Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata
Abstract
1. Introduction
2. Materials and Methods
2.1. Pyrolysis of Biochar
2.2. Chemical Modifications with FeCl3 and NaOH
2.3. Evaluation of Physical Properties of Biochars
2.4. Evaluation of As and Cd Adsorption Capacities of Biochars
2.5. Data Analysis
3. Results
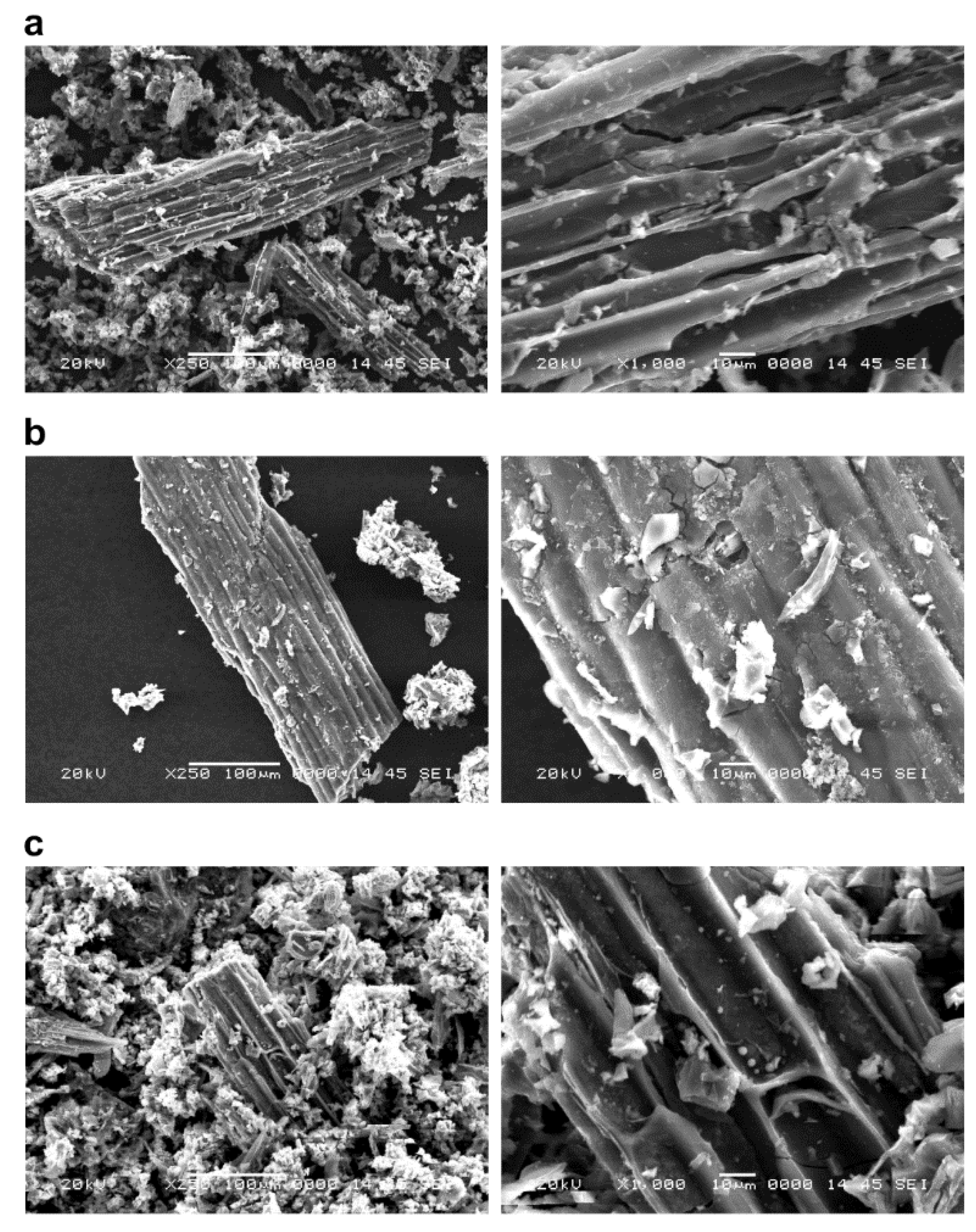
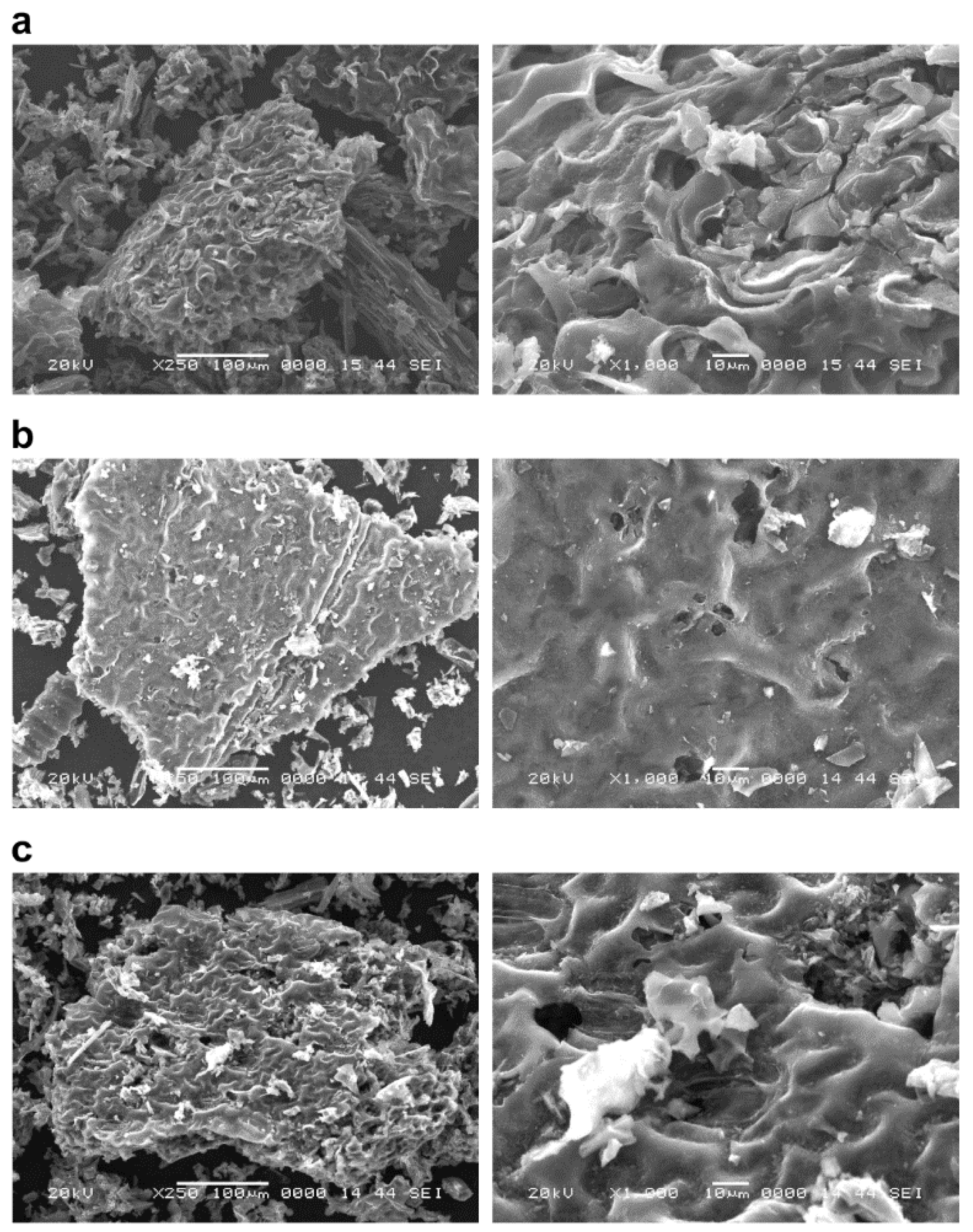
3.1. Evaluation of Surface Structure and Specific Surface Area of Biochar
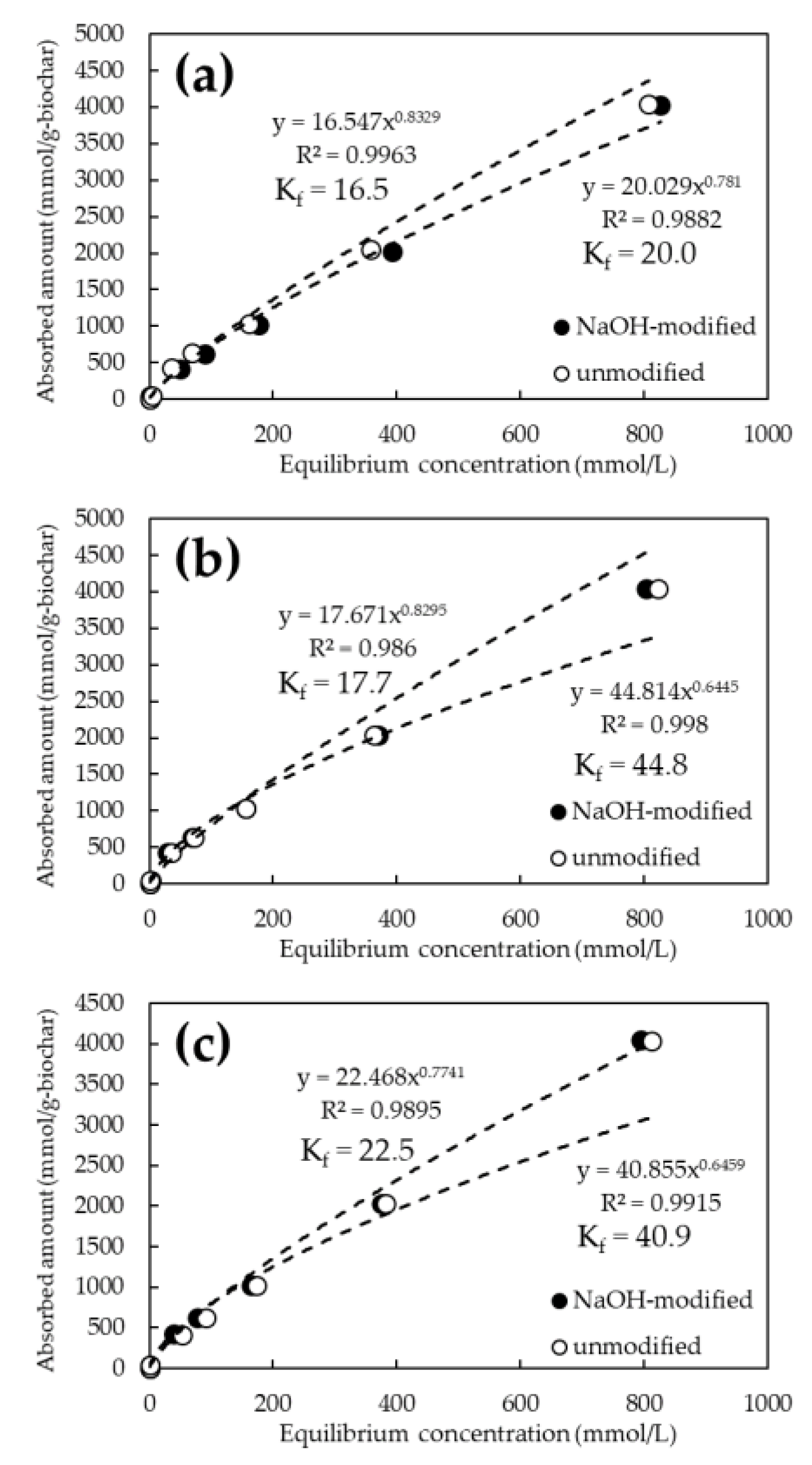
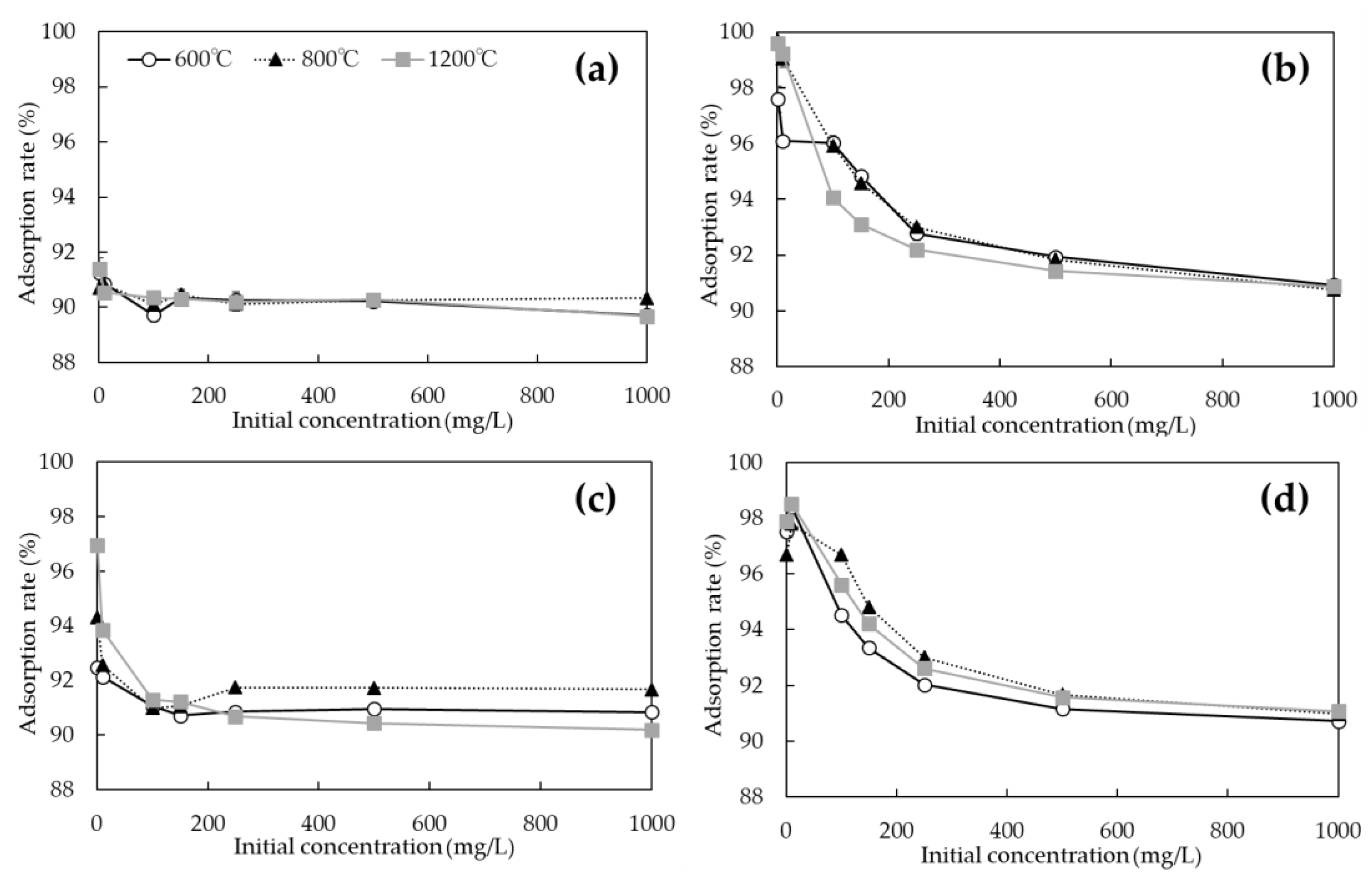
3.2. Evaluation of As and Cd Adsorption Capacities of P. vittata Biochar
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duker, A.; Carranza, E.; Hale, M. Arsenic geochemistry and health. Environ. Int. 2005, 31, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Hans Wedepohl, K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Hasan, R.; et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. Lancet 2010, 376, 252–258. [Google Scholar] [CrossRef]
- Bagchi, S. Arsenic threat reaching global dimensions. Can. Med. Assoc. J. 2007, 177, 1344–1345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernhoft, R.A. Cadmium Toxicity and Treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef]
- Zwicker, R.; Promsawad, A.; Zwicker, B.M.; Laoharojanaphand, S. Cadmium Content of Commercial and Contaminated Rice, Oryza sativa, in Thailand and Potential Health Implications. Bull. Environ. Contam. Toxicol. 2010, 84, 285–288. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Bulat, Z.; Đukić-Ćosić, D. Cadmium Toxicity Revisited: Focus on Oxidative Stress Induction and Interactions with Zinc and Magnesium. Arch. Ind. Hyg. Toxicol. 2011, 62, 65–76. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Nedelkoska, T.V.; Doran, P.M. Hyperaccumulation of cadmium by hairy roots of Thlaspi caerulescens. Biotechnol. Bioeng. 2000, 67, 607–615. [Google Scholar] [CrossRef]
- Ingle, R.A.; Smith, J.A.C.; Sweetlove, L.J. Responses to Nickel in the Proteome of the Hyperaccumulator Plant Alyssum lesbiacum. BioMetals 2005, 18, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of Cadmium Mobility and Accumulation in Indian Mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef]
- Tognacchini, A.; Rosenkranz, T.; van der Ent, A.; Machinet, G.E.; Echevarria, G.; Puschenreiter, M. Nickel phytomining from industrial wastes: Growing nickel hyperaccumulator plants on galvanic sludges. J. Environ. Manag. 2020, 254, 109798. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management; Routledge: London, UK, 2012. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W. Adsorption of Pb(II) and Cu(II) by ginkgo-leaf-derived biochar produced under various carbonization temperatures and times. Int. J. Environ. Res. Public Health 2017, 14, 1528. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(III) and As(V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.; Zhang, Q.; Liu, Q.; Li, Y.; Xuao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Datta, R.; Das, P.; Tappero, R.; Punamiya, P.; Sahi, S.; Feng, H.; Kiiskila, J.; Sarkar, D. Evidence for exocellular Arsenic in Fronds of Pteris vittata. Sci. Rep. 2017, 7, 2839. [Google Scholar] [CrossRef]
- Sugawara, K.; Kitajima, N.; Suzuki, S. Analysis of essential elements behavior accompanying with arsenic accumulation in arsenic hyper accumulator and non-accumulating plants by multivariate analysis. Environ. Biotechnol. 2018, 18, 73–80. [Google Scholar]
- Guillory, J.K. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals Edited by Maryadele J. O’Neil, Patricia E. Heckelman, Cherie B. Koch, and Kristin J. Roman. Merck, John Wiley & Sons, Inc., Hoboken, NJ. 2006. xiv + 2564 pp. 18 × 26 cm. ISBN-13 978-0-911910-001. $125.00. J. Med. Chem. 2007, 50, 590. [Google Scholar]
- Yang, C.; Ho, Y.N.; Inoue, C.; Chien, M.F. Long-term effectiveness of microbe-assisted arsenic phytoremediation by Pteris vittata in field trials. Sci. Total Environ. 2020, 740, 140137. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, M.; Yang, F.; Ali, I.; Naz, I.; Peng, C. Remediation of Chromium (VI) from Groundwater by Metal-Based Biochar under Anaerobic Conditions. Water 2022, 14, 894. [Google Scholar] [CrossRef]
- Shirakashi, T. Reduction Reaction of Fe3+ Ion by Activated Carbon. Nippon Kagaku Kaishi 1993, 8, 924–930. [Google Scholar] [CrossRef][Green Version]
- Choo, W.; Hayashi, T.; Ohashi, C.; Sugawara, K.; Ito, T.; Suzuki, S.; Kato, S.; Kojima, T. Formulation of gasification rate of char from Eucalyptus camaldulensis grown in arid land on Western Australia. J. Arid. Land Stud. 2018, 28, 131–134. [Google Scholar]
- Sasaki, M.; Tamai, H.; Yoshida, T.; Yasuda, H. Dye Adsorption on Mesoporous Activated Carbon Fiber Obtained from Pitc Containing Yttrium Complex and tha Acid Treatment Effects. Tanso 1998, 181, 20–26. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Zhang, S.; Yang, H.; Feng, Y.; Chen, Y.; Wang, X.; Chen, H. Nitrogen enriched biochar modified by high temperature CO2-ammonia treatment: Characterization and adsorption of CO2. Chem. Eng. J. 2014, 257, 20–27. [Google Scholar] [CrossRef]
- Basta, A.H.; Fierro, V.; El-Saied, H.; Celzard, A. 2-Steps KOH activation of rice straw: An efficient method for preparing high-performance activated carbons. Bioresour. Technol. 2009, 100, 3941–3947. [Google Scholar] [CrossRef]
- Cope, C.O.; Webster, D.S.; Sabatini, D.A. Arsenate adsorption onto iron oxide amended rice husk char. Sci. Total Environ. 2014, 488–489, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jing, Y.; Wang, Y.; Meng, X.; Zhang, C.; Chen, Z.; Zhou, J.; Oiu, R.; Zhang, X. Enhanced removal of aqueous Cd(II) by a biochar derived from salt-sealing pyrolysis coupled with NaOH treatment. Appl. Surf. Sci. 2020, 511, 145619. [Google Scholar] [CrossRef]
- Baig, S.A.; Sheng, T.; Hu, Y.; Xu, J.; Xu, X. Arsenic Removal from Natural Water Using Low Cost Granulated Adsorbents: A Review. Clean Soil Air Water 2015, 43, 13–26. [Google Scholar] [CrossRef]
- Yang, T.; Sheng, L.; Wang, Y.; Wyckoff, K.N.; He, C.; He, Q. Characteristics of Cadmium Sorption by Heat-Activated Red Mud in Aqueous Solution. Sci. Rep. 2018, 8, 13558. [Google Scholar] [CrossRef]
- Kano, F.; Abe, I.; Kamaya, H.; Ueda, I. Fractal model for adsorption on activated carbon surfaces: Langmuir and Freundlich adsorption. Surf. Sci. 2000, 467, 131–138. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Role of surface chemistry in adsorption of phenol on activated carbons. J. Colloid Interface Sci. 2003, 264, 307–312. [Google Scholar] [CrossRef]
- Radovic, L.R.; Silva, I.F.; Ume, J.I.; Menéndez, J.A.; Leon, C.A.; Leon, Y.; Scaroni, A.W. An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon 1997, 35, 1339–1348. [Google Scholar] [CrossRef]
- Kato, Y.; Machida, M.; Tatsumoto, H. Influence of Surface Functional Groups and Solvent on Adsorption of Dissolved Aromatics by Activated Carbon. J. Environ. Chem. 2007, 17, 387–394. [Google Scholar] [CrossRef][Green Version]
- Couture, R.M.; Rose, J.; Kumar, N.; Mitchell, K.; Wallschläger, D.; Van Cappellen, P. Sorption of arsenite, arsenate, and thioarsenates to iron oxides and iron sulfides: A kinetic and spectroscopic investigation. Environ. Sci. Technol. 2013, 47, 5652–5659. [Google Scholar] [CrossRef]



| Modification | — | FeCl3 | NaOH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pyrolysis Temperature | 600 °C | 800 °C | 1200 °C | 600 °C | 800 °C | 1200 °C | 600 °C | 800 °C | 1200 °C |
| As(V) | ○ | ○ | ○ | ○ | ○ | ○ | |||
| Cd | ○ | ○ | ○ | ○ | ○ | ○ | |||
| Pyrolysis Temperature | |||
|---|---|---|---|
| Treatment | 600 °C | 800 °C | 1200 °C |
| Unmodified | 6.57 ± 0.22 | 4.31 ± 0.13 | 34.54 ± 0.92 |
| FeCl3-modified | 160.83 ± 2.54 | 114.78 ± 1.83 | 129.35 ± 1.98 |
| NaOH-modified | 72.23 ± 1.65 | 31.92 ± 0.85 | 65.38 ± 1.26 |
| Absorption Rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Initial Concentration (mg/L) | 1 | 10 | 100 | 150 | 250 | 500 | 1000 | |
| Activate carbon | As(V) | 99.31 ± 0.01 | 92.72 ± 0.37 | 90.15 ± 0.31 | 90.14 ± 0.20 | 90.16 ± 0.16 | 89.82 ± 0.14 | 89.68 ± 0.20 |
| Cd | 93.84 ± 0.05 | 92.52 ± 0.14 | 91.38 ± 0.01 | 91.01 ± 0.32 | 90.79 ± 0.23 | 90.64 ± 0.13 | 90.33 ± 0.32 | |
| Biochar | As(V) | 90.72 ± 0.23 | 90.80 ± 0.10 | 90.07 ± 0.14 | 90.52 ± 0.18 | 90.13 ± 0.25 | 90.26 ± 0.09 | 90.32 ± 0.11 |
| Cd | 99.71 ± 0.04 | 99.13 ± 0.32 | 95.97 ± 0.22 | 94.64 ± 0.13 | 92.95 ± 0.09 | 91.80 ± 0.07 | 90.79 ± 0.11 | |
| FeCl3-modified | As(V) | 94.34 ± 0.33 | 92.60 ± 0.09 | 91.01 ± 0.03 | 91.11 ± 0.22 | 91.82 ± 0.27 | 91.67 ± 0.08 | 91.66 ± 0.11 |
| NaOH-modified | Cd | 96.73 ± 0.02 | 97.82 ± 0.01 | 96.73 ± 0.20 | 94.81 ± 0.19 | 93.01 ± 0.08 | 91.67 ± 0.07 | 91.04 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugawara, K.; Ichio, K.; Ichikawa, Y.; Ogawa, H.; Suzuki, S. Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata. Int. J. Environ. Res. Public Health 2022, 19, 5226. https://doi.org/10.3390/ijerph19095226
Sugawara K, Ichio K, Ichikawa Y, Ogawa H, Suzuki S. Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata. International Journal of Environmental Research and Public Health. 2022; 19(9):5226. https://doi.org/10.3390/ijerph19095226
Chicago/Turabian StyleSugawara, Kazuki, Kouhei Ichio, Yumiko Ichikawa, Hitoshi Ogawa, and Seiichi Suzuki. 2022. "Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata" International Journal of Environmental Research and Public Health 19, no. 9: 5226. https://doi.org/10.3390/ijerph19095226
APA StyleSugawara, K., Ichio, K., Ichikawa, Y., Ogawa, H., & Suzuki, S. (2022). Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata. International Journal of Environmental Research and Public Health, 19(9), 5226. https://doi.org/10.3390/ijerph19095226






