Strategy to Promote the Biodegradation of Phenanthrene in Contaminated Soil by a Novel Bacterial Consortium in Slurry Bioreactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Soil
2.3. Activated Sludge Domestication
2.4. Reactor Configuration and Operation
- (1)
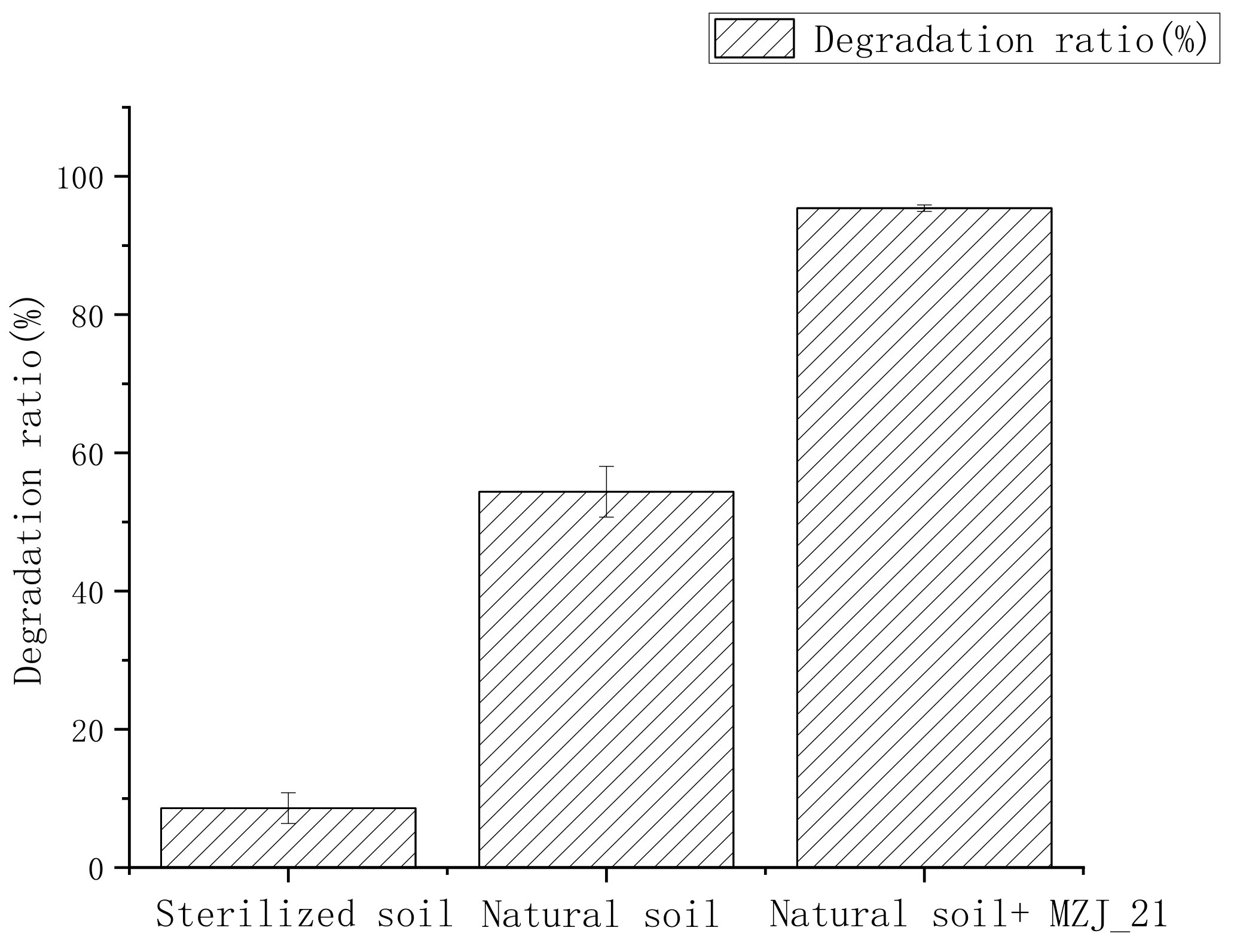
- Natural soil + MZJ_21: Firstly, we accurately weighed 200 g of natural soil with phenanthrene concentration of 200 mg/kg and added it to the aerobic slurry bioreactor. Secondly, we accurately measured 360 mL of inorganic salt medium and 40 mL MZJ_21 bacteria liquid, mixed them evenly, and put the mixture into the aerobic slurry bioreactor. The water–soil ratio in the aerobic slurry bioreactor was 2:1.
- (2)
- Natural soil: Firstly, we accurately weighed 200 g of natural soil with phenanthrene concentration of 200 mg/kg and added it to the aerobic slurry bioreactor. Secondly, we accurately measured 400 mL of inorganic salt medium and put it into the aerobic slurry bioreactor. The water–soil ratio in the aerobic slurry bioreactor was 2:1.
- (3)
- Sterile soil: Firstly, the soil samples used for the tests were sterilized at 121 °C for 30 min. Secondly, we accurately weighed 200 g of sterile soil with phenanthrene concentration of 200 mg/kg and added it to the aerobic slurry bioreactor. The phenanthrene content and soil–water ratio were the same as those in step (2).
2.5. Extraction Method of Phenanthrene
2.5.1. Solid Phase
2.5.2. Liquid Phase
2.6. Extraction Methods of EPS
2.7. Analysis Methods
2.7.1. Determination of Phenanthrene
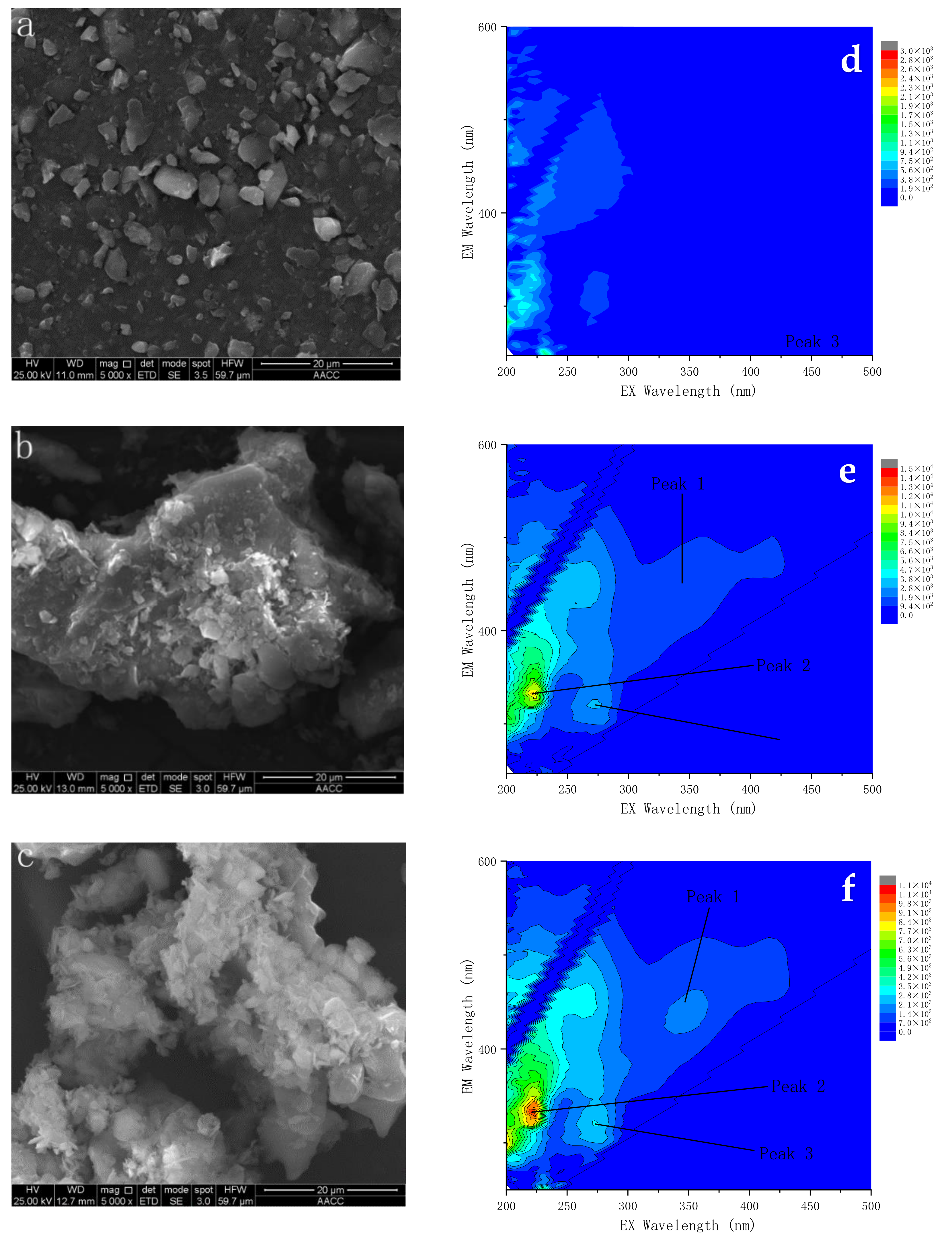
2.7.2. Fluorescent Component of EPS
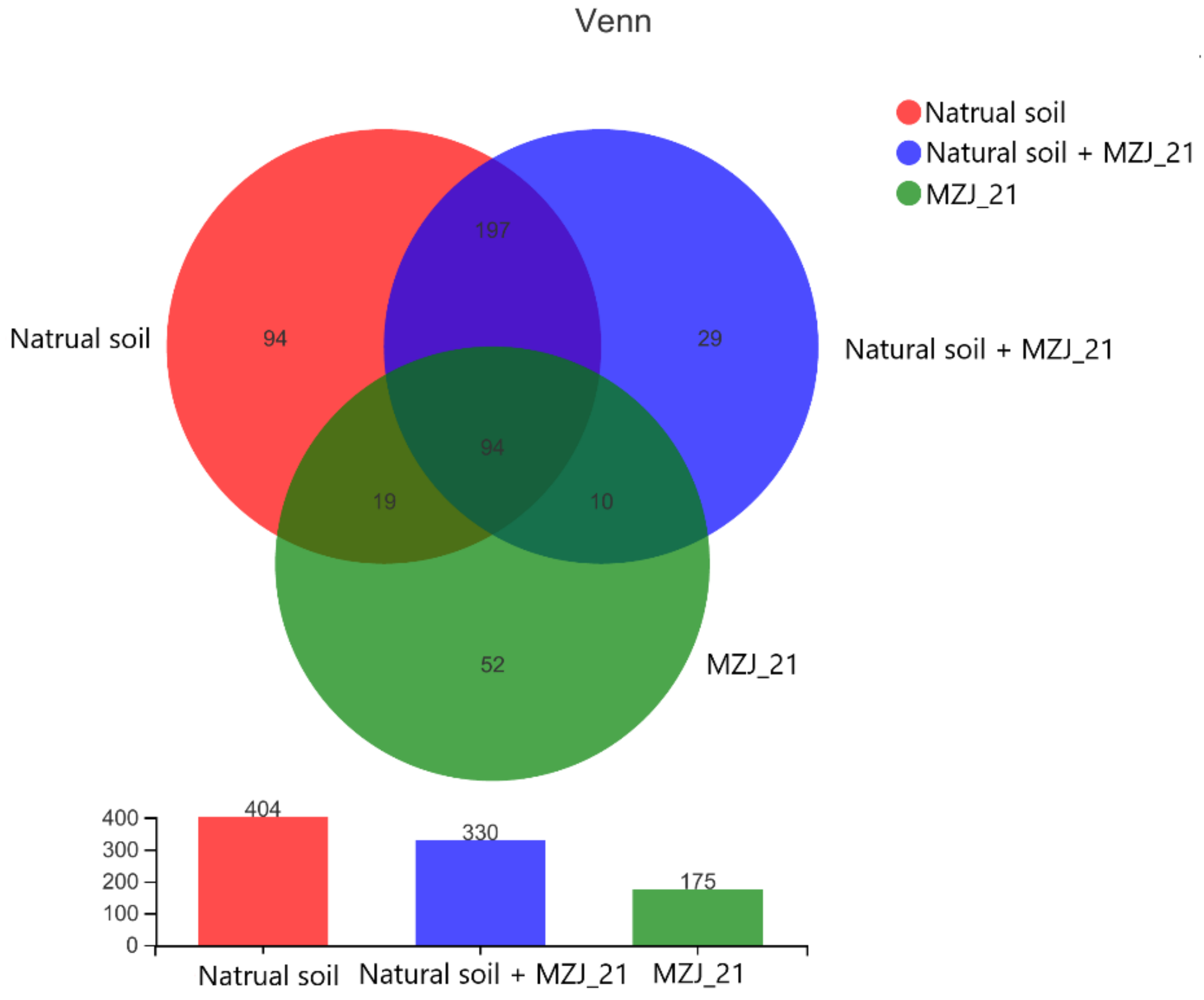
2.7.3. Microbial Diversity Analysis
2.7.4. Analysis of Other Indicators
3. Results
3.1. Degradation of Phenanthrene in Aerobic Slurry Bioreactor
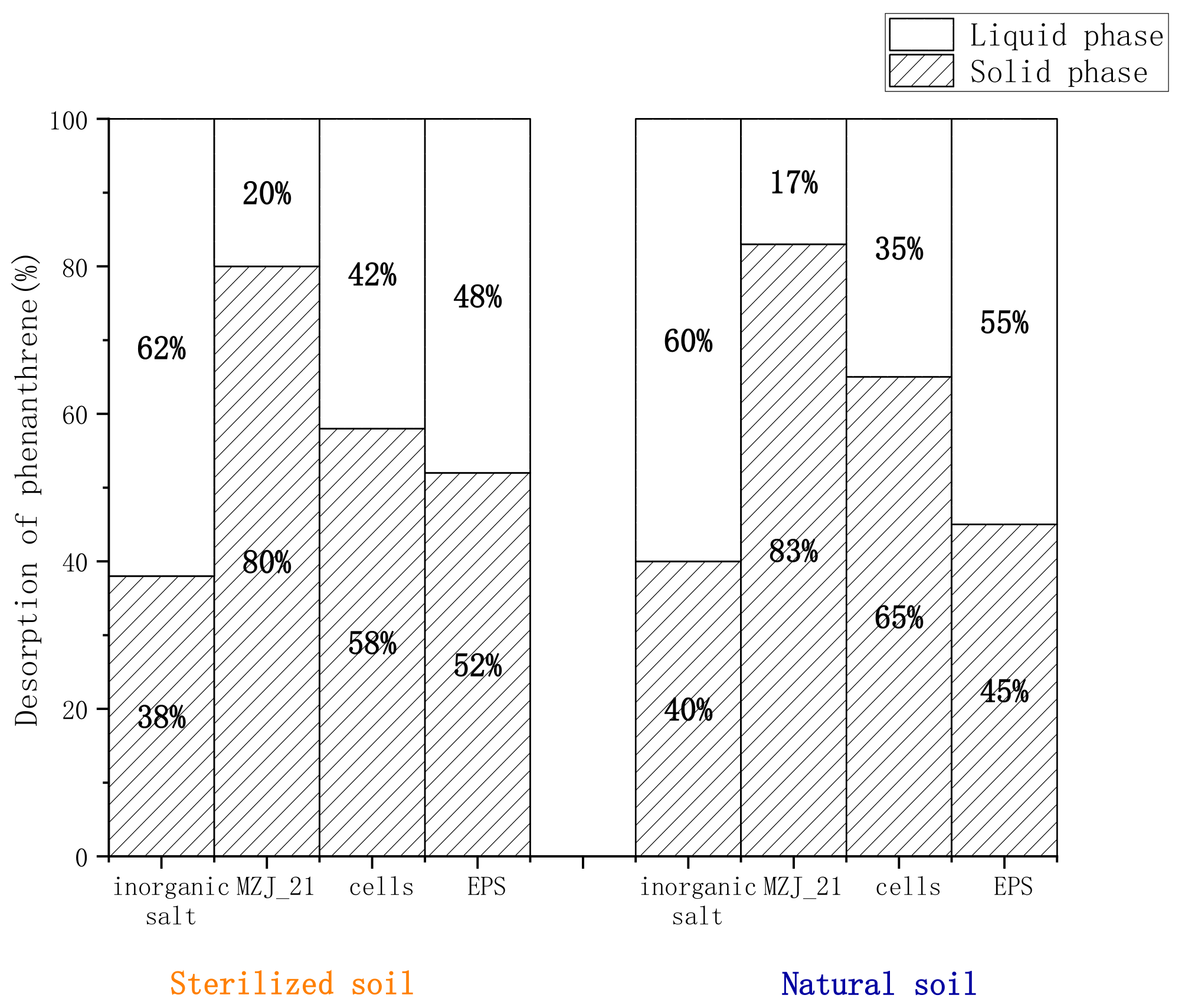
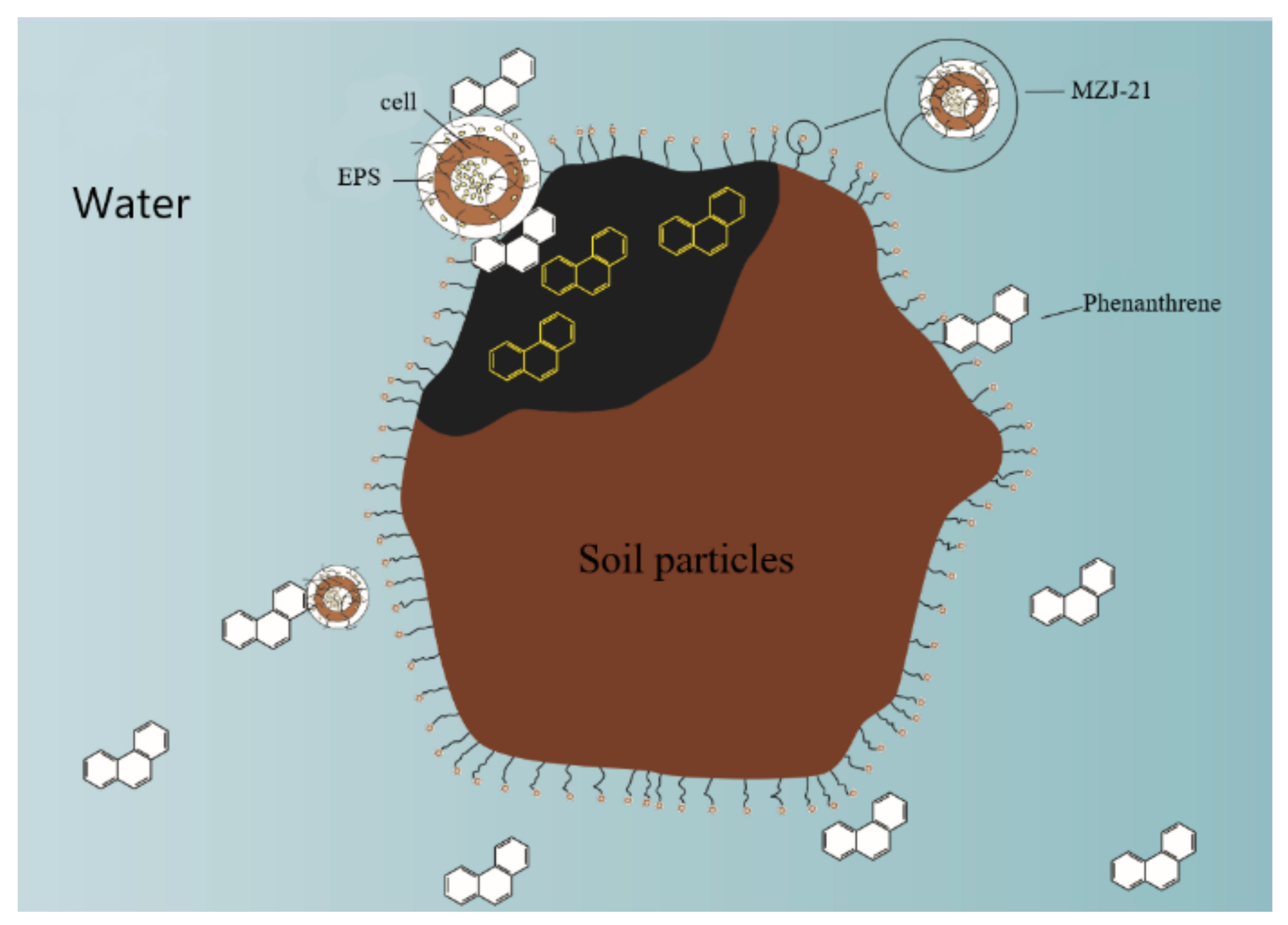
3.2. Distribution of Phenanthrene in the Aerobic Slurry Bioreactor
3.3. Effect of Extracellular Polymers on the Degradation Rate of Phenanthrene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Wang, M.; Cui, H.; Qiao, J.; Guo, D.; Wang, B.; Li, X.; Huang, H. How humic acid and Tween80 improve the phenanthrene biodegradation efficiency: Insight from cellular characteristics and quantitative proteomics. J. Hazard. Mater. 2022, 421, 126685. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, S.; Deschaux, P. The effects of polycyclic aromatic hydrocarbons on the immune system of fish: A review. Aquat. Toxicol. 2006, 77, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zedeck, M.S. Polycyclic aromatic hydrocarbons: A review. J. Environ. Pathol. Toxicol. 1980, 3, 537–567. [Google Scholar] [PubMed]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Tsibart, A.S.; Gennadiev, A.N. Polycyclic aromatic hydrocarbons in soils: Sources, behavior, and indication significance (a review). Eurasian Soil Sci. 2013, 46, 728–741. [Google Scholar] [CrossRef]
- Han, M.J.; Choi, H.T.; Song, H.G. Degradation of phenanthrene by Trametes versicolor and its laccase. J. Microbiol. 2004, 42, 94–98. [Google Scholar]
- Geng, S.; Qin, W.; Cao, W.; Wang, Y.; Ding, A.; Zhu, Y.; Fan, F.; Dou, J. Pilot-scale bioaugmentation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil using an indigenous bacterial consortium in soil-slurry bioreactors. Chemosphere 2022, 287, 132183. [Google Scholar] [CrossRef]
- Samsami, R.; Marandi, R.; Sepahi, A.A.; Sohrabi, M.R.; Mazhar, F. Bioremediation Combined with Ozonation Treatment of Polycyclic Aromatic Hydrocarbon Contaminated Soil of an Aged Oil Refinery Using Local Bacteria. Asian J. Chem. 2011, 23, 44–48. [Google Scholar]
- Zhou, Z.; Cui, J.; Xu, P.; Tang, H. Progress in biodegradation of low molecular weight polycyclic aromatic hydrocarbons. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2019, 35, 2069–2080. [Google Scholar] [CrossRef]
- Mougin, C. Bioremediation and phytoremediation of industrial PAH-polluted soils. Polycycl. Aromat. Compd. 2002, 22, 1011–1043. [Google Scholar] [CrossRef]
- Treatment Technologies for Site Cleanup: Annual Status Report. 2007. Available online: http://cfpub.epa.gov/asr/ (accessed on 26 April 2022).
- Roundtable, F. Remediation Case Studies: Bioremediation. 1995. Available online: https://www.osti.gov/biblio/56403 (accessed on 26 April 2022).
- Cai, L.; Shen, T.; Li, Z.; Cao, J.; Zuo, L.; Shen, L. Study on Degradation Characteristics of Petroleum Hydrocarbons during In-situ Bioremediation. Resour. Environ. Eng. 2018, 32, 606. [Google Scholar]
- Cassidy, D.P.; Hudak, A.J. Microorganism selection and performance in bioslurry reactors treating PAH-contaminated soil. Environ. Technol. 2002, 23, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Forjan, R.; Lores, I.; Sierra, C.; Baragano, D.; Gallego, J.L.R.; Isabel Pelaez, A. Bioaugmentation Treatment of a PAH-Polluted Soil in a Slurry Bioreactor. Appl. Sci. 2020, 10, 2837. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhang, Z.; Sun, S.; Wang, Q.; Zhong, W. Enhanced degradation of bioremediation residues in petroleum-contaminated soil using a two-liquid-phase bioslurry reactor. Chemosphere 2009, 77, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Shailaja, S.; Ramakrishna, M.; Mohan, S.V.; Sarma, P.N. Biodegradation of di-n-butyl phthalate (DnBP) in bioaugmented bioslurry phase reactor. Bioresour. Technol. 2007, 98, 1561–1566. [Google Scholar] [CrossRef]
- Nano, G.; Borroni, A.; Rota, R. Combined slurry and solid-phase bioremediation of diesel contaminated soils. J. Hazard. Mater. 2003, 100, 79–94. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Zehnder, A.J. Influence of interfaces on microbial activity. Microbiol. Rev. 1990, 54, 75–87. [Google Scholar] [CrossRef]
- Pino-Herrera, D.O.; Pechaud, Y.; Huguenot, D.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A. Removal mechanisms in aerobic slurry bioreactors for remediation of soils and sediments polluted with hydrophobic organic compounds: An overview. J. Hazard. Mater. 2017, 339, 427–449. [Google Scholar] [CrossRef]
- Strieth, D.; Schwarz, A.; Stiefelmaier, J.; Erdmann, N.; Muffler, K.; Ulber, R. New procedure for separation and analysis of the main components of cyanobacterial EPS. J. Biotechnol. 2021, 328, 78–86. [Google Scholar] [CrossRef]
- Xia, P.-F.; Li, Q.; Tan, L.-R.; Sun, X.-F.; Song, C.; Wang, S.-G. Extracellular polymeric substances protect Escherichia coli from organic solvents. Rsc Adv. 2016, 6, 59438–59444. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef]
- Jia, C.; Li, P.; Li, X.; Tai, P.; Liu, W.; Gong, Z. Degradation of pyrene in soils by extracellular polymeric substances (EPS) extracted from liquid cultures. Process Biochem. 2011, 46, 1627–1631. [Google Scholar] [CrossRef]
- Liu, A.; Ahn, I.S.; Mansfield, C.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Phenanthrene desorption from soil in the presence of bacterial extracellular polymer: Observations and model predictions of dynamic behavior. Water Res. 2001, 35, 835–843. [Google Scholar] [CrossRef]
- Jia, C.; Li, X.; Zhang, L.; Francis, D.; Tai, P.; Gong, Z.; Liu, W. Extracellular Polymeric Substances from a Fungus Are More Effective than Those from a Bacterium in Polycyclic Aromatic Hydrocarbon Biodegradation. Water Air Soil Pollut. 2017, 228, 195. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Zhu, X.; Zeng, J.; Zhao, Q.; Jiang, X. Extracellular polymeric substances govern the development of biofilm and mass transfer of polycyclic aromatic hydrocarbons for improved biodegradation. Bioresour. Technol. 2015, 193, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, X.; Hao, G.; Guo, Y.; Ren, Y.; Yu, L.; Bao, X.; Zhang, Y. Insight into the roles of tightly and loosely bound extracellular polymeric substances on a granular sludge in ammonium nitrogen removal. Bioresour. Technol. 2016, 222, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Nie, M.Q.; Wang, X.C.; Su, J.M.; Cao, W. Analysis of phenanthrene biodegradation by using FTIR, UV and GC-MS. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1047–1050. [Google Scholar] [CrossRef]
- Zhu, P.; Liao, H.-Q.; Hua, Z.-L.; Xie, F.-Z.; Tang, Z.; Zhang, L. Parallel Factor Analysis as an Analysis Technique for the Ratio of Three-Dimensional Fluorescence Peak in Taihu Lake. Spectrosc. Spectr. Anal. 2012, 32, 152–156. [Google Scholar] [CrossRef]
- Jacquin, C.; Lesage, G.; Traber, J.; Pronk, W.; Heran, M. Three-dimensional excitation and emission matrix fluorescence (3DEEM) for quick and pseudo-quantitative determination of protein- and humic-like substances in full-scale membrane bioreactor (MBR). Water Res. 2017, 118, 82–92. [Google Scholar] [CrossRef]
- Ouyang, E.-m.; Wang, W.; Long, N.; Li, H. Three-Dimensional Excitation Emission Matrix Fluorescence Spectroscopic Characterization of Loosely Bound and Tightly Bound Extracellular Polymeric Substances of Sludge. Spectrosc. Spectr. Anal. 2009, 29, 1313–1318. [Google Scholar] [CrossRef]
- Jie, Z.; Wang, J.J.; Baudon, A.; Chow, A.T. Improved Fluorescence Excitation-Emission Matrix Regional Integration to Quantify Spectra for Fluorescent Dissolved Organic Matter. J. Environ. Qual. 2013, 42, 925–930. [Google Scholar]
- Lu, C.; Hong, Y.; Liu, J.; Gao, Y.; Ma, Z.; Yang, B.; Ling, W.; Waigi, M.G. A PAH-degrading bacterial community enriched with contaminated agricultural soil and its utility for microbial bioremediation. Environ. Pollut. 2019, 251, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Bacosa, H.P.; Inoue, C. Polycyclic aromatic hydrocarbons (PAHs) biodegradation potential and diversity of microbial consortia enriched from tsunami sediments in Miyagi, Japan. J. Hazard. Mater. 2015, 283, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Martirani-Von Abercron, S.-M.; Marin, P.; Solsona-Ferraz, M.; Castaneda-Catana, M.-A.; Marques, S. Naphthalene biodegradation under oxygen-limiting conditions: Community dynamics and the relevance of biofilm-forming capacity. Microb. Biotechnol. 2017, 10, 1781–1796. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, Y.; Wu, H.; Yang, M.; Zhang, H.; Hao, Z.; Jiang, H. Isolation and characterization of a bacterial strain Hydrogenophaga sp PYR1 for anaerobic pyrene and benzo a pyrene biodegradation. RSC Adv. 2017, 7, 46690–46698. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Sharma, R.; Singh, S.B.; Nain, L. Bioremediation of PAH by Streptomyces sp. Bull. Environ. Contam. Toxicol. 2011, 86, 268–271. [Google Scholar] [CrossRef]
- Zhong, L.; Zhou, L.X.; Wang, S.M. Isolation and Identification of Phenanthrene Degrading Bacteria and Their Roles in Bioremediation of Phenanthrene-contaminated Soil. J. Agro-Environ. Sci. 2010, 29, 465–470. [Google Scholar]
- Hudson, N.; Baker, A.; Ward, D.; Reynlds, D.M.; Brunsdon, C.; Carliell-Marquet, C.; Browning, S. Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 2008, 391, 149–158. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation—Emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Gong, B.; Wu, P.; Ruan, B.; Zhang, Y.; Lai, X.; Yu, L.; Li, Y.; Dang, Z. Differential regulation of phenanthrene biodegradation process by kaolinite and quartz and the underlying mechanism. J. Hazard. Mater. 2018, 349, 51–59. [Google Scholar] [CrossRef]



| Sterilized Soil | Natural Soil | Natural Soil + MZI_21 | |
|---|---|---|---|
| Liquid phase (CFU/mL) | 3.65 ± 0.04 × 103 | 3.78 ± 0.16 × 105 | 6.45 ± 0.23 × 105 |
| Solid phase (CFU/g) | 2.02 ± 0.07 × 103 | 1.11 ± 0.33 × 107 | 6.25 ± 0.36 × 107 |
| Sample | Sobs | Shannon | Simpson | ACE | Chao | Coverage |
|---|---|---|---|---|---|---|
| MZJ_21 | 377 | 3.159 | 0.0849 | 489.159 | 484.018 | 0.997 |
| Natural Soil | 1493 | 5.697 | 0.0122 | 1633.885 | 1643.707 | 0.994 |
| MZJ_21 + Natural Soil | 949 | 4.1001 | 0.0415 | 1318.331 | 1362.255 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Mao, Z.; Zhong, L.; Yu, J.; Tang, Y. Strategy to Promote the Biodegradation of Phenanthrene in Contaminated Soil by a Novel Bacterial Consortium in Slurry Bioreactors. Int. J. Environ. Res. Public Health 2022, 19, 5515. https://doi.org/10.3390/ijerph19095515
Jiang X, Mao Z, Zhong L, Yu J, Tang Y. Strategy to Promote the Biodegradation of Phenanthrene in Contaminated Soil by a Novel Bacterial Consortium in Slurry Bioreactors. International Journal of Environmental Research and Public Health. 2022; 19(9):5515. https://doi.org/10.3390/ijerph19095515
Chicago/Turabian StyleJiang, Xuyang, Zhen Mao, Licun Zhong, Jinbiao Yu, and Yan Tang. 2022. "Strategy to Promote the Biodegradation of Phenanthrene in Contaminated Soil by a Novel Bacterial Consortium in Slurry Bioreactors" International Journal of Environmental Research and Public Health 19, no. 9: 5515. https://doi.org/10.3390/ijerph19095515





