Folate Status as a Nutritional Indicator among People with Substance Use Disorder; A Prospective Cohort Study in Norway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Characteristics; Design, Population, Data Collection and Study Sample
2.2. Measuring Serum Folate; Laboratory Assays and Definitions
2.3. Study Variables; Baseline, OAT, Clinical and Sociodemographic Factors
2.4. Statistical Analyses
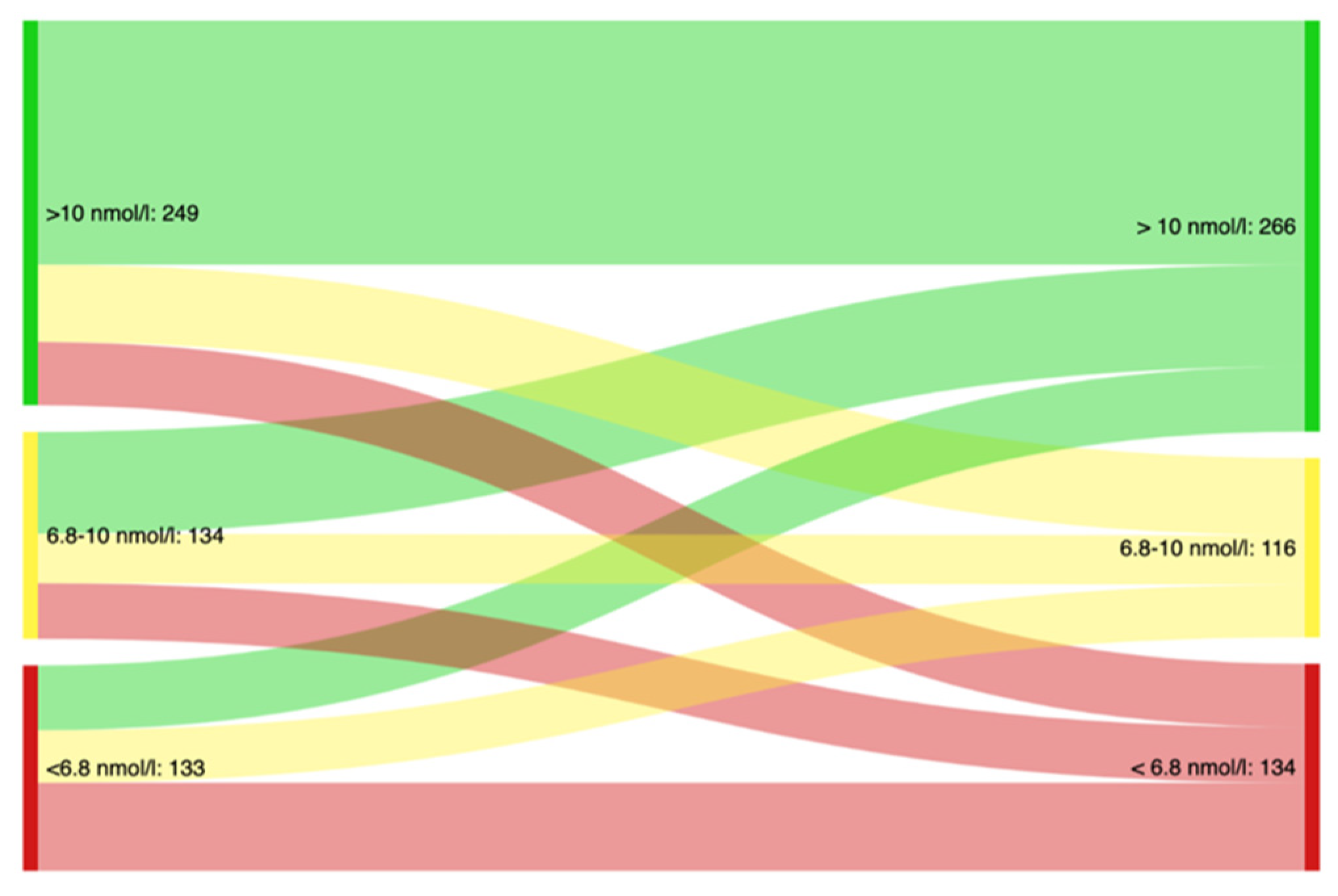
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 2008, 29, S20–S34; discussion S27–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halsted, C.H.; Villanueva, J.A.; Devlin, A.M.; Chandler, C.J. Metabolic interactions of alcohol and folate. J. Nutr. 2002, 132, 2367s–2372s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboub, N.; Rizk, R.; Karavetian, M.; de Vries, N. Nutritional status and eating habits of people who use drugs and/or are undergoing treatment for recovery: A narrative review. Nutr. Rev. 2020, 79, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.; Nettleton, S.; Pickering, L.; Fischer, J. Eating patterns among heroin users: A qualitative study with implications for nutritional interventions. Addiction 2012, 107, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, A.; Kheradmand, A. Nutritional status in patients under methadone maintenance treatment. J. Subst. Use 2020, 25, 173–176. [Google Scholar] [CrossRef]
- Saeland, M.; Haugen, M.; Eriksen, F.L.; Wandel, M.; Smehaugen, A.; Böhmer, T.; Oshaug, A. High sugar consumption and poor nutrient intake among drug addicts in Oslo, Norway. Br. J. Nutr. 2011, 105, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Zador, D.; Lyons Wall, P.M.; Webster, I. High sugar intake in a group of women on methadone maintenance in south western Sydney, Australia. Addiction 1996, 91, 1053–1061. [Google Scholar] [CrossRef]
- Shrestha, S.; Jimenez, E.; Garrison, L.; Pribis, P.; Raisch, D.W.; Stephen, J.M.; Bakhireva, L.N. Dietary intake among opioid- and alcohol-using pregnant women. Subst. Use Misuse 2018, 53, 260–269. [Google Scholar] [CrossRef]
- Varela, P.; Marcos, A.; Ripoll, S.; Santacruz, I.; Requejo, A.M. Effects of human immunodeficiency virus infection and detoxification time on anthropometric measurements and dietary intake of male drug addicts. Am. J. Clin. Nutr. 1997, 66, 509s–514s. [Google Scholar] [CrossRef] [Green Version]
- El-Nakah, A.; Frank, O.; Louria, D.B.; Quinones, M.A.; Baker, H. A vitamin profile of heroin addiction. Am. J. Public Health 1979, 69, 1058–1060. [Google Scholar] [CrossRef]
- Tomedi, L.E.; Bogen, D.L.; Hanusa, B.H.; Wisner, K.L.; Bodnar, L.M. A pilot study of the nutritional status of opiate-using pregnant women on methadone maintenance therapy. Subst. Use Misuse 2012, 47, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazici, A.B.; Akcay Ciner, O.; Yazici, E.; Cilli, A.S.; Dogan, B.; Erol, A. Comparison of vitamin B12, vitamin D and folic acid blood levels in patients with schizophrenia, drug addiction and controls. J. Clin. Neurosci. 2019, 65, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of nutrition for development-folate review. J. Nutr. 2015, 145, 1636s–1680s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brevik, A.; Vollset, S.E.; Tell, G.S.; Refsum, H.; Ueland, P.M.; Loeken, E.B.; Drevon, C.A.; Andersen, L.F. Plasma concentration of folate as a biomarker for the intake of fruit and vegetables: The hordaland homocysteine study. Am. J. Clin. Nutr. 2005, 81, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benoist, B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef]
- Bender, A.; Hagan, K.E.; Kingston, N. The association of folate and depression: A meta-analysis. J. Psychiatr. Res. 2017, 95, 9–18. [Google Scholar] [CrossRef]
- Jang, S.; Han, J.W.; Shin, J.; Kim, T.H.; Kwak, K.P.; Kim, K.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Kim, T.H.; et al. Normal-but-low serum folate levels and the risks for cognitive impairment. Psychiatry Investig. 2019, 16, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and its impact on cancer risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, E.H. The neurology of folic acid deficiency. Handb. Clin. Neurol. 2014, 120, 927–943. [Google Scholar] [CrossRef]
- Degenhardt, L.; Grebely, J.; Stone, J.; Hickman, M.; Vickerman, P.; Marshall, B.D.L.; Bruneau, J.; Altice, F.L.; Henderson, G.; Rahimi-Movaghar, A.; et al. Global patterns of opioid use and dependence: Harms to populations, interventions, and future action. Lancet 2019, 394, 1560–1579. [Google Scholar] [CrossRef]
- Connery, H.S. Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harv. Rev. Psychiatry 2015, 23, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karow, A.; Verthein, U.; Pukrop, R.; Reimer, J.; Haasen, C.; Krausz, M.; Schäfer, I. Quality of life profiles and changes in the course of maintenance treatment among 1015 patients with severe opioid dependence. Subst. Use Misuse 2011, 46, 705–715. [Google Scholar] [CrossRef]
- Sordo, L.; Barrio, G.; Bravo, M.J.; Indave, B.I.; Degenhardt, L.; Wiessing, L.; Ferri, M.; Pastor-Barriuso, R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ 2017, 357, j1550. [Google Scholar] [CrossRef] [Green Version]
- Aas, C.F.; Vold, J.H.; Gjestad, R.; Skurtveit, S.; Lim, A.G.; Gjerde, K.V.; Løberg, E.M.; Johansson, K.A.; Fadnes, L.T. Substance use and symptoms of mental health disorders: A prospective cohort of patients with severe substance use disorders in Norway. Subst. Abus. Treat. Prev. Policy 2021, 16, 20. [Google Scholar] [CrossRef]
- Rosca, P.; Haklai, Z.; Goldberger, N.; Zohar, P.; Margolis, A.; Ponizovsky, A.M. Mortality and causes of death among users of methadone maintenance treatment in Israel, 1999–2008. Drug Alcohol Depend. 2012, 125, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Vold, J.H.; Gjestad, R.; Aas, C.F.; Chalabianloo, F.; Skurtveit, S.; Løberg, E.M.; Johansson, K.A.; Fadnes, L.T. Impact of clinical and sociodemographic factors on fatigue among patients with substance use disorder: A cohort study from Norway for the period 2016–2020. Subst. Abus. Treat. Prev. Policy 2020, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Bech, A.B.; Clausen, T.; Waal, H.; Šaltytė Benth, J.; Skeie, I. Mortality and causes of death among patients with opioid use disorder receiving opioid agonist treatment: A national register study. BMC Health Serv. Res. 2019, 19, 440. [Google Scholar] [CrossRef]
- Degenhardt, L.; Charlson, F.; Stanaway, J.; Larney, S.; Alexander, L.T.; Hickman, M.; Cowie, B.; Hall, W.D.; Strang, J.; Whiteford, H.; et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: Findings from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 1385–1398. [Google Scholar] [CrossRef] [Green Version]
- Antony, T.; Alzaharani, S.Y.; El-Ghaiesh, S.H. Opioid-induced hypogonadism: Pathophysiology, clinical and therapeutics review. Clin. Exp. Pharm. Physiol. 2020, 47, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Elman, I.; Howard, M.; Borodovsky, J.T.; Mysels, D.; Rott, D.; Borsook, D.; Albanese, M. Metabolic and addiction indices in patients on opioid agonist medication-assisted treatment: A comparison of buprenorphine and methadone. Sci. Rep. 2020, 10, 5617. [Google Scholar] [CrossRef] [PubMed]
- Vallecillo, G.; Robles, M.J.; Torrens, M.; Samos, P.; Roquer, A.; Martires, P.K.; Sanvisens, A.; Muga, R.; Pedro-Botet, J. Metabolic syndrome among individuals with heroin use disorders on methadone therapy: Prevalence, characteristics, and related factors. Subst. Abus. 2018, 39, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Helse Bergen. ATLAS4LAR: Kartlegging og Behandling av Lungesykdom i Legemiddelassistert Behandling. Available online: https://helse-bergen.no/avdelinger/rusmedisin/rusmedisin-seksjon-forsking/bar/atlas4lar-kartlegging-og-behandling-av-lungesykdom-i-legemiddelassistert-behandling (accessed on 10 May 2021).
- Fadnes, L.T.; Aas, C.F.; Vold, J.H.; Ohldieck, C.; Leiva, R.A.; Chalabianloo, F.; Skurtveit, S.; Lygren, O.J.; Dalgård, O.; Vickerman, P.; et al. Integrated treatment of hepatitis C virus infection among people who inject drugs: Study protocol for a randomised controlled trial (INTRO-HCV). BMC Infect. Dis. 2019, 19, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Analyseoversikten. Folat. Available online: https://analyseoversikten.no/analyse/110 (accessed on 17 March 2021).
- Helse Stavanger. Folater. Available online: https://labhandbok.sus.no/docs/doc_17511/index.html (accessed on 17 March 2021).
- World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. Available online: http://apps.who.int/iris/bitstream/10665/162114/1/WHO_NMH_NHD_EPG_15.01.pdf?ua=1 (accessed on 17 March 2021).
- Analyseoversikten. s-Folat—Endring av Aksjonsgrense for Folatmangel. Available online: https://analyseoversikten.no/labinfo/24 (accessed on 17 March 2021).
- Nasjonal Brukerhåndbok i Medisinsk Biokjemi. Folat, P. Available online: https://www.prosedyrer.no/index.php?action=showtopic&topic=4ad7a834f5b503c9b9f6 (accessed on 17 March 2021).
- World Health Organization. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence; WHO: Gevenva, Switzerland, 2009. Available online: https://www.who.int/publications/i/item/9789241547543 (accessed on 12 April 2022).
- Enders, C.K. Applied Missing Data Analysis; The Guilford Press: New York, NY, USA, 2010. [Google Scholar]
- Öhrvik, V.; Lemming, E.W.; Nälsén, C.; Becker, W.; Ridefelt, P.; Lindroos, A.K. Dietary intake and biomarker status of folate in Swedish adults. Eur. J. Nutr. 2018, 57, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.M.; Cordero, A.M.; Pfeiffer, C.M.; Hausman, D.B.; Tsang, B.L.; De-Regil, L.M.; Rosenthal, J.; Razzaghi, H.; Wong, E.C.; Weakland, A.P.; et al. Global folate status in women of reproductive age: A systematic review with emphasis on methodological issues. Ann. N. Y. Acad. Sci. 2018, 1431, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuesen, B.H.; Husemoen, L.L.; Ovesen, L.; Jørgensen, T.; Fenger, M.; Linneberg, A. Lifestyle and genetic determinants of folate and vitamin B12 levels in a general adult population. Br. J. Nutr. 2010, 103, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Monteagudo, C.; Scander, H.; Nilsen, B.; Yngve, A. Folate intake in a Swedish adult population: Food sources and predictive factors. Food Nutr. Res. 2017, 61, 1328960. [Google Scholar] [CrossRef] [Green Version]
- Santolaria-Fernández, F.J.; Gómez-Sirvent, J.L.; González-Reimers, C.E.; Batista-López, J.N.; Jorge-Hernández, J.A.; Rodríguez-Moreno, F.; Martínez-Riera, A.; Hernández-García, M.T. Nutritional assessment of drug addicts. Drug Alcohol Depend. 1995, 38, 11–18. [Google Scholar] [CrossRef]
- Nazrul Islam, S.K.; Jahangir Hossain, K.; Ahmed, A.; Ahsan, M. Nutritional status of drug addicts undergoing detoxification: Prevalence of malnutrition and influence of illicit drugs and lifestyle. Br. J. Nutr. 2002, 88, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.M.; Forrester, J.E.; Spiegelman, D.; Flanigan, T.; Dobs, A.; Skinner, S.; Wanke, C. Heavy injection drug use is associated with lower percent body fat in a multi-ethnic cohort of HIV-positive and HIV-negative drug users from three U.S. cities. Am. J. Drug Alcohol Abus. 2010, 36, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, L.J.; Scagnelli, L.M. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst. Use Misuse 2007, 42, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Medved, D.; Clausen, T.; Bukten, A.; Bjørnestad, R.; Muller, A.E. Large and non-specific somatic disease burdens among ageing, long-term opioid maintenance treatment patients. Subst. Abus. Treat. Prev. Policy 2020, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Kozeniecki, M.; Ludke, R.; Kerner, J.; Patterson, B. Micronutrients in liver disease: Roles, risk factors for deficiency, and recommendations for supplementation. Nutr. Clin. Pract. 2020, 35, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Muro, N.; Bujanda, L.; Sarasqueta, C.; Gil, I.; Hijona, E.; Cosme, A.; Arenas, J., Jr.; Elosegui, M.E.; Sarasola, M.; Calpasoro, J.; et al. Plasma levels of folate and vitamin B(12) in patients with chronic liver disease. Gastroenterol. Hepatol. 2010, 33, 280–287. [Google Scholar] [CrossRef]

| Characteristic | N (%) | S-Folate (nmol/L) Median (IQR 1) | Low S-Folate % of Group (CI 2) | Deficient S-Folate % of Group (CI 2) |
|---|---|---|---|---|
| Gender | ||||
| Male | 466 (70) | 13 (9.6) | 48 (43–52) | 22 (20–32) |
| Female | 197 (30) | 15 (11) | 48 (41–55) | 26 (18–25) |
| Age group | ||||
| <30 years | 78 (12) | 9.1 (7.1) | 59 (48–69) | 24 (16–35) |
| 30–39 years | 188 (28) | 10 (9.3) | 50 (43–57) | 27 (21–33) |
| 40–49 years | 202 (30) | 10 (12) | 48 (41–54) | 22 (17–28) |
| 50–59 years | 155 (23) | 12 (13) | 45 (38–53) | 21 (15–28) |
| ≥60 years | 40 (6) | 14 (12) | 25 (14–40) | 15 (8–34) |
| Education level | ||||
| Primary school | 39 (6) | 11 (13) | 49 (34–64) | 28 (17–44) |
| Middle school | 297 (45) | 10 (10) | 50 (44–54) | 26 (21–31) |
| High school | 263 (40) | 11 (10) | 46 (40–52) | 21 (16–26) |
| ≤3 years higher education | 51 (8) | 13 (14) | 43 (30–57) | 20 (11–32) |
| >3 years higher education | 13 (2) | 13 (19) | 46 (23–71) | 0 (0–23) |
| Housing conditions 3 | ||||
| Unstable | 81 (12) | 10 (11) | 49 (39–60) | 20 (13–29) |
| Stable | 582 (88) | 10 (8.3) | 47 (43–51) | 23 (20–27) |
| HCV infection 4 | 311 (47) | 11 (10) | 45 (40–51) | 23 (19–28) |
| Injecting substances 5 | 318 (48) | 9.2 (8.0) | 55 (50–60) | 24 (20–29) |
| Opioid agonist therapy | ||||
| Buprenorphine | 352 (53) | 11 (11) | 44 (39–49) | 18 (14–22) |
| Methadone | 229 (35) | 9.1 (11) | 54 (48–60) | 32 (26–38) |
| Not in OAT | 73 (11) | 11 (11) | 45 (34–57) | 15 (9–25) |
| Weekly substance use6 | ||||
| Alcohol | 148 (25) | 13 (11) | 32 (25–40) | 12 (7–18) |
| Cannabis | 297 (49) | 9.8 (10) | 52 (46–57) | 28 (23–33) |
| Stimulants 7 | 157 (26) | 9.4 (6.9) | 54 (46–62) | 21 (15–28) |
| Benzodiazepines | 230 (38) | 9.6 (9.1) | 52 (45–58) | 26 (21–32) |
| Non-OAT opioids | 85 (14) | 11 (10) | 47 (37–58) | 17 (10–26) |
| No weekly substance use | 144 (24) | 11 (12) | 44 (37–53) | 23 (17–30) |
| Overall | 663 (100) | 10 (11) | 48 (44–51) | 23 (20–26) |
| Fixed Effects | Partly Adjusted 1 | Adjusted | ||
|---|---|---|---|---|
| Effect Estimate | Time Trend (per Year) | Effect Estimate | Time Trend (per Year) | |
| Estimate (CI) | Slope (CI) | Estimate (CI) | Slope (CI) | |
| s-folate | 12.8 (10.3, 15.3) | 2.1 (0.79, 3.4) | ||
| Gender | ||||
| Male | Reference (0.0) | |||
| Female | 0.85 (−0.49, 2.2) | |||
| Age | ||||
| <30 | Reference (0.0) | |||
| 30–39 | 0.71 (−1.3, 2.6) | |||
| 40–49 | 1.5 (−0.46, 3.6) | |||
| 50–59 | 2.4 (0.32, 4.5) | |||
| ≥60 | 4.5 (1.5, 7.4) | |||
| OAT dose ratio2 | −0.018 (−1.5, 1.5) | −0.71 (−1.7, 0.33) | 0.06 (−1.5, 1.6) | −1.1 (−2.2, −0.024) |
| Injecting substances3 | −1.6 (−3.1, −0.13) | −0.93 (−1.9, 0.014) | −1.2 (−2.9, 0.41) | −1.2 (−2.3, −0.14) |
| Weekly substance use4 | ||||
| Alcohol | 1.9 (0.13, 3.6) | −0.53 (−1.6, 0.57) | 1.9 (0.15, 3.6) | −0.60 (−1.7, 0.50) |
| Cannabis | −2.1 (−3.6, −0.63) | 0.12 (−0.82, 1.1) | −1.8 (−3.3, −0.25) | 0.14 (−0.84, 1.1) |
| Non-OAT opioids | 0.32 (−1.9, 2.5) | 0.16 (−1.3, 1.6) | 0.80 (−1.5, 3.1) | 0.22 (−1.3, 1.8) |
| Stimulants 5 | −0.93 (−2.6, 0.77) | −0.54 (−1.7, 0.61) | −0.29 (−2.2, 1.6) | −0.38 (−1.7, 0.94) |
| Benzodiazepines | −1.1 (−2.6, 0.45) | 0.47 (−0.50, 1.4) | −0.50 (−2.1, 1.2) | 0.84 (−0.24, 1.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bemanian, M.; Vold, J.H.; Chowdhury, R.; Aas, C.F.; Gjestad, R.; Johansson, K.A.; Fadnes, L.T. Folate Status as a Nutritional Indicator among People with Substance Use Disorder; A Prospective Cohort Study in Norway. Int. J. Environ. Res. Public Health 2022, 19, 5754. https://doi.org/10.3390/ijerph19095754
Bemanian M, Vold JH, Chowdhury R, Aas CF, Gjestad R, Johansson KA, Fadnes LT. Folate Status as a Nutritional Indicator among People with Substance Use Disorder; A Prospective Cohort Study in Norway. International Journal of Environmental Research and Public Health. 2022; 19(9):5754. https://doi.org/10.3390/ijerph19095754
Chicago/Turabian StyleBemanian, Mitra, Jørn Henrik Vold, Ranadip Chowdhury, Christer Frode Aas, Rolf Gjestad, Kjell Arne Johansson, and Lars Thore Fadnes. 2022. "Folate Status as a Nutritional Indicator among People with Substance Use Disorder; A Prospective Cohort Study in Norway" International Journal of Environmental Research and Public Health 19, no. 9: 5754. https://doi.org/10.3390/ijerph19095754






