Urban Air Pollution and Emergency Department Visits for Neoplasms and Outcomes of Blood Forming and Metabolic Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Health Data
2.2. Environmental Data
2.3. Statistical Methods
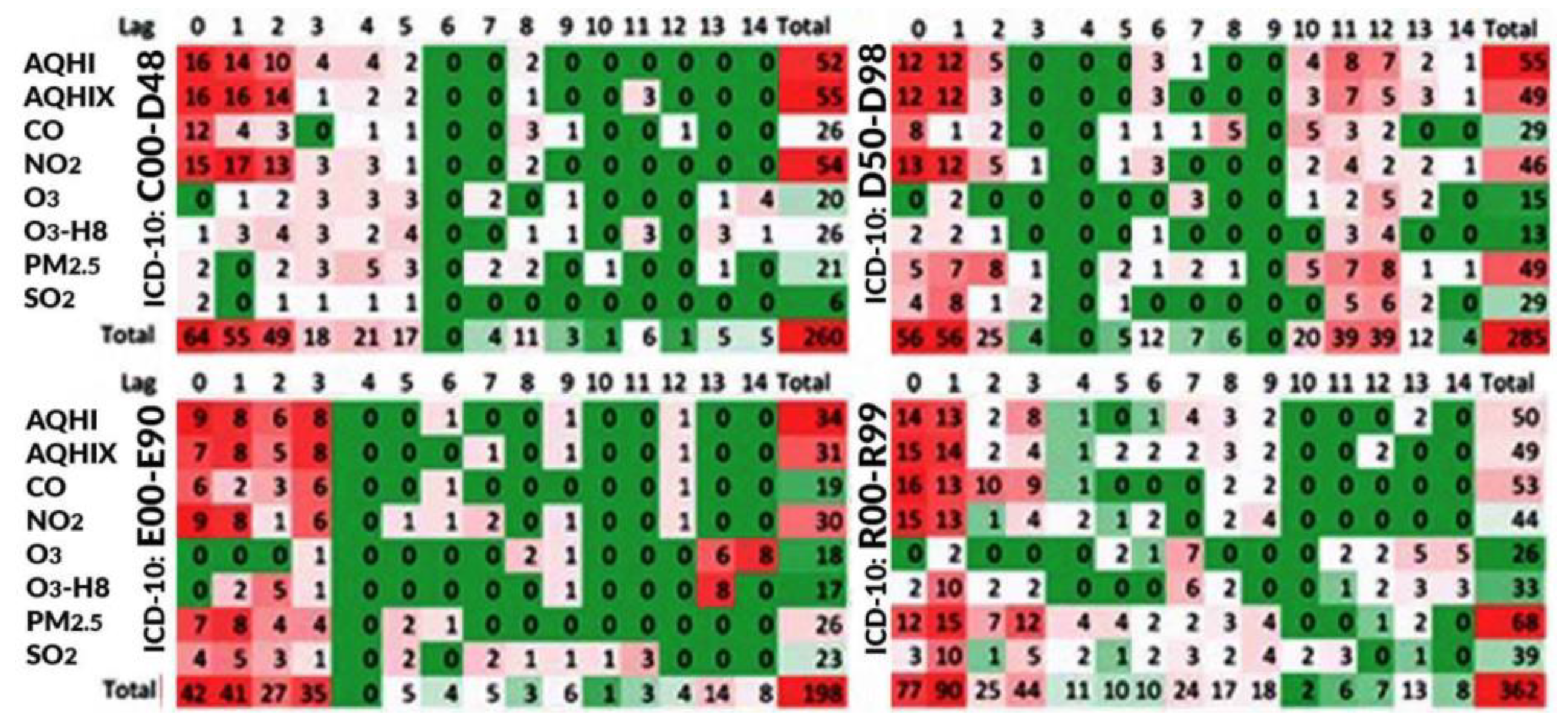
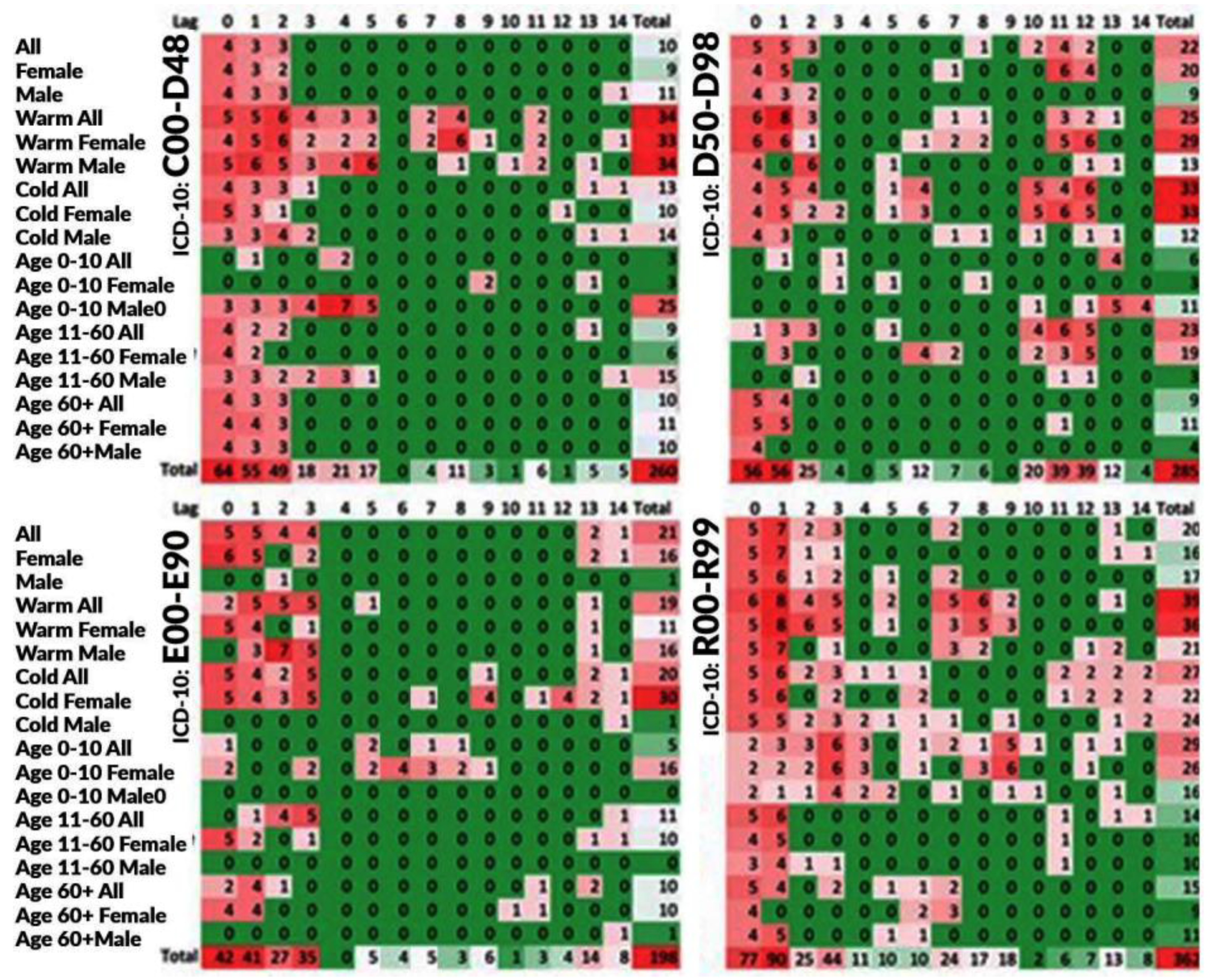
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, J.; Shi, Y.; Zhu, Y.; Chen, N.; Wang, H.; Zhang, Z.; Chen, T. Ambient air pollution and cause-specific risk of hospital admission in China: A nationwide time-series study. PLoS Med. 2020, 17, e1003188. [Google Scholar] [CrossRef] [PubMed]
- Szyszkowicz, M.; de Angelis, N. Ambient air pollution and emergency department visits in Toronto, Canada. Environ. Sci. Pollut. Res. 2021, 28, 28789–28796. [Google Scholar] [CrossRef] [PubMed]
- Szyszkowicz, M.; de Angelis, N. Urban Air Pollution and Emergency Department Visits for Cardiac and Respiratory Diseases. Adv. Environ. Eng. Res. 2021, 3, 1. [Google Scholar] [CrossRef]
- Szyszkowicz, M.; Schoen, S.; de Angelis, N. Air Pollution and Emergency Department Visits for Disease of the Genitourinary System. Environ. Health Insights 2021, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization. International Statistical Classification of Diseases and Related Health Problems; 10th Revision; World Health Organization: Geneva, Switzerland, 2016; Available online: https://icd.who.int/browse10/2016/en (accessed on 17 January 2022).
- Wang, N.; Mengersen, K.; Tong, S.; Kimlin, M.; Zhou, M.; Wang, L.; Yin, P.; Xu, Z.; Cheng, J.; Zhang, Y.; et al. Short-term association between ambient air pollution and lung cancer mortality. Environ. Res. 2019, 179, 108748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ding, R.; Lei, R.; Cheng, H.; Liu, J.; Shen, C.; Zhang, C.; Xu, Y.; Xiao, C.; Li, X.; et al. The short-term effects of air pollution on respiratory diseases and lung cancer mortality in Hefei: A time-series analysis. Respir. Med. 2019, 146, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ancajima, V.C.; Tapia, V.; Vu, B.N.; Liu, Y.; Alarcón-Yaquetto, D.E.; Gonzales, G.F. Increased Outdoor PM2.5 Concentration Is Associated with Moderate/Severe Anemia in Children Aged 6–59 Months in Lima, Peru. J. Environ. Public Health 2019, 2019, 16127845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Chen, X.; Zhang, L.; Yao, Y.; Chen, W.; Zhang, H.; Han, Y.; Xue, T.; Wang, J.; Lu, L.; et al. Associations between differences in anemia-related blood cell parameters and short-term exposure to ambient particle pollutants in middle-aged and elderly residents in Beijing, China. Sci. Total Environ. 2021, 816, 151520. [Google Scholar] [CrossRef] [PubMed]
- Zaccarelli-Marino, M.A.; Alessi, R.; Balderi, T.Z.; Martins, M.A.G. Association between the Occurrence of Primary Hypothyroidism and the Exposure of the Population Near to Industrial Pollutants in São Paulo State, Brazil. Int. J. Environ. Res. Public Health 2019, 16, 3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhu, G.; Zhang, L.; Chen, K. Particulate matter pollution and hospital outpatient visits for endocrine, digestive, urological, and dermatological diseases in Nanjing, China. Environ. Pollut. 2020, 261, 114205. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, A.; Schindeler, S.; Jalaludin, B.; Smith, W. Health effects of the September 2009 dust storm in Sydney, Australia: Did emergency department visits and hospital admissions increase? Environ. Health 2013, 12, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NACRS. The National Ambulatory Care Reporting System, Canadian Institute for Health Information (CIHI), Canada. Available online: https://www.cihi.ca/en/national-ambulatory-care-reporting-system-metadata (accessed on 30 September 2020).
- Gibson, D.; Richards, H.; Chapman, A. The National Ambulatory Care Reporting System: Factors that affect the quality of its emergency data. Int. J. Inf. Qual. 2008, 2, 97. [Google Scholar] [CrossRef]
- NAPS. National Air Pollution Surveillance Program. Available online: https://www.canada.ca/en/environment-climate-change/services/air-pollution/monitoring-networks-data/national-air-pollution-program.html (accessed on 30 September 2020).
- Vicedo-Cabrera, A.M.; Sera, F.; Liu, C.; Armstrong, B.; Milojevic, A.; Guo, Y.; Tong, S.; Lavigne, E.; Kyselý, J.; Urban, A.; et al. Short term association between ozone and mortality: Global two stage time series study in 406 locations in 20 countries. BMJ 2020, 368, m108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieb, D.M.; Burnett, R.T.; Smith-Doiron, M.; Brion, O.; Shin, H.H.; Economou, V. A New Multipollutant, No-Threshold Air Quality Health Index Based on Short-Term Associations Observed in Daily Time-Series Analyses. J. Air Waste Manag. Assoc. 2008, 58, 435–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maclure, M. The Case-Crossover Design: A Method for Studying Transient Effects on the Risk of Acute Events. Am. J. Epidemiol. 1991, 133, 144–153. [Google Scholar] [CrossRef]
- Janes, H.; Sheppard, L.; Lumley, T. Case-crossover analyses of air pollution exposure data: Referent selection strategies and their implications for bias. Epidemiology 2005, 16, 717–726. [Google Scholar] [CrossRef]
- Szyszkowicz, M. Use of generalized linear mixed models to examine the association between air pollution and health out-comes. Int. J. Occup. Med. Environ. Health. 2006, 19, 224–227. [Google Scholar] [CrossRef]
- Armstrong, B.G.; Gasparrini, A.; Tobias, A. Conditional Poisson models: A flexible alternative to conditional logistic case cross-over analysis. BMC Med. Res. Methodol. 2014, 14, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, H.; Firth, D. Generalized Nonlinear Models in R: An Overview of the gnm Package. (Version 1.1-1, 2020-02-02). Available online: https://cran.r-project.org/web/packages/gnm/vignettes/gnmOverview.pdf (accessed on 16 October 2021).
- Lukina, A.O.; Maquiling, A.; Burstein, B.; Szyszkowicz, M. Exposure to urban air pollution and emergency department visits for diseases of the ear and mastoid processes. Atmos. Pollut. Res. 2021, 12, 101198. [Google Scholar] [CrossRef]

| C00D48 | Frequency | % | D50D89 | Frequency | % |
| D12 | 96,198 | 28.9 | D64 | 36,305 | 48.4 |
| C44 | 26,088 | 7.8 | D57 | 10,261 | 13.7 |
| C50 | 18,970 | 5.7 | D50 | 8490 | 11.3 |
| C67 | 16,003 | 4.8 | D70 | 6390 | 8.5 |
| D25 | 13,043 | 3.9 | D69 | 4350 | 5.8 |
| C34 | 12,117 | 3.6 | D68 | 3152 | 4.2 |
| D41 | 11,075 | 3.3 | D61 | 1473 | 2.0 |
| C18 | 8324 | 2.5 | D86 | 659 | 0.9 |
| D17 | 8269 | 2.5 | D72 | 589 | 0.8 |
| C61 | 7776 | 2.3 | D75 | 500 | 0.7 |
| D24 | 7413 | 2.2 | D53 | 387 | 0.5 |
| D22 | 7259 | 2.2 | D66 | 367 | 0.5 |
| C78 | 5382 | 1.6 | D56 | 358 | 0.5 |
| D05 | 4703 | 1.4 | D58 | 312 | 0.4 |
| C79 | 4654 | 1.4 | D59 | 292 | 0.4 |
| D37 | 4462 | 1.3 | D73 | 217 | 0.3 |
| D06 | 4310 | 1.3 | D52 | 194 | 0.3 |
| D23 | 3964 | 1.2 | D62 | 142 | 0.2 |
| C20 | 3855 | 1.2 | D51 | 123 | 0.2 |
| D27 | 3798 | 1.1 | D80 | 94 | 0.1 |
| E00E90 | Frequency | % | R00R99 | Frequency | % |
| E11 | 27,299 | 25.6 | R10 | 512,573 | 22.0 |
| E87 | 19,190 | 18.0 | R07 | 398,248 | 17.1 |
| E86 | 13,929 | 13.0 | R51 | 111,339 | 4.8 |
| E14 | 13,686 | 12.8 | R50 | 111,089 | 4.8 |
| E10 | 13,302 | 12.5 | R42 | 99,923 | 4.3 |
| E16 | 4524 | 4.2 | R06 | 94,296 | 4.1 |
| E04 | 3926 | 3.7 | R11 | 92,029 | 4.0 |
| E83 | 3827 | 3.6 | R55 | 85,902 | 3.7 |
| E05 | 1105 | 1.0 | R31 | 75,426 | 3.2 |
| E03 | 775 | 0.7 | R00 | 55,931 | 2.4 |
| E61 | 539 | 0.5 | R53 | 55,390 | 2.4 |
| E06 | 494 | 0.5 | R56 | 52,139 | 2.2 |
| E27 | 430 | 0.4 | R04 | 51,807 | 2.2 |
| E88 | 372 | 0.4 | R05 | 50,436 | 2.2 |
| E28 | 294 | 0.3 | R33 | 44,464 | 1.9 |
| E85 | 296 | 0.3 | R21 | 42,862 | 1.8 |
| E65 | 275 | 0.3 | R45 | 36,870 | 1.6 |
| E07 | 271 | 0.3 | R22 | 31,312 | 1.4 |
| E84 | 250 | 0.2 | R20 | 28,711 | 1.2 |
| E80 | 228 | 0.2 | R41 | 26,015 | 1.1 |
| Lags | Lag 0 | Lag 1 | Lag 2 | |||
|---|---|---|---|---|---|---|
| Strata | RR | 95%CI | RR | 95%CI | RR | 95%CI |
| All | 1.048 | (1.032, 1.063) | 1.032 | (1.016, 1.047) | 1.022 | (1.006, 1.037) |
| Female | 1.050 | (1.034, 1.067) | 1.031 | (1.014, 1.047) | 1.021 | (1.004, 1.038) |
| Male | 1.045 | (1.028, 1.062) | 1.033 | (1.016, 1.050) | 1.022 | (1.005, 1.040) |
| Warm All | 1.031 | (1.019, 1.044) | 1.020 | (1.008, 1.033) | 1.025 | (1.012, 1.038) |
| Warm Female | 1.031 | (1.017, 1.045) | 1.020 | (1.006, 1.034) | 1.027 | (1.013, 1.042) |
| Warm Male | 1.032 | (1.018, 1.046) | 1.021 | (1.007, 1.035) | 1.023 | (1.008, 1.037) |
| Cold All | 1.070 | (1.052, 1.090) | 1.048 | (1.029, 1.067) | 1.021 | (1.003, 1.040) |
| Cold Female | 1.078 | (1.058, 1.098) | 1.046 | (1.027, 1.066) | 1.017 | (0.998, 1.037) |
| Cold Male | 1.063 | (1.042, 1.083) | 1.049 | (1.029, 1.070) | 1.025 | (1.005, 1.045) |
| Age 0–10 All | 1.023 | (0.958, 1.092) | 1.063 | (0.996, 1.134) | 1.059 | (0.991, 1.133) |
| Age 0–10 Female | 0.955 | (0.879, 1.037) | 1.025 | (0.943, 1.115) | 1.020 | (0.937, 1.110) |
| Age 0–10 Male0 | 1.094 | (1.010, 1.184) | 1.099 | (1.017, 1.188) | 1.099 | (1.013, 1.192) |
| Age 11–60 All | 1.050 | (1.033, 1.067) | 1.025 | (1.008, 1.042) | 1.019 | (1.002, 1.036) |
| Age 11–60 Female | 1.047 | (1.028, 1.066) | 1.020 | (1.002, 1.039) | 1.015 | (0.996, 1.034) |
| Age 11–60 Male | 1.055 | (1.034, 1.076) | 1.032 | (1.011, 1.054) | 1.025 | (1.004, 1.047) |
| Age 60+ All | 1.046 | (1.030, 1.063) | 1.037 | (1.020, 1.053) | 1.023 | (1.007, 1.040) |
| Age 60+ Female | 1.055 | (1.036, 1.074) | 1.042 | (1.023, 1.061) | 1.027 | (1.008, 1.046) |
| Age 60+Male | 1.040 | (1.022, 1.058) | 1.032 | (1.014, 1.051) | 1.020 | (1.002, 1.038) |
| Lags | Lag 0 | Lag 1 | Lag 2 | |||
|---|---|---|---|---|---|---|
| Strata | RR | 95%CI | RR | 95%CI | RR | 95%CI |
| All | 1.025 | (1.011, 1.039) | 1.026 | (1.012, 1.040) | 1.014 | (1.000, 1.028) |
| Female | 1.024 | (1.007, 1.042) | 1.030 | (1.012, 1.048) | 1.007 | (0.989, 1.025) |
| Male | 1.026 | (1.006, 1.046) | 1.021 | (1.001, 1.042) | 1.023 | (1.003, 1.043) |
| Warm All | 1.025 | (1.012, 1.038) | 1.022 | (1.009, 1.035) | 1.013 | (1.000, 1.026) |
| Warm Female | 1.029 | (1.012, 1.046) | 1.027 | (1.010, 1.045) | 0.999 | (0.982, 1.016) |
| Warm Male | 1.021 | (1.002, 1.039) | 1.015 | (0.997, 1.034) | 1.031 | (1.012, 1.050) |
| Cold All | 1.027 | (1.012, 1.042) | 1.032 | (1.017, 1.047) | 1.017 | (1.002, 1.032) |
| Cold Female | 1.022 | (1.004, 1.042) | 1.035 | (1.015, 1.054) | 1.018 | (0.999, 1.037) |
| Cold Male | 1.034 | (1.012, 1.056) | 1.028 | (1.006, 1.050) | 1.015 | (0.993, 1.037) |
| Age 0–10 All | 1.037 | (0.983, 1.094) | 1.032 | (0.978, 1.089) | 1.001 | (0.948, 1.057) |
| Age 0–10 Female | 1.036 | (0.964, 1.115) | 1.015 | (0.943, 1.093) | 0.953 | (0.885, 1.027) |
| Age 0–10 Male0 | 1.039 | (0.969, 1.115) | 1.048 | (0.977, 1.124) | 1.042 | (0.970, 1.119) |
| Age 11–60 All | 1.014 | (0.994, 1.034) | 1.026 | (1.005, 1.046) | 1.024 | (1.004, 1.045) |
| Age 11–60 Female | 1.017 | (0.991, 1.043) | 1.034 | (1.008, 1.061) | 1.022 | (0.996, 1.049) |
| Age 11–60 Male | 1.010 | (0.979, 1.041) | 1.014 | (0.983, 1.046) | 1.028 | (0.997, 1.060) |
| Age 60+ All | 1.033 | (1.014, 1.052) | 1.026 | (1.007, 1.045) | 1.007 | (0.988, 1.026) |
| Age 60+ Female | 1.030 | (1.005, 1.055) | 1.028 | (1.003, 1.053) | 0.999 | (0.975, 1.024) |
| Age 60+Male | 1.036 | (1.009, 1.063) | 1.024 | (0.997, 1.052) | 1.017 | (0.990, 1.044) |
| Lags | Lag 0 | Lag 1 | Lag 2 | |||
|---|---|---|---|---|---|---|
| Strata | RR | 95%CI | RR | 95%CI | RR | 95%CI |
| All | 1.014 | (1.003, 1.025) | 1.013 | (1.003, 1.024) | 1.011 | (1.001, 1.022) |
| Female | 1.024 | (1.009, 1.039) | 1.018 | (1.004, 1.033) | 1.009 | (0.995, 1.024) |
| Male | 1.004 | (0.989, 1.019) | 1.008 | (0.993, 1.023) | 1.014 | (0.999, 1.029) |
| Warm All | 1.007 | (0.997, 1.018) | 1.013 | (1.003, 1.023) | 1.012 | (1.002, 1.022) |
| Warm Female | 1.019 | (1.006, 1.033) | 1.016 | (1.002, 1.030) | 1.003 | (0.989, 1.016) |
| Warm Male | 0.995 | (0.981, 1.009) | 1.009 | (0.995, 1.024) | 1.022 | (1.008, 1.037) |
| Cold All | 1.023 | (1.011, 1.035) | 1.012 | (1.001, 1.024) | 1.012 | (1.000, 1.024) |
| Cold Female | 1.030 | (1.014, 1.047) | 1.020 | (1.003, 1.036) | 1.021 | (1.004, 1.037) |
| Cold Male | 1.015 | (0.999, 1.031) | 1.005 | (0.989, 1.021) | 1.003 | (0.987, 1.019) |
| Age 0–10 All | 1.065 | (1.001, 1.133) | 1.026 | (0.963, 1.092) | 1.010 | (0.948, 1.075) |
| Age 0–10 Female | 1.098 | (1.016, 1.187) | 0.988 | (0.912, 1.072) | 0.974 | (0.898, 1.057) |
| Age 0–10 Male0 | 1.034 | (0.955, 1.120) | 1.061 | (0.980, 1.147) | 1.040 | (0.960, 1.127) |
| Age 11–60 All | 1.014 | (0.998, 1.030) | 1.010 | (0.994, 1.026) | 1.018 | (1.002, 1.035) |
| Age 11–60 Female | 1.028 | (1.005, 1.051) | 1.016 | (0.994, 1.039) | 1.021 | (0.998, 1.045) |
| Age 11–60 Male | 1.001 | (0.979, 1.023) | 1.004 | (0.982, 1.026) | 1.016 | (0.993, 1.038) |
| Age 60+ All | 1.012 | (0.998, 1.027) | 1.015 | (1.001, 1.030) | 1.006 | (0.992, 1.021) |
| Age 60+ Female | 1.019 | (1.000, 1.038) | 1.021 | (1.002, 1.040) | 1.003 | (0.984, 1.022) |
| Age 60+Male | 1.005 | (0.984, 1.025) | 1.009 | (0.988, 1.030) | 1.011 | (0.990, 1.032) |
| Lags | Lag 0 | Lag 1 | Lag 2 | |||
|---|---|---|---|---|---|---|
| Strata | RR | 95%CI | RR | 95%CI | RR | 95%CI |
| All | 1.005 | (1.002, 1.008) | 1.006 | (1.003, 1.009) | 1.004 | (1.001, 1.007) |
| Female | 1.005 | (1.002, 1.009) | 1.006 | (1.002, 1.009) | 1.004 | (1.001, 1.008) |
| Male | 1.004 | (1.001, 1.008) | 1.006 | (1.002, 1.009) | 1.003 | (0.999, 1.007) |
| Warm All | 1.002 | (0.999, 1.005) | 1.005 | (1.002, 1.008) | 1.002 | (0.999, 1.005) |
| Warm Female | 1.002 | (0.999, 1.006) | 1.005 | (1.002, 1.009) | 1.004 | (1.001, 1.008) |
| Warm Male | 1.001 | (0.997, 1.004) | 1.004 | (1.001, 1.008) | 1.000 | (0.996, 1.003) |
| Cold All | 1.007 | (1.004, 1.010) | 1.007 | (1.004, 1.009) | 1.004 | (1.001, 1.007) |
| Cold Female | 1.008 | (1.004, 1.011) | 1.006 | (1.003, 1.010) | 1.004 | (1.000, 1.007) |
| Cold Male | 1.006 | (1.003, 1.010) | 1.007 | (1.003, 1.010) | 1.005 | (1.002, 1.009) |
| Age 0–10 All | 1.013 | (1.005, 1.022) | 1.011 | (1.003, 1.020) | 1.011 | (1.002, 1.019) |
| Age 0–10 Female | 1.011 | (1.000, 1.023) | 1.009 | (0.998, 1.020) | 1.011 | (1.000, 1.022) |
| Age 0–10 Male0 | 1.015 | (1.005, 1.025) | 1.013 | (1.003, 1.023) | 1.010 | (1.000, 1.021) |
| Age 11–60 All | 1.004 | (1.001, 1.007) | 1.005 | (1.002, 1.009) | 1.003 | (1.000, 1.006) |
| Age 11–60 Female | 1.006 | (1.002, 1.010) | 1.006 | (1.002, 1.010) | 1.003 | (0.999, 1.007) |
| Age 11–60 Male | 1.002 | (0.998, 1.006) | 1.004 | (1.000, 1.008) | 1.003 | (0.998, 1.007) |
| Age 60+ All | 1.004 | (1.000, 1.008) | 1.006 | (1.001, 1.010) | 1.003 | (0.998, 1.007) |
| Age 60+ Female | 1.004 | (0.999, 1.009) | 1.004 | (0.999, 1.010) | 1.004 | (0.999, 1.009) |
| Age 60+Male | 1.005 | (0.999, 1.010) | 1.007 | (1.001, 1.012) | 1.001 | (0.996, 1.007) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyszkowicz, M.; Lukina, A.; Dinu, T. Urban Air Pollution and Emergency Department Visits for Neoplasms and Outcomes of Blood Forming and Metabolic Systems. Int. J. Environ. Res. Public Health 2022, 19, 5603. https://doi.org/10.3390/ijerph19095603
Szyszkowicz M, Lukina A, Dinu T. Urban Air Pollution and Emergency Department Visits for Neoplasms and Outcomes of Blood Forming and Metabolic Systems. International Journal of Environmental Research and Public Health. 2022; 19(9):5603. https://doi.org/10.3390/ijerph19095603
Chicago/Turabian StyleSzyszkowicz, Mieczysław, Anna Lukina, and Tatiana Dinu. 2022. "Urban Air Pollution and Emergency Department Visits for Neoplasms and Outcomes of Blood Forming and Metabolic Systems" International Journal of Environmental Research and Public Health 19, no. 9: 5603. https://doi.org/10.3390/ijerph19095603
APA StyleSzyszkowicz, M., Lukina, A., & Dinu, T. (2022). Urban Air Pollution and Emergency Department Visits for Neoplasms and Outcomes of Blood Forming and Metabolic Systems. International Journal of Environmental Research and Public Health, 19(9), 5603. https://doi.org/10.3390/ijerph19095603






