Lifelong Lung Sequelae of Prematurity
Abstract
:1. Introduction
2. Lifelong Symptoms in Ex-Preterm Subjects
2.1. Respiratory Symptoms in Infancy and Preschool Age
2.2. Respiratory Symptoms in School Age
2.3. Respiratory Symptoms in Adolescence
2.4. Respiratory Symptoms in Adulthood
2.5. The Exercise Tolerance
3. Lifelong Lung Function in Ex-Preterm Subjects
3.1. The Relationship between Birth Weight and Lung Function
3.2. Lung Function in Infancy and Preschool Age
3.3. Lung Function in School Age and Adolescence
3.4. Lung Function in Adulthood
3.5. Gas Diffusion Impairment
3.6. Focus on Lung Function Impairment Related to Prematurity
3.7. Focus on Lung Function Impairment Related to Bronchopulmonary Dysplasia
3.8. Focus on Lung Function Improvement over Time
4. Lifelong Lung Structural Abnormalities in Ex-Preterm Subjects
4.1. Lung Structural Abnormalities in Infancy and Preschool Age
4.2. Lung Structural Abnormalities in School Age
4.3. Lung Structural Abnormalities in Adolescence and Adulthood
4.4. Focus on the Role of Magnetic Resonance Imaging
5. Follow-Up of Ex-Preterm Children
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, 37–46. [Google Scholar] [CrossRef] [Green Version]
- McGrath-Morrow, S.A.; Collaco, J.M. Bronchopulmonary dysplasia: What are its links to COPD? Ther. Adv. Respir. Dis. 2019, 13, 1753466619892492. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.; Kantar, A.; Masini, B.; Nuzzi, G.; Ragazzo, V.; Peroni, D. Structural and functional development in airways throughout childhood: Children are not small adults. Pediatr. Pulmonol. 2021, 56, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Savran, O.; Ulrik, C.S. Early life insults as determinants of chronic obstructive pulmonary disease in adult life. Int. J. Chron Obstr. Pulmon. Dis. 2018, 13, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Narang, I.; Baraldi, E.; Silverman, M.; Bush, A. Airway function measurement and the long-term follow-up of survivors of preterm birth with and without chronic lung disease. Pediatr. Pulmonol. 2006, 41, 497–508. [Google Scholar] [CrossRef]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respiratory therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef]
- Di Filippo, P.; Giannini, C.; Attanasi, M.; Dodi, G.; Scaparrotta, A.; Petrosino, M.I.; Di Pillo, S.; Chiarelli, F. Pulmonary Outcomes in Children Born Extremely and Very Preterm at 11 Years of Age. Front. Pediatr. 2021, 9, 635503. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Edwards, M.O.; Watkins, W.J.; Henderson, A.J.; Paranjothy, S.; Dunstan, F.D.; Kotecha, S. Effect of preterm birth on later FEV1: A systematic review and metaanalysis. Thorax 2013, 68, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Carraro, S.; Piacentini, G.; Lusiani, M.; Uyan, Z.S.; Filippone, M.; Schiavon, M.; Boner, A.L.; Baraldi, E. Exhaled air temperature in bronchopulmonary dysplasia. Pediatr. Pulmonol. 2010, 45, 1240–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Mazloum, D.; Moschino, L.; Bozzetto, S.; Baraldi, E. Chronic Lung Disease of Prematurity: Long-Term Respiratory Outcome. Neonatology 2014, 105, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Sillers, L.; Alexiou, S.; Jensen, E.A. Lifelong pulmonary sequelae of bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2020, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Narang, I. Long-term follow-up of infants with lung disease of prematurity. Chronic Respir. Dis. 2010, 7, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Robin, B.; Kim, Y.L.; Huth, J.; Klocksieben, J.; Torres, M.; Tepper, R.S.; Castile, R.G.; Solway, J.; Hershenson, M.B.; Goldstein-Filbrun, A. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. 2004, 37, 236–242. [Google Scholar] [CrossRef]
- Chandran, A.; Sagar, P.; Bhalla, A.S.; Kumar, R. Mounier-Kuhn syndrome. BMJ Case Rep. 2021, 14, e239876. [Google Scholar] [CrossRef]
- Noriega Aldave, A.P.; William Saliski, D. The clinical manifestations, diagnosis and management of williams-campbell syndrome. N. Am. J. Med. Sci. 2014, 6, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Behrendt, A.; Lee, Y. Swyer-James-MacLeod Syndrome. 25 July 2021. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Chan, K.N.; Elliman, A.; Bryan, E.; Silverman, M. Respiratory symptoms in children of low birth weight. Arch. Dis. Child. 1989, 64, 1294–1304. [Google Scholar] [CrossRef] [Green Version]
- Greenough, A.; Giffin, F.J.; Yuksel, B.; Dimitriou, G. Respiratory morbidity in young school children born prematurely—Chronic lung disease is not a risk factor? Eur. J. Pediatr. 1996, 155, 823–826. [Google Scholar] [CrossRef]
- McLeod, A.; Ross, P.; Mitchell, S.; Tay, D.; Hunter, L.; Hall, A.; Paton, J.; Mutch, L. Respiratory health in a total very low birthweight cohort and their classroom controls. Arch. Dis. Child. 1996, 74, 188–194. [Google Scholar] [CrossRef]
- Rona, R.J.; Gulliford, M.C.; Chinn, S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. Br. Med. J. 1993, 306, 817–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palta, M.; Sadek-Badawi, M.; Sheehy, M.; Albanese, A.; Weinstein, M.; McGuinness, G.; Peters, M.E. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am. J. Epidemiol. 2001, 154, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Fawke, J.; Lum, S.; Kirkby, J.; Hennessy, E.; Marlow, N.; Rowell, V.; Thomas, S.; Stocks, J. Lung function and respiratory symptoms at 11 years in children born extremely premature. The EPICure study. Am. J. Respir. Crit. Care Med. 2010, 182, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Welsh, L.; Kirkby, J.; Lum, S.; Odendaal, D.; Marlow, N.; Derrick, G.; Stocks, J. The EPICure study: Maximal exercise and physical activity in school children born extremely preterm. Thorax 2010, 65, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.; van Asperen, P.; McKay, K.; Selvaurai, H.; Fitzgerald, D. Reduced exercise capacity in children born very preterm. Pediatrics 2008, 122, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, M.; Wilson, A.C.; Pillow, J.J.; Stick, S.M.; Hall, G.L. Respiratory function and symptoms in young preterm children in the contemporary era. Pediatr. Pulmonol. 2016, 51, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, E.; Bracewell, M.; Wood, N.; Wolke, D.; Costeloe, K.; Gibson, A.; Marlow, N. Respiratory health in pre-school and school age children following extremely preterm birth. Arch. Dis. Child. 2008, 93, 1037–1043. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheung, M.M.; Ford, G.W.; Olinsky, A.; Davis, N.M.; Callanan, C. Birth weight <1501 g and respiratory health at age 14. Arch. Dis. Child. 2001, 84, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Anand, D.; Stevenson, C.J.; West, C.R.; Pharoah, P.O. Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child. 2003, 88, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Narang, I.; Rosenthal, M.; Cremonesini, D.; Silverman, M.; Bush, A. Longitudinal evaluation of airway function 21 years after preterm birth. Am. J. Respir. Crit. Care Med. 2008, 178, 74–80. [Google Scholar] [CrossRef]
- Baraldi, E.; Filippone, M. Chronic lung disease after premature birth. N. Engl. J. Med. 2007, 357, 1946–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Community Respiratory Health Survey. Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur. Respir. J. 1996, 9, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Gough, A.; Linden, M.; Spence, D.; Patterson, C.C.; Halliday, H.L.; McGarvey, L.P. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur. Respir. J. 2014, 43, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caskey, S.; Gough, A.; Rowan, S.; Gillespie, S.; Clarke, J.; Riley, M.; Megarry, J.; Nicholls, P.; Patterson, C.; Halliday, H.L.; et al. Structural and Functional Lung Impairment in Adults Survivors of Bronchopulmonary Dysplasia. Ann. Am. Thorac. Soc. 2016, 13, 1262–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, M.; Sozo, F.; Harding, R. Impact of preterm birth and bronchopulmonary dysplasia on the developing lung: Long-term consequences for respiratory health. Clin. Exp. Pharmacol. Physiol. 2013, 40, 765–773. [Google Scholar] [CrossRef]
- Vrijlandt, E.J.L.E.; Gerritsen, J.; Boezen, H.M.; Grevink, R.G.; Duiverman, E.J. Lung function and exercise capacity in young adults born prematurely. Am. J. Respir. Crit. Care Med. 2006, 173, 890–896. [Google Scholar] [CrossRef]
- Lovering, A.T.; Elliott, J.E.; Laurie, S.S.; Beasley, K.M.; Gust, C.E.; Mangum, T.S.; Gladstone, I.M.; Duke, J.W. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann. Am. Thorac. Soc. 2014, 11, 1528–1537. [Google Scholar] [CrossRef]
- Mitchell, S.H.; Teague, W.G.; Robinson, A. Reduced gas transfer at rest and during exercise in school-age survivors of bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 1998, 157, 1406–1412. [Google Scholar] [CrossRef]
- Landry, J.S.; Tremblay, G.M.; Li, P.Z.; Wong, C.; Benedetti, A.; Taivassalo, T. Lung function and bronchial hyperresponsiveness in adults born prematurely: A cohort study. Ann. Am. Thorac. Soc. 2016, 13, 17–24. [Google Scholar] [CrossRef]
- Vollsaeter, M.; Roksund, O.D.; Eide, G.E.; Markestad, T.; Halvorsen, T. Lung function after preterm birth: Development from mid-childhood to adulthood. Thorax 2013, 68, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.M.; Reddington, C.; McBride, L.; Callanan, C.; Robertson, C.; Doyle, L.W. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr. Pulmonol. 2015, 50, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.N.; Noble-Jamieson, C.M.; Elliman, A.; Bryan, E.M.; Silverman, M. Lung function in children of low birth weight. Arch. Dis. Child. 1989, 64, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzato, S.; Ridolfi, L.; Bernardi, F.; Faldella, G.; Bertelli, L. Lung function outcome at school age in very low birth weight children. Pediatr. Pulmonol. 2013, 48, 830–837. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Kwok, M.K.; Au Yeung, S.L.; Lin, S.L.; Leung, J.Y.Y.; Hui, L.L.; Li, A.M.; Leung, G.M.; Schooling, C.M. Birth weight and prematurity with lung function at ~17.5 years: “Children of 1997” birth cohort. Sci. Rep. 2020, 10, 341. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Ebrahim, S.; Davey Smith, G. Association of birth weight with adult lung function: Findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax 2005, 60, 851–885. [Google Scholar] [CrossRef] [Green Version]
- Saad, N.J.; Patel, J.; Burney, P.; Minelli, C. Birth Weight and Lung Function in Adulthood: A Systematic Review and Meta-analysis. Ann. Am. Thorac. Soc. 2017, 14, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Den Dekker, H.T.; Sonnenschein-van der Voort, A.M.M.; de Jongste, J.C.; Anessi-Maesano, I.; Arshad, S.H.; Barros, H.; Beardsmore, C.S.; Bisgaard, H.; Phar, S.C.; Craig, L.; et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J. Allergy Clin. Immunol. 2016, 137, 1026–1035. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.; Mamun, A.A.; O’Callaghan, M.; Sly, P.D. The impact of birth weight on peak lung function in young adults. Chest 2012, 142, 1603–1610. [Google Scholar] [CrossRef]
- Vrijlandt, E.J.L.E.; Boezen, H.M.; Gerritsen, J.; Stremmelaar, E.F.; Duiverman, E.J. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J. Pediatr. 2007, 150, 256–261. [Google Scholar] [CrossRef]
- Udomittipong, K.; Sly, P.; Patterson, H.; Gangell, C.; Stick, S.; Hall, G. Forced oscillations in the clinical setting in young children with neonatal lung disease. Eur. Respir. J. 2008, 31, 1292–1299. [Google Scholar] [CrossRef] [Green Version]
- Thunqvist, P.; Tufvesson, E.; Bjermer, L.; Winberg, A.; Fellman, V.; Domellöf, M.; Melén, E.; Norman, M.; Hallberg, J. Lung function after extremely preterm birth-A population-based cohort study (EXPRESS). Pediatr. Pulmonol. 2018, 53, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, E.; Bar-Yishay, E.; Prais, D.; Klinger, G.; Mei-Zahav, M.; Mussaffi, H.; Steuer, G.; Hananya, S.; Matyashuk, Y.; Gabarra, N.; et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest 2012, 142, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Logie, K.M.; O’Dea, C.A.; Banton, G.L.; Murray, C.; Wilson, A.C.; Pillow, J.J.; Hall, G.L. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 2017, 72, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Filippone, M.; Sartor, M.; Zacchello, F.; Baraldi, E. Flow limitation in infants with bronchopulmonary dysplasia and respiratory function at school age. Lancet 2003, 361, 753–754. [Google Scholar] [CrossRef]
- Moschino, L.; Stocchero, M.; Filippone, M.; Carraro, S.; Baraldi, E. Longitudinal Assessment of Lung Function in Survivors of Bronchopulmonary Dysplasia from Birth to Adulthood. The Padova BPD Study. Am. J. Respir. Crit. Care Med. 2018, 19, 134–137. [Google Scholar] [CrossRef]
- Satrell, E.; Røksund, O.; Thorsen, E.; Halvorsen, T. Pulmonary gas transfer in children and adolescents born extremely preterm. Eur. Respir. J. 2013, 42, 1536–1544. [Google Scholar] [CrossRef] [Green Version]
- Lum, S.; Kirkby, J.; Welsh, L.; Marlow, N.; Hennessy, E.; Stocks, J. Nature and severity of lung function abnormalities in extremely pre-term children at 11 years of age. Eur. Respir. J. 2011, 37, 1199–1207. [Google Scholar] [CrossRef]
- Hakulinen, A.L.; Jarvenpaa, A.; Turpeinen, M.; Sovijarvi, A. Diffusing capacity of the lung in school-aged children born very preterm, with and without bronchopulmonary dysplasia. Pediatr. Pulmonol. 1996, 360, 353–360. [Google Scholar] [CrossRef]
- Um-Bergström, P.; Hallberg, J.; Pourbazargan, M.; Berggren-Broström, E.; Ferrara, G.; Eriksson, M.J.; Nyrén, S.; Gao, J.; Lilja, G.; Lindén, A.; et al. Pulmonary outcomes in adults with a history of Bronchopulmonary Dysplasia differ from patients with asthma. Respir. Res. 2019, 20, 102. [Google Scholar] [CrossRef] [Green Version]
- De Paepe, M.E.; Mao, Q.; Powell, J.; Rubin, S.E.; Dekoninck, P.; Appel, N.; Dixon, M.; Gundogan, F. Growth of pulmonary microvasculature in ventilated preterm infants. Am. J. Respir. Crit. Care Med. 2006, 173, 204–211. [Google Scholar] [CrossRef]
- Sørensen, J.K.; Buchvald, F.; Berg, A.K.; Robinson, P.D.; Nielsen, K.G. Ventilation inhomogeneity and NO and CO diffusing capacity in ex-premature school children. Respir. Med. 2018, 140, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Bush, A.; van den Berge, M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2015, 385, 899–909. [Google Scholar] [CrossRef]
- McGeachie, M.J.; Yates, K.P.; Zhou, X.; Guo, F.; Sternberg, A.L.; Van Natta, M.L.; Wise, R.; Szefler, S.J.; Sharma, S.; Kho, A.T.; et al. Patterns of growth and decline in lung function in persistent childhood asthma. N. Engl. J. Med. 2016, 374, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, W.H.; Olinsky, A.; Doyle, L.W.; Ford, G.W.; Murton, L.J.; Callanan, C.; Slonim, L. Respiratory health and lung function in 8-year-old children of very low birth weight: A cohort study. Pediatrics 1992, 89, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, K.F.; Sellers, C.; Smith, E.O.; Rama, J.A.; Fan, L.L. Serial measurements of lung function in a cohort of young children with bronchopulmonary dysplasia. Pediatrics 2010, 125, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, W.; Huysman, M.W.; van der Wiel, E.C.; Holland, W.P.; Hop, W.C.; Brinkhorst, G.; de Jongste, J.C.; Merkus, P.J. Worsening of V0 max FRC in infants with chronic lung disease in the first year of life: A more favorable outcome after high-frequency oscillation ventilation. Am. J. Respir. Crit. Care Med. 2002, 166, 1539–1543. [Google Scholar] [CrossRef]
- Friedrich, L.; Pitrez, P.M.; Stein, R.T.; Goldani, M.; Tepper, R.; Jones, M.H. Growth rate of lung function in healthy preterm infants. Am. J. Respir. Crit. Care Med. 2007, 176, 1269–1273. [Google Scholar] [CrossRef]
- Parat, S.; Moriette, G.; Delaperche, M.F.; Escourrou, P.; Denjean, A.; Gaultier, C. Long-term pulmonary functional outcome of bronchopulmonary dysplasia and premature birth. Pediatr. Pulmonol. 1995, 20, 289–296. [Google Scholar] [CrossRef]
- Chang, H.Y.; Chang, J.H.; Chi, H.; Hsu, C.H.; Lin, C.-Y.; Jim, W.T.; Peng, C.-C. Reduced lung function at preschool age in survivors of very low birth weight preterm infants. Front. Pediatr. 2020, 8, 577–673. [Google Scholar] [CrossRef]
- Kulasekaran, K.; Gray, P.H.; Masters, B. Chronic lung disease of prematurity and respiratory outcome at eight years of age. J. Paediatr. Child Health 2007, 43, 44–48. [Google Scholar] [CrossRef]
- Thunqvist, P.; Gustafsson, P.; Norman, M.; Wickman, M.; Hallberg, J. Lung function at 6 and 18 months after preterm birth in relation to severity of bronchopulmonary dysplasia. Pediatr. Pulmonol. 2015, 50, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.K.; McEvoy, C.T. Trajectories of lung function in infants and children: Setting a course for lifelong lung health. Pediatrics 2020, 146, e2020024588. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; Hutchinson, J.L.; Donoghue, D.A.; Evans, N.J.; Simpson, J.M.; Wright, I. Prenatal predictors of chronic lung disease in very preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F40–F45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onland, W.; Debray, T.P.; Laughon, M.M.; Miedema, M.; Cools, F.; Askie, L.M.; Asselin, J.M.; Calvert, S.; Courtney, E.S.; Dani, C.; et al. Clinical prediction models for bronchopulmonary dysplasia: A systematic review and external validation study. BMC Pediatr. 2013, 13, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, S.-H.; Chiang, M.-C.; Chu, S.-M.; Hsu, J.-F.; Yao, T.-C.; Tsai, M.-H.; Hua, M.-C.; Chiu, C.-Y.; Yeh, K.-W.; Huang, J.-L.; et al. Evolution and determinants of lung function until late infancy among infants born preterm. Sci. Rep. 2020, 10, 490. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, M.; Beardsmore, C.S.; Owers-Bradley, J.; Dogaru, C.M.; Mada, M.; Ball, I.; Garipov, R.R.; Kuehni, C.E.; Spycher, B.D.; Silverman, M. Catch-up alveolarization in ex preterm children: Evidence from the magnetic resonance. Am. J. Respir. Crit. Care Med. 2013, 187, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.D. Lung Function outcome in children of premature birth. J. Paediatr. Child Health 1999, 35, 516–521. [Google Scholar] [CrossRef]
- Mahut, B.; De Blic, J.; Emond, S.; Benoist, M.R.; Jarreau, P.H.; Lacaze-Masmonteil, T.; Magny, J.F.; Delacourt, C. Chest computed tomography findings in bronchopulmonary dysplasia and correlation with lung function. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, C.; Mamou-Mani, T.; Sayegh, N.; De Blic, J.; Scheinmann, P.; Lallemand, D. Bronchopulmonary dysplasia: Value of CT in identifying pulmonary sequelae. Am. J. Roentgenol. 1994, 163, 169–172. [Google Scholar] [CrossRef]
- Howling, S.J.; Northway, W.H., Jr.; Hansell, D.M.; Moss, R.B.; Ward, S.; Müller, N.L. Pulmonary sequelae of bronchopulmonary dysplasia survivors: High-resolution CT findings. Am. J. Roentgenol. 2000, 174, 1323–1326. [Google Scholar] [CrossRef]
- Ronkainene, E.; Perhomaa, M.; Mattila, L.; Hallman, M.; Dunder, T. Structural Pulmonary Abnormalities Still Evident in Schoolchildren with New Bronchopulmonary Dysplasia. Neonatology 2018, 113, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Aukland, S.M.; Halvorsen, T.; Fosse, K.R.; Daltveit, A.K.; Rosendahl, K. High-resolution CT of the chest in children and young adults who were born prematurely: Findings in a population-based study. Am. J. Roentgenol. 2006, 187, 1012–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Mastrigt, E.; Logie, K.; Ciet, P.; Reiss, I.K.M.; Duiijts, L.; Pijnenburg, W.M.; Tiddens, H.A. Lung CT imaging in patients with bronchopulmonary dysplasia: A systematic review. Pediatr. Pulmonol. 2016, 51, 975–986. [Google Scholar] [CrossRef]
- Semple, T.; Akhtar, M.R.; Owens, C.M. Imaging Bronchopulmonary Dysplasia-A Multimodality Update. Front. Med. 2017, 4, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, E.W.; Counsell, S.J.; Hajnal, J.V.; Cox, P.N.; Kennea, N.L.; Thornton, A.S.; Bryan, A.C.; Edwards, A.D. Magnetic resonance imaging of lung water content and distribution in term and preterm infants. Am. J. Respir. Crit. Care Med. 2002, 166, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Förster, K.; Ertl-Wagner, B.; Ehrhardt, H.; Busen, H.; Sass, S.; Pomschar, A.; Naehrlich, L.; Schulze, A.; Flemmer, A.W.; Hübener, C.; et al. Altered relaxation times in MRI indicate bronchopulmonary dysplasia. Thorax 2020, 75, 184–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walkup, L.L.; Woods, J.C. Newer Imaging Techniques for Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Higano, N.S.; Spielberg, D.R.; Fleck, R.J.; Schapiro, A.H.; Walkup, L.L.; Hahn, A.D.; Tkach, J.A.; Kingma, P.S.; Merhar, S.L.; Fain, S.B.; et al. Neonatal Pulmonary Magnetic Resonance Imaging of Bronchopulmonary Dysplasia Predicts Short-Term Clinical Outcomes. Am. J. Respir. Crit. Care Med. 2018, 198, 1302–1311. [Google Scholar] [CrossRef]
- Blanken, M.O.; Rovers, M.M.; Molenaar, J.M.; Winkler-Seinstra, P.L.; Meijer, A.; Kimpen, J.L.; Bont, L. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013, 368, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Patria, M.F.; Tagliabue, C.; Longhi, B.; Esposito, S. Influenza vaccination in children at high risk of respiratory disease. Ther. Adv. Vaccines 2013, 1, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Duijts, L.; van Meel, E.R.; Moschino, L.; Baraldi, E.; Barnhoorn, M.; Brameret, W.M.; Bolton, C.E.; Boyd, J.; Buchvald, F.; Del Cerro, M.J.; et al. European Respiratory Society guideline on long-term management of children with bronchopulmonary dysplasia. Eur. Respir. J. 2020, 55, 1900788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzato, S.; Picca, M.; Romagnoli, V.; Mazzoleni, S.; Buttè, C.; Fama, M.; Pasinato, A.; Priante, E.; Cutrera, R.; Baraldi, E. Follow-up del bambino con dysplasia broncopolmonare. Area Pediatrica 2017, 18, 158–170. [Google Scholar]
- Baraldi, E.; Bonetto, G.; Zacchello, F.; Filippone, M. Low exhaled nitric oxide in school-age children with bronchopulmonary dysplasia and airflow limitation. Am. J. Respir. Crit. Care Med. 2005, 171, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Loi, B.; Vigo, G.; Baraldi, E.; Raimondi, F.; Carnielli, V.P.; Mosca, F.; De Luca, D. Lung Ultrasound to Monitor Extremely Preterm Infants and Predict Bronchopulmonary Dysplasia. A Multicenter Longitudinal Cohort Study. Am. J. Respir. Crit. Care Med. 2021, 203, 1398–1409. [Google Scholar] [CrossRef]
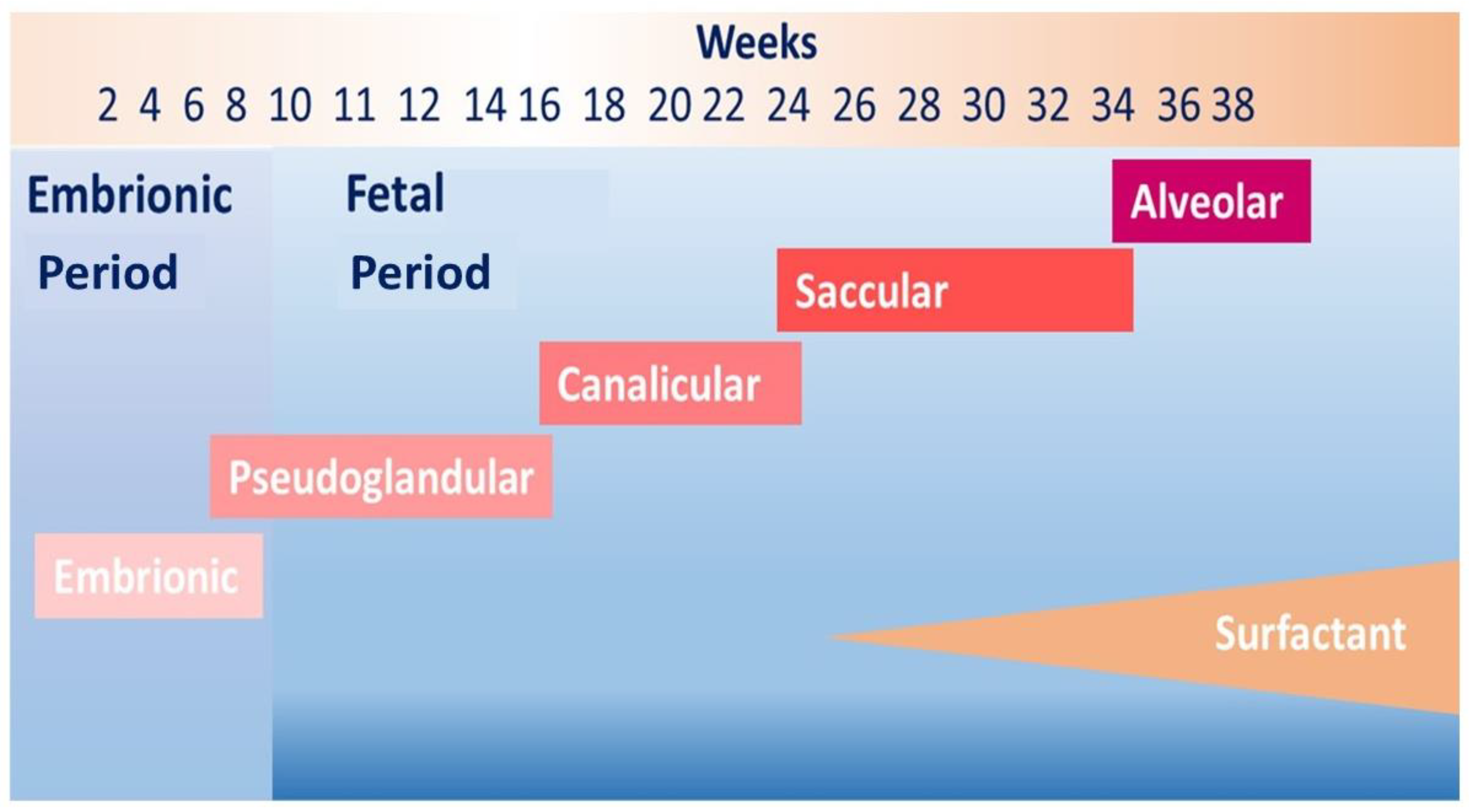


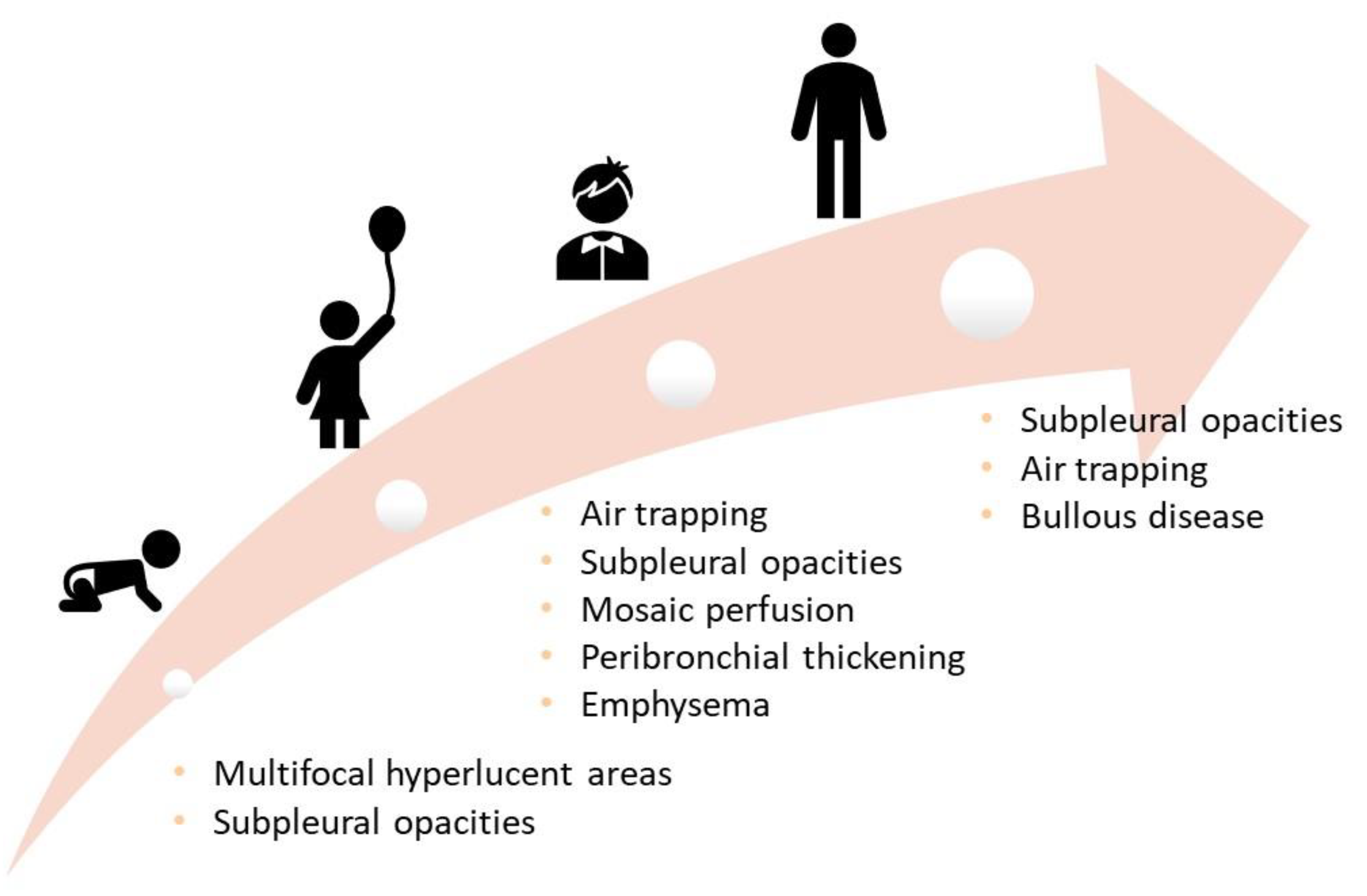

| Symptoms | Lung Function | Structural Abnormalities | |
|---|---|---|---|
| Toddler | Wheezing | Tidal flow-volume technique Rapid thoraco-abdominal compression technique (RVRTC) ↓Respiratory System Compliance | HRCT Multifocal hyperlucent areas Subpleural opacities |
| Preschool-age | Wheezing | Forced oscillation technique, multiple breath wash out tests, spirometry if compliant ↓FVC, FEV1, reactance and resistance | HRCT Air trapping Subpleural opacities Mosaic perfusion Peribronchial thickening Emphysema |
| School-age | Wheezing Cough | Spirometry and DLCO ↑RV, RV/TLC ↓FEV1, FEF25-75, DLCO | |
| Adolescence and adulthood | Breathlessness Wakening with cough Sedentary life Leg discomfort during exercise | Spirometry and DLCO, stress test ↓FEV1, FEF25-75, DLCO Fixed airflow obstruction | HRCT Air trapping Subpleural opacities Bullous disease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, P.; Dodi, G.; Ciarelli, F.; Di Pillo, S.; Chiarelli, F.; Attanasi, M. Lifelong Lung Sequelae of Prematurity. Int. J. Environ. Res. Public Health 2022, 19, 5273. https://doi.org/10.3390/ijerph19095273
Di Filippo P, Dodi G, Ciarelli F, Di Pillo S, Chiarelli F, Attanasi M. Lifelong Lung Sequelae of Prematurity. International Journal of Environmental Research and Public Health. 2022; 19(9):5273. https://doi.org/10.3390/ijerph19095273
Chicago/Turabian StyleDi Filippo, Paola, Giulia Dodi, Francesca Ciarelli, Sabrina Di Pillo, Francesco Chiarelli, and Marina Attanasi. 2022. "Lifelong Lung Sequelae of Prematurity" International Journal of Environmental Research and Public Health 19, no. 9: 5273. https://doi.org/10.3390/ijerph19095273
APA StyleDi Filippo, P., Dodi, G., Ciarelli, F., Di Pillo, S., Chiarelli, F., & Attanasi, M. (2022). Lifelong Lung Sequelae of Prematurity. International Journal of Environmental Research and Public Health, 19(9), 5273. https://doi.org/10.3390/ijerph19095273








