083. https://doi.org/10.3390/ijerph19095083
Chicago/Turabian StyleKalabiska, Irina, Annamaria Zsakai, Dorina Annar, Robert M. Malina, and Tamas Szabo. 2022. "Sport Activity Load and Skeletomuscular Robustness in Elite Youth Athletes" International Journal of Environmental Research and Public Health 19, no. 9: 5083. https://doi.org/10.3390/ijerph19095083
APA StyleKalabiska, I., Zsakai, A., Annar, D., Malina, R. M., & Szabo, T. (2022). Sport Activity Load and Skeletomuscular Robustness in Elite Youth Athletes. International Journal of Environmental Research and Public Health, 19(9), 5083. https://doi.org/10.3390/ijerph19095083







