Chronic Microplastic Exposure and Cadmium Accumulation in Blue Crabs
Abstract
:1. Introduction
2. Material and Methods
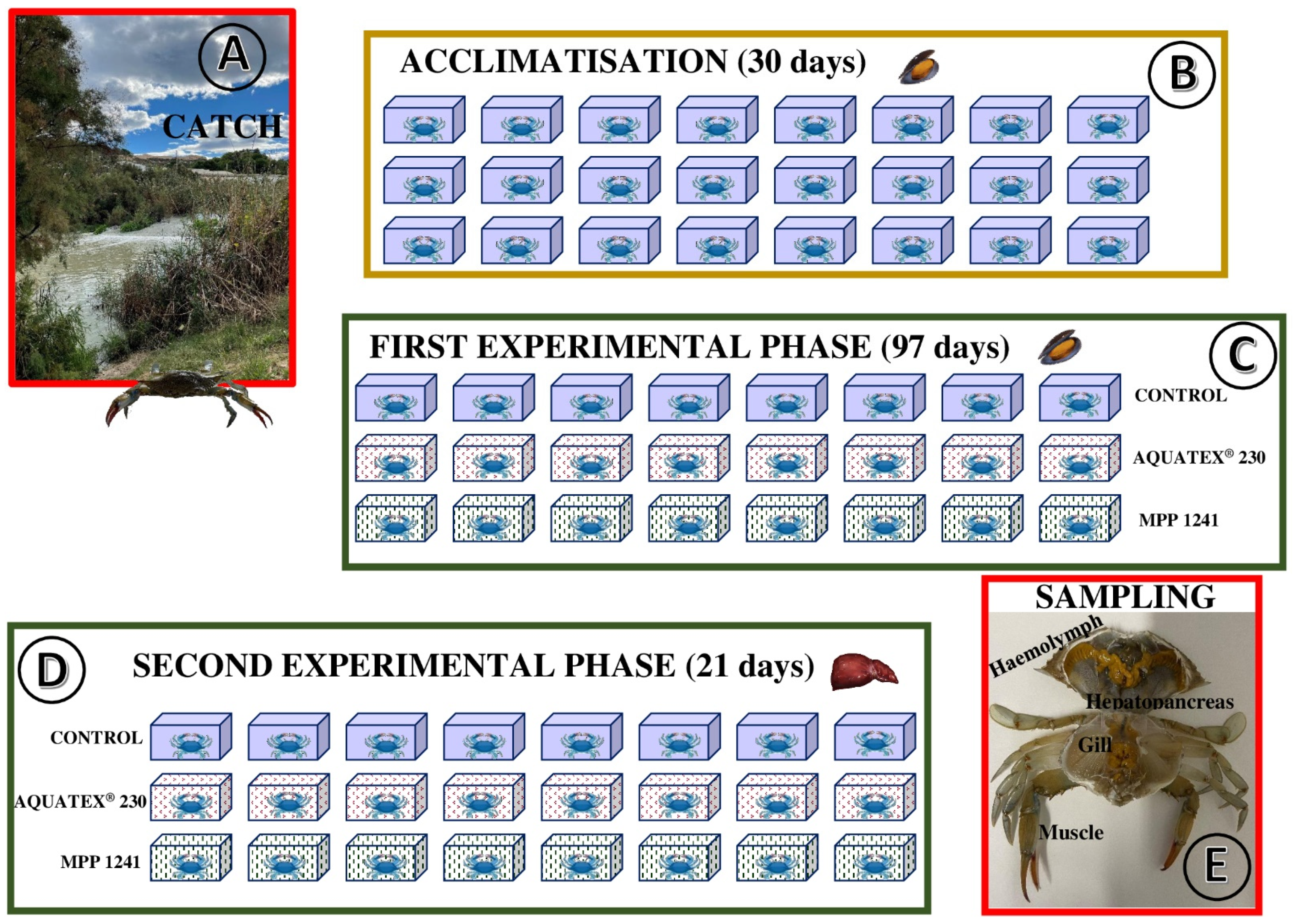
2.1. Capture and Acclimatisation
2.2. Experimental Procedure
2.3. Cd Analysis
2.4. Data Analysis
3. Results
4. Discussion
4.1. Effects of Microplastics
4.2. Distribution, Accumulation, and Excretion of Cd
4.3. Food Safety
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henkel, G.; Krebs, B. Metallothioneins: Zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features-structural aspects and biological implications. Chem. Rev. 2004, 104, 801–824. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Q.; Cao, T.M.; Lin, Q.M. Characteristics of trapping copper ions with scrolled ferritin reactor in the flowing seawater. Environ. Sci. Technol. 2004, 38, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; An, K.W.; Nelson, E.R.; Habibi, H.R. Cadmium affects the expression of metallothionein (MT) and glutathione peroxidase (GPX) mRNA in goldfish, Carassius auratus. Comp. Bioquímica. Physiol. Part C Toxicol. Farmacol. 2007, 145, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Agnello, M.; Bosco, L.; Roccheri, M.C. Sea urchin embryos exposed to cadmium as an experimental model for studying the relationship between autophagy and apoptosis. Mar. Environ. Res. 2014, 93, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Morrow, H. Cadmium and cadmium alloys. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Othmer, K., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2001; pp. 471–507. [Google Scholar]
- Falcó, G.; Nadal, M.; Llobet, J.M.; Domingo, J.L. Riesgo tóxico por metales presentes en alimentos. In Toxicología Alimentaria; Cameán, A.M., Repetto, M., Eds.; Diaz de Santos: Madrid, Spain, 2006; pp. 309–326. [Google Scholar]
- Cullen, J.T.; Maldonado, M.T. Biogeochemistry of cadmium and its release to the environment. Met. Ions Life Sci. 2013, 11, 31–62. [Google Scholar]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health. 2020, 17, 3782. [Google Scholar] [CrossRef]
- Commission Regulation (EC) Nº 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24.
- Commission Regulation (EU) Nº 488/2014 of 12 May 2014 amending Regulation (EC) Nº 1881/2006 as regards maximum levels of cadmium in foodstuff. Off. J. Eur. Union 2014, L138, 75–79.
- Commission Regulation (EU) 2021/1323 of 10 August 2021 amending Regulation (EC) Nº 1881/2006 as regards maximum levels of cadmium in certain foodstuffs. Off. J. Eur. Union 2021, L288, 13–18.
- European Food Safety Authority. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food. EFSA J. 2009, 980, 1–139. [Google Scholar]
- European Food Safety Authority. EFSA Panel on Contaminants in the Food Chain (CONTAM).; Scientific Opinion on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- European Commission. Information Note. Consumption of Brown Crab Meat; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Rhodes, C.J. Plastic pollution and potential solutions. Sci. Pro. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Agamuthu, P.; Mehran, S.B.; Norkhairah, A.; Norkhairiyah, A. Marine debris: A review of impacts and global initiatives. Waste Manag. Res. 2019, 37, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- Nachev, M.; Sures, B. Environmental parasitology: Parasites as accumulation bioindicators in the marine environment. J. Sea Res. 2015, 113, 45–50. [Google Scholar] [CrossRef]
- Munuera, P.; Salvat-Leal, I.; Belmonte, A.; Romero, D. Can Microplastics Influence the Accumulation of Pb in Tissues of Blue Crab? Int. J. Environ. Res. Public Health. 2021, 18, 3599. [Google Scholar] [CrossRef] [PubMed]
- Menezes, E.J.; Cruz, B.P.; Martins, C.M.G.; Maciel, F.E. Copper exposure alters the metabolism of the blue crab Callinectes sapidus submitted to osmotic shock. Mar. Pollut. 2020, 150, 110743. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Mohammadi, R.; Farjadfard, S.; Esmaeili, H.; Saberi, M.; Sahebi, S.; Dobaradaran, S.; Ramavandi, B. Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: Real wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 6336–6347. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Boots, B.; O’Connor, N.E.; Thompson, R. Microplastics affect the ecological functioning of an important biogenic habitat. Environ. Sci. Technol. 2017, 51, 68–77. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Calò, M.; Naccari, F. Heavy metals in liver and muscle of bluefin tuna (Thunnus thynnus) caught in the Straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef]
- Vizzini, S.; Tramati, C.; Mazzola, A. Comparison of stable isotope composition and inorganic and organic contaminant levels in wild and farmed bluefin tuna, Thunnus thynnus, in the Mediterranean Sea. Chemosphere 2010, 78, 1236–1243. [Google Scholar] [CrossRef]
- World Health Organization. GEMS/Food-EURO. Second Workshop on Reliable Evaluation of Low-Level Contamination of Food: Report on a Workshop in the Frame of GEMS/Food-Euro; WHO: Kulmbach, Germany, 1995. [Google Scholar]
- Salvat-Leal, I.; Verdiell, D.; Parrondo, P.; Barcala, E.; Romero, D. Assessing lead and cadmium pollution at the mouth of the river Segura (SE Spain) using the invasive blue crab (Callinectes sapidus Rathbun, 1896, Crustacea, Decapoda, Portunidae) as a bioindicator organism. Reg. Stud. Mar. Sci. 2020, 40, 101521. [Google Scholar] [CrossRef]
- Genç, T.O.; Yilmaz, F. Metal accumulations in water, sediment, crab (Callinectes sapidus) and two fish species (Mugil cephalus and Anguilla anguilla) from the Köyceğiz Lagoon System– Turkey: An Index Analysis Approach. Bull. Environ. Contam. Toxicol. 2017, 99, 173–181. [Google Scholar] [CrossRef]
- Çoğun, H.Y.; First, Ö.; Aytekin, T.; Firidin, G.; Varkal, H.; Temiz, Ö.; Kargin, F. Heavy Metals in the Blue Crab (Callinectes sapidus) in Mersin Bay, Turkey. Bull. Environ. Contam. Toxicol. 2017, 98, 824–829. [Google Scholar] [CrossRef]
- Karouna-Renier, N.K.; Snyder, R.A.; Allison, J.G.; Wagner, M.G.; Ranga Rao, K. Accumulation of organic and inorganic contaminants in shellfish collected in estuarine waters near Pensacola, Florida: Contamination profiles and risks to human consumers. Environ. Pollut. 2007, 145, 474–488. [Google Scholar] [CrossRef]
- Mutlu, C.; Türkmen, M.; Türkmen, A.; Tepe, Y. Comparison of metal concentrations in tissues of blue crab, Callinectes sapidus from Mediterranean Lagoons. Bull. Environ. Contam. Toxicol. 2011, 87, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, A.; Türkmen, M.; Tepe, Y.; Mazlum, Y.; Oymael, S. Metal Concentrations in Blue Crab (Callinectes sapidus) and Mullet (Mugil cephalus) in Iskenderun Bay, Northern East Mediterranean, Turkey. Bull. Environ. Contam. Toxicol. 2006, 77, 186–193. [Google Scholar] [CrossRef]
- Adams, D.H.; Engel, M.E. Mercury, lead, and cadmium in blue crabs, Callinectes sapidus, from the Atlantic coast of Florida, USA: A multipredator approach. Ecotoxicol. Environ. Saf. 2014, 102, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Ayas, D.; Özogul, Y. The effects of sex and seasonality on the metal levels of different muscle tissues of mature Atlantic blue crabs (Callinectes sapidus) in Mersin Bay, north-eastern Mediterranean. Int. J. Food Sci. Technol. Nutr. 2011, 46, 2030–2034. [Google Scholar] [CrossRef]
- Zotti, M.; Coco, L.D.; Pascali, S.A.; Migoni, D.; Vizzini, S.; Mancinelli, G.; Fanizzi, F.P. Comparative analysis of the proximate and elemental composition of the blue crab Callinectes sapidus, the warty crab Eriphia verrucosa, and the edible crab Cancer pagurus. Heliyon 2016, 2, e00075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, P.; Vitorino, H.A.; Green, S.; Zanotto, F.P.; Chung, J.S.; Moreira, R.G. Experimental effects of cadmium on physiological response of Callinectes danae (Crustacea, Portunidae) from environments with different levels of Cd contamination. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 251, 109210. [Google Scholar] [CrossRef] [PubMed]
- Falcó, G.; Llobet, J.M.; Bocio, A.; Domingo, J.L. Daily intake of arsenic, cadmium, mercury, and lead by consumption of edible marine species. J. Agric. Food Chem. 2006, 54, 6106–6112. [Google Scholar] [CrossRef]
- Zhelyazkov, G.; Yankovska-Stefanova, T.; Mineva, E.; Stratev, D.; Vashin, I.; Dospatliev, L.; Valkova, E.; Popova, T. Risk assessment of some heavy metals in mussels (Mytilus galloprovincialis) and veined rapa whelks (Rapana venosa) for human health. Mar. Pollut. 2018, 128, 197–201. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Content of mercury and cadmium in fish (Thunnus alalunga) and cephalopods (Eledone moschata) from the south-eastern Mediterranean Sea. Food Addit. Contam. 2005, 21, 1051–1056. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Cedeño-Macias, L.A. Heavy metals in yellowfin tuna (Thunnus albacares) and common dolphinfish (Coryphaena hippurus) landed on the Ecuadorian coast. Sci. Total Environ. 2016, 541, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.J.; Zachary, F.S.; Malinich, T.D.; Höök, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, A.J.R.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of Plastic Microfibers by the Crab Carcinus maenas and Its Effect on Food Consumption and Energy Balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.N.; Liu, J.H.; Feng, X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, S.; Wang, Z.; Zhang, C.; Pan, Z.; Sun, D.; Xu, G.; Zou, J. Single and Combined Effects of Microplastics and Cadmium on the Cadmium Accumulation and Biochemical and Immunity of Channa argus. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Wakkaf, T.; Allouche, M.; Harrath, A.H.; Mansour, L.; Alwasel, S.; Mohamed-Thameemul-Ansari, K.G.; Beyrem, H.; Sellami, B.; Boufahja, F. The individual and combined effects of cadmium, polyvinyl chloride (PVC) microplastics and their polyalkylamines modified forms on meiobenthic features in a microcosm. Environ. Pollut. 2020, 266, 115263. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Legras, S.; Mouneyrac, C.; Amiard, J.C.; Triquet-Amiard, C.; Rainbow, P. Changes in metallothionein concentrations in response to variation in natural factors (salinity, sex, weight) and metal concentration in crabs from a metal-rich estuary. J. Exp. Mar. Biol. Ecol. 2000, 246, 259–279. [Google Scholar] [CrossRef]
- Reichmuth, J.M.; Weis, P.; Weris, J.S. Bioaccumulation and depuration of metals in blue crabs (Callinectes sapidus Rathbun) from a contaminated and clean estuary. Environ. Pollut. 2010, 158, 361–368. [Google Scholar] [CrossRef]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.M.G.; Barcarolli, I.F.; Menezes, E.J.; Giacomin, M.M.; Wood, C.M.; Bianchini, A. Acute toxicity, accumulation and tissue distribution of copper in the blue crab Callinectes sapidus acclimated to different salinities: In vivo and in vitro studies. Aquat. Toxicol. 2009, 101, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Wiech, M.; Amlund, H.; Jensen, K.A.; Aldenberg, T.; Duinker, A.; Maage, A. Tracing simultaneous cadmium accumulation from different uptake routes in brown crab Cancer pagurus by the use of stable isotopes. Aquat. Toxicol. 2018, 201, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.F. Biology of Crabs; Elek: London, UK, 1977. [Google Scholar]
- Bjerregaard, P. Influence of physiological condition on cadmium transport from haemolymph to hepatopancreas in Carcinus maenas. Mar. Biol. 1990, 106, 199–209. [Google Scholar] [CrossRef]
- Bjerregaard, P.; Depledge, M. Cadmium accumulation in Littorina littorea, Mytilus edulis and Carcinus maenas: The influence of salinity and calcium ion concentrations. Mar. Biol. 1994, 119, 385–395. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 297–507. [Google Scholar] [CrossRef]
- Truchet, D.M.; Buzzi, N.S.; Simonetti, P.; Marcovecchio, J.E. Uptake and detoxification of trace metals in estuarine crabs: Insights into the role of metallothioneins. Environ. Sci. Pollut. Res. 2020, 27, 31905–31917. [Google Scholar] [CrossRef]
- Bjerregaard, P. Accumulation of cadmium and selenium and their mutual interaction in the shore crab Carcinus maenas (L.). Aquat. Toxicol. 1982, 2, 113–125. [Google Scholar] [CrossRef]
- Wiech, M.; Vik, E.; Duinker, A.; Frantzen, S.; Bakke, S.; Maage, A. Effects of cooking and freezing practices on the distribution of cadmium in different tissues of the brown crab (Cancer pagurus). Food Control. 2017, 75, 14–20. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Wayman, C.; Niemann, H. The fate of plastic in the ocean environment–a minireview. Environ. Sci Process. Impacts. 2021, 23, 198–212. [Google Scholar] [CrossRef] [PubMed]

| Weight | Length | Width | |
|---|---|---|---|
| Control | 32.959 ± 12.416 | 3.963 ± 0.490 | 7.838 ± 1.194 |
| AQUATEX® 230 | 30.202 ± 8.930 | 3.963 ± 0.403 | 7.425 ± 0.924 |
| MPP 1241 | 33.648 ± 16.506 | 3.988 ± 0.696 | 7.550 ± 1.606 |
| Zero-hour | 25.664 ± 7.407 | 3.563 ± 0.385 | 7.038 ± 0.852 |
| Whole population | 30.618 ± 11.700 | 3.838 ± 0.507 | 7.463 ± 1.160 |
| Haemolymph | Hepatopancreas | Gills | Muscles | Faeces | |
|---|---|---|---|---|---|
| Control | 0.011 ± 0.010 A,B,(C) (nd-0.031) | 10.424 ± 3.137 A,D,E (3.639–13.694) | 0.503 ± 0.137 B,D,F (0.223–0.647) | 0.032 ± 0.020 (C),E,F (0.010–0.076) | 8.250 ± 0.014 (8.240–8.259) |
| Aquatex | 0.095 ± 0.227 A,B (0.002–0.654) | 13.666 ± 4.729 A,D,E (7.025–21.208) | 0.539 ± 0.156 B,D,F (0.320–0.871) | 0.031 ± 0.012 E,F (0.019–0.058) | 8.953 ± 1.339 (8.123–10.498) |
| MPP | 0.014 ± 0.010 A,B,(C) (0.002–0.029) | 10.950 ± 2.771 A,D,E (5.010–14.123) | 0.501 ± 0.129 B,D,F (0.285–0.681) | 0.037 ± 0.021 (C),E,F (0.020–0.083) | 12.181 ± 5.776 (5.821–17.102) |
| Zero-hour | nd | 0.121 ± 0.080 (0.037–0.293) | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-López, M.; Romero, D. Chronic Microplastic Exposure and Cadmium Accumulation in Blue Crabs. Int. J. Environ. Res. Public Health 2022, 19, 5631. https://doi.org/10.3390/ijerph19095631
Hernández-López M, Romero D. Chronic Microplastic Exposure and Cadmium Accumulation in Blue Crabs. International Journal of Environmental Research and Public Health. 2022; 19(9):5631. https://doi.org/10.3390/ijerph19095631
Chicago/Turabian StyleHernández-López, María, and Diego Romero. 2022. "Chronic Microplastic Exposure and Cadmium Accumulation in Blue Crabs" International Journal of Environmental Research and Public Health 19, no. 9: 5631. https://doi.org/10.3390/ijerph19095631