Long-Lasting Olfactory Dysfunction in Hospital Workers Due to COVID-19: Prevalence, Clinical Characteristics, and Most Affected Odorants
Abstract
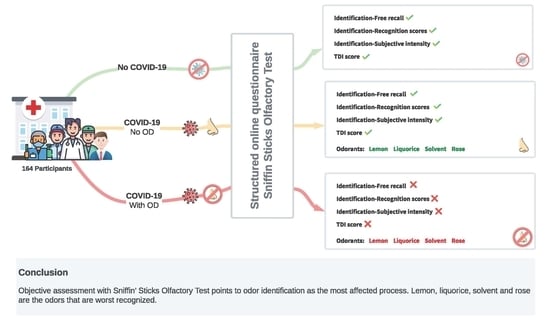
:1. Introduction
2. Method
2.1. Participants
2.2. Measures and Testing Procedure
2.3. Study Design
2.4. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Lu, Y.; Lure, F.; Jaeger, S.; Lu, P. Clinical and radiological features of novel coronavirus pneumonia. J. X-Ray Sci. Technol. 2020, 28, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Drozdzik, A.; Drozdzik, M. Oral Pathology in COVID-19 and SARS-CoV-2 Infection—Molecular Aspects. Int. J. Mol. Sci. 2022, 23, 1431. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Yang, L.; You, R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020, 157, 104833. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Bilinska, K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem. Neurosci. 2020, 11, 1200–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherry, G.; Rocke, J.; Chu, M.; Liu, J.; Lechner, M.; Lund, V.J.; Kumar, B.N. Loss of smell and taste: A new marker of COVID-19? Tracking reduced sense of smell during the coronavirus pandemic using search trends. Expert Rev. Anti-Infect. Ther. 2020, 18, 1165–1170. [Google Scholar] [CrossRef]
- Vetter, P.; Vu, D.L.; L’Huillier, A.G.; Schibler, M.; Kaiser, L.; Jacquerioz, F. Clinical features of covid-19. BMJ 2020, 369, 1470. [Google Scholar] [CrossRef] [Green Version]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Speth, M.M.; Singer-Cornelius, T.; Obere, M.; Gengler, I.; Brockmeier, S.J.; Sedaghat, A.R. Olfactory dysfunction and sinonasal symptomatology in covid-19: Prevalence, severity, timing, and associated characteristics. Otolaryngol. Head Neck Surg. 2020, 163, 114–120. [Google Scholar] [CrossRef]
- Niklassen, A.S.; Draf, J.; Huart, C.; Hintschich, C.; Bocksberger, S.; Trecca, E.M.C.; Klimek, L.; Le Bon, S.D.; Altundag, A.; Hummel, T.; et al. COVID-19: Recovery from chemosensory dysfunction. A multicentre study on smell and taste. Laryngoscope 2021, 131, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.L.B.; Campbell, M.; Hopkins, C.; Smith, B.; Kelly, C.; Deary, V. Altered smell and taste: Anosmia, parosmia and the impact of long Covid-19. PLoS ONE 2021, 16, e0256998. [Google Scholar] [CrossRef]
- Keller, A.; Malaspina, D. Hidden consequences of olfactory dysfunction: A patient report series. BMC Ear Nose Throat Disord. 2013, 13, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leopold, D. Distortion of olfactory perception: Diagnosis and treatment. Chem. Senses 2002, 27, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Ohla, K.; Veldhuizen, M.G.; Green, T.; Hannum, M.E.; Bakke, A.J.; Moein, S.; Niv, M.Y. Increasing incidence of parosmia and phantosmia in patients recovering from COVID-19 smell loss. medRxiv 2021. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef]
- Gómez-Iglesias, P.; Porta-Etessam, J.; Montalvo, T.; Valls-Carbó, A.; Gajate, V.; Matías-Guiu, J.A.; Parejo-Carbonell, B.; González-García, N.; Ezpeleta, D.; Láinez, J.M.; et al. An Online Observational Study of Patients with Olfactory and Gustory Alterations Secondary to SARS-CoV-2 Infection. Front. Public Health 2020, 8, 243. [Google Scholar] [CrossRef]
- Moein, S.T.; Hashemian, S.M.R.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 944–950. [Google Scholar] [CrossRef]
- Wong, D.K.C.; Gendeh, H.S.; Thong, H.K.; Lum, S.G.; Gendeh, B.S.; Saim, A.; Salina, H. A review of smell and taste dysfunction in COVID-19 patients. Med. J. Malays. 2020, 75, 574–581. [Google Scholar]
- Sedaghat, A.R.; Gengler, I.; Speth, M.M. Olfactory dysfunction: A highly prevalent symptom of COVID-19 with public health significance. Otolaryngol. Head Neck Surg. 2020, 163, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, C.; Surda, P.; Whitehead, E.; Kumar, B.N. Early recovery following new onset anosmia during the COVID-19 pandemic—An observational cohort study. J. Otolaryngol. Head Neck Surg. 2020, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Domínguez, A.; Rojas-Lechuga, M.J.; Mullol, J.; Alobid, I. Pérdida del sentido del olfato durante la pandemia COVID-19. Med. Clin. 2020, 155, 403–408. [Google Scholar] [CrossRef]
- Auinger, A.B.; Besser, G.; Liu, D.T.; Renner, B.; Mueller, C.A. Long-term impact of olfactory dysfunction on daily life. Wien. Klin. Wochenschr. 2020, 133, 1004–1011. [Google Scholar] [CrossRef]
- Beltran-Corbellini, A.; Chico-Garcia, J.L.; Martinez-Poles, J.; Rodriguez-Jorge, F.; Natera-Villalba, E.; Gomez-Corral, J.; Gómez-López, A.; Monreal, E.; Parra-Díaz, P.; Cortés-Cuevas, J.L.; et al. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicentre polymerase chain reaction based case-control study. Eur. J. Neurol. 2020, 27, 1738–1741. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of anosmia in COVID-19. Med. Mal. Infect. 2020, 50, 436–439. [Google Scholar] [CrossRef]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, 174. [Google Scholar] [CrossRef]
- Levinson, R.; Elbaz, M.; Ben-Ami, R.; Shasha, D.; Levinson, T.; Choshen, G.; Petrov, K.; Gadoth, A.; Paran, Y. Time course of anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. J. Infect. Dis. 2020, 52, 600–602. [Google Scholar] [CrossRef]
- Chiesa-Estomba, C.; Lechien, J.; Radulesco, T.; Michel, J.; Sowerby, L.J.; Hopkins, C.; Saussez, S. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur. J. Neurol. 2020, 27, 2318–2321. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Guida, F.; Polesel, J.; Marcuzzo, A.V.; Antonucci, P.; Capriotti, V.; Sacchet, E.; Cragnolini, F.; D’Alessandro, A.; Zanelli, E.; et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: A one-year prospective study. Eur. Arch. Otorhinolaryngol. 2021, 279, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez de Limia Ramírez, K.; Ruiz-Robledillo, N.; Duro-Torrijos, J.L.; García-Román, V.; Albaladejo-Blázquez, N.; Ferrer-Cascales, R. Prevalence of SARS-CoV-2 Infection in a Sample of Health Workers in Two Health Departments of the Valencian Community in Spain. Int. J. Environ. Res. 2022, 19, 66. [Google Scholar] [CrossRef]
- Garcia-Basteiro, A.L.; Moncunill, G.; Tortajada, M.; Vidal, M.; Guinovart, C.; Jiménez, A.; Santano, R.; Sanz, S.; Méndez, S.; Llupià, A.; et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Garralda Fernandez, J.; Molero Vilches, I.; Bermejo Rodríguez, A.; Cano Torres, I.; Colino Romay, E.I.; García Arata, I.; Jaqueti Aroca, J.; Lillo Rodríguez, R.; López Lacomba, D.; Mazón Cuadrado, L.; et al. Impact of SARS-CoV-2 pandemic among health care workers in a secondary teaching hospital in Spain. PLoS ONE 2021, 16, e0245001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Xiang, M.; Li, S.; Zhao, D.; Huang, C.; Cheng, S. Protecting healthcare personnel from 2019-nCoV infection risks: Lessons and suggestions. Front. Med. 2020, 14, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, H.; McKenney, M.; Elkbuli, A. Protecting our healthcare workers during the COVID-19 pandemic. Am. J. Emerg. Med. 2020, 38, 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Informe Sobre la Situación de COVID-19 en Personal Sanitario en España. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx (accessed on 25 February 2022).
- Kaye, R.C.C.; Kazahaya, K.; Brereton, J.; Denneny, J.C. III. COVID-19 anosmia reporting tool: Initial findings. Otolaryngol. Head Neck Surg. 2020, 163, 132–134. [Google Scholar] [CrossRef]
- Kempker, R.R.; Kempker, J.A.; Peters, M.; Rebolledo, P.A.; Carroll, K.; Toomer, L.; Hunter, M. Loss of smell and taste among healthcare personnel screened for coronavirus 2019. Arch. Clin. Infect. Dis. 2021, 72, 1244–1246. [Google Scholar] [CrossRef]
- Van Loon, N.; Verbrugghe, M.; Cartuyvels, R.; Ramaekers, D. Diagnosis of COVID-19 based on symptomatic analysis of hospital healthcare workers in Belgium: Observational study in a large Belgian tertiary care center during early COVID-19 outbreak. J Occup. Environ. Med. 2021, 63, 27. [Google Scholar] [CrossRef]
- Iversen, K.; Bundgaard, H.; Hasselbalch, R.B.; Kristensen, J.H.; Nielsen, P.B.; Pries-Heje, M.; Knudsen, A.D.; Christensen, C.E.; Fogh, K.; Norsk, J.B.; et al. Risk of COVID-19 in health-care workers in Denmark: An observational cohort study. Lancet Infect. Dis. 2020, 20, 1401–1408. [Google Scholar] [CrossRef]
- Ollarves-Carrero, M.F.; Rodriguez-Morales, A.G.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Anosmia in a healthcare worker with COVID-19 in Madrid, Spain. Travel Med. Infect. Dis. 2020, 35, 101666. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, I.; Martínez de Aramayona López, M.J.; Sáez Vicente, A.; Lobo Abascal, P. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J. Hosp. Infect. 2020, 106, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Deschamps-Perdomo, Á.; Garrafa-Núñez, M.; Meza-Caballero, M.E.; Patricio-Villanueva, G.; Salgado-Balbas, Y.; Sánchez-Paniagua-Castillo, J. Características clínicas de COVID-19 en trabajadores sanitarios de tres hospitales de Madrid durante la primera ola de la pandemia. Med. Segur. Trab. 2021, 67, 11–23. [Google Scholar] [CrossRef]
- Delgado-Losada, M.L.; Delgado-Lima, A.H.; Bouhaben, J. Spanish validation for olfactory function testing using the sniffin’sticks olfactory test: Threshold, discrimination, and identification. Brain Sci. 2020, 10, 943. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin’ Sticks”: Olfactory performance assessed by the combined testing of odor identification, odor discrimination, and olfactory thresholds. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 21 February 2022).
- Delgado-Losada, M.L.; Bouhaben, J.; Delgado-Lima, A.H. Development of the Spanish Version of Sniffin’s Sticks Olfactory Identification Test: Normative Data and Validity of Parallel Measures. Brain Sci. 2021, 11, 216. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Folgueira, M.D.; Munoz-Ruiperez, C.; Alonso-Lopez, M.A.; Delgado, R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.04.07.20055723v2.full.pdf (accessed on 10 July 2020).
- Moreno Borraz, L.A.; Giménez López, M.; Carrera Lasfuentes, P.; González Pérez, E.; Ortiz Domingo, C.; Bonafonte Marteles, J.L.; Vicente Gaspar, C.; Amorós de la Nieta, F.; Sastre Heres, A.; García Forcada, Á.L.; et al. Prevalencia de infección por coronavirus SARS-CoV-2 en pacientes y profesionales de un hospital de media y larga estancia en España [Prevalence of SARS-CoV-2 coronavirus infection in patients and professional staff at a medium or long-stay hospital in Spain]. Rev. Esp. Geriatr. Gerontol. 2021, 56, 75–80. [Google Scholar] [CrossRef]
- Pollan, M.; Perez-Gomez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernan, M.A.; Perez-Olmeda, M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Olalla, J.; Correa, A.M.; Martín-Escalante, M.D.; Hortas, M.L.; Martín-Sendarrubias, M.J.; Fuentes, V.; Sena, G.; García-Alegría, J. Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: A Spanish experience. QJM 2020, 113, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Breazzano, M.P.; Shen, J.; Abdelhakim, A.H.; Glass, L.R.D.; Horowitz, J.D.; Xie, S.X.; de Moraes, C.G.; Chen-Plotkin, A.; Chen, R.W.; New York City Residency Program Directors COVID-19 Research Group. New York City COVID-19 resident physician exposure during exponential phase of pandemic. J. Clin. Investig. 2020, 130, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, G.; Jacob, S.; Barrett, P.M.; Gallagher, J. Covid-19 presentation among symptomatic healthcare workers in Ireland. Occup. Med. 2021, 71, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Vahidy, F.; Sostman, H.D.; Bernardet, D.; Boom, M.; Drews, A.; Christensen, P. Prevalence of SARS-CoV-2 infection among asymptomatic healthcare workers in greater Houston: A cross-sectional analysis of surveillance data from a large healthcare system. JAMA 2020, 3, e2016451-e2016451. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Buckley, D.; Selph, S.; Fu, R. Epidemiology of and risk factors for coronavirus infection in health care workers: A living rapid review. Ann. Intern. Med. 2020, 173, 120–136. [Google Scholar] [CrossRef]
- Felice, C.; Di Tanna, G.L.; Zanus, G.; Grossi, U. Impact of COVI D-19 Outbreak on Healthcare Workers in Italy: Results from a National E-Survey. J. Community Health 2020, 45, 675–683. [Google Scholar] [CrossRef]
- Tabah, A.; Ramanan, M.; Laupland, K.B.; Buetti, N.; Cortegiani, A.; Mellinghoff, J. Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): An international survey. J. Crit. Care 2020, 59, 70–75. [Google Scholar] [CrossRef]
- Iqbal, M.R.; Chaudhuri, A. COVID-19: Results of a national survey of United Kingdom healthcare professionals’ perceptions of current management strategy—A crosssectional questionnaire study. Int. J. Surg. 2020, 79, 156–161. [Google Scholar] [CrossRef]
- Varona, J.F.; Madurga, R.; Peñalver, F.; Abarca, E.; Almirall, C.; Cruz, M.; Castellano Vázquez, J.M. Seroprevalence of SARS-CoV-2 antibodies in over 6000 healthcare workers in Spain. Int. J. Epidemiol. 2021, 50, 400–409. [Google Scholar] [CrossRef]
- Andrews, P.J.; Pendolino, A.L.; Ottaviano, G.; Scarpa, B.; Grant, J.; Gaudioso, P.; Andrews, J.A. Olfactory and taste dysfunction among mild-to-moderate symptomatic COVID-19 positive health care workers: An international survey. Laryngoscope Investig. Otolaryngol. 2020, 5, 1019–1028. [Google Scholar] [CrossRef]
- Clemency, B.M.; Varughese, R.; Scheafer, D.K.; Ludwig, B.; Welch, J.V.; McCormack, R.F.; Ma, C.; Nan, N.; Giambra, T.; Raab, T. Symptom Criteria for COVID-19 Testing of Heath Care Workers. Acad. Emerg. Med. 2020, 27, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Rudberg, A.S.; Havervall, S.; Manberg, A.; Falk, A.J.; Aguilera, K.; Ng, H.; Gabrielsson, L.; Salomonsson, A.C.; Hanke, L.; Murrell, B.; et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat. Commun. 2020, 11, 5064. [Google Scholar] [CrossRef]
- Xiong, Y.; Peng, L. Focusing on health-care providers’ experiences in the COVID-19 crisis. Lancet Glob. Health 2020, 8, 740–741. [Google Scholar] [CrossRef]
- Hannum, M.E.; Koch, R.J.; Ramirez, V.A.; Marks, S.S.; Toskala, A.K.; Herriman, R.D.; Lin, C.; Joseph, P.V.; Reed, D.R. Taste loss as a distinct symptom of COVID-19: A systematic review and meta-analysis. Chem. Senses 2022, 47, bjac001. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, C.R.; Seale, H.; Yang, P.; Zhang, Y.; Shi, W.; Almatroudi, A. Quantifying the risk of respiratory infection in healthcare workers performing high-risk procedures. Epidemiol. Infect. 2014, 142, 1802–1808. [Google Scholar] [CrossRef] [Green Version]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study. Arch. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Lefèvre, N.; Corazza, F.; Valsamis, J.; Delbaere, A.; De Maertelaer, V.; Duchateau, J. The number of X chromosomes influences inflammatory cytokine production following toll-like receptor stimulation. Front. Immunol. 2019, 10, 1052. [Google Scholar] [CrossRef]
- Paderno, A.; Schreiber, A.; Grammatica, A.; Raffetti, E.; Tomasoni, M.; Gualtieri, T.; Taboni, S.; Zorzi, S.; Lombardi, D.; Deganello, A.; et al. Smell and taste alterations in COVID-19: A cross-sectional analysis of different cohorts. Int. Forum Allergy Rhinol. 2020, 10, 955–962. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, February 12–March 28, 2020. MMWR 2020, 69, 382–386. [Google Scholar] [CrossRef]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandersteen, C.; Payne, M.; Dumas, L.É.; Plonka, A.; D’Andréa, G.; Chirio, D.; Demonchy, É.; Risso, K.; Robert, P.; Fernandez, X.; et al. What about using sniffin’sticks 12 Items test to screen post-COVID-19 olfactory disorders? Eur. Arch. Oto-Rhino-L 2021, 1–8, online ahead of print. [Google Scholar] [CrossRef]
- Karamali, K.; Elliott, M.; Hopkins, C. COVID-19 related olfactory dysfunction. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 19–25. [Google Scholar] [CrossRef]
- Vandersteen, C.; Payne, M.; Dumas, L.E.; Metelkina-Fernandez, V.; Plonka, A.; Chirio, D.; Demonchy, É.; Risso, K.; Askenazy-Gittard, F.; Guevara, N.; et al. Persistent olfactory complaints after COVID-19: A new interpretation of the psychophysical olfactory scores. Rhinol. Online 2021, 4, 66–72. [Google Scholar] [CrossRef]
- Iannuzzi, L.; Salzo, A.E.; Angarano, G.; Palmieri, V.O.; Portincasa, P.; Saracino, A.; Gelardi, M.; Dibattista, M.; Quaranta, N. Gaining Back What Is Lost: Recovering the Sense of Smell in Mild to Moderate Patients After COVID-19. Chem. Senses 2020, 45, 875–881. [Google Scholar] [CrossRef]
- Amoore, J.E. Specific anosmia and the concept of primary odors. Chem. Senses 1977, 2, 267–281. [Google Scholar] [CrossRef]
- Castro, J.B.; Ramanathan, A.; Chennubhotla, C.S. Categorical Dimensions of Human Odor Descriptor Space Revealed by Non-Negative Matrix Factorization. PLoS ONE 2013, 8, e73289. [Google Scholar] [CrossRef] [Green Version]


| No-COV | COV-without-Symp | COV-with-Symp | |||
|---|---|---|---|---|---|
| Sample size | 76 | 29 | 59 | ||
| Mean (SD) or count | Mean (SD) or count | Mean (SD) or count | chi or F | p | |
| Female | 62 | 24 | 49 | 0.054 | 0.973 |
| Age | 48.3 (11.7) | 43.3 (14.6) | 44.7 (13) | 2.235 | 0.11 |
| Health hospital workers | 44 | 21 | 44 | 4.706 | 0.095 |
| Allergies | 26 | 11 | 20 | 0.137 | 0.933 |
| Other pathologies | 24 | 10 | 14 | 2.273 | 0.321 |
| None | 58 | 20 | 54 | ||
| Allergies/hay fever | 10 | 5 | 7 | ||
| High blood preasure | 10 | 5 | 3 | ||
| Obesity | 5 | 1 | 3 | ||
| Cardiac issues | 0 | 1 | 0 | ||
| Neurological issues | 1 | 1 | 0 | ||
| Respiratory issues | 4 | 1 | 3 | ||
| Cancer | 0 | 0 | 1 | ||
| Frequent smoking | 25 | 4 | 6 | 11.154 | 0.003 ** |
| Frequent alcohol consumption | 7 | 7 | 4 | 5.99 | 0.06 |
| Pre-COVID subjective smell performance | - | 7.8 (1.58) | 7.71 (1.46) | 0.052 | 0.82 |
| Post-COVID subjective smell performance | - | ||||
| Sniffin’ Sticks Olfactory Test scores | |||||
| Threshold | 6.47 (2.6) | 6.46 (2.56) | 5.19 (3.56) | - | - |
| Discrimination | 10.71 (2.71) | 10.75 (2.29) | 9.52 (3.12) | - | - |
| Identification–Free recall | 2.82 (2.36) | 2.76 (2.41) | 1.88 (1.74) | - | - |
| Identification–Recognition | 12.93 (2.14) | 13.28 (2.55) | 11.05 (3.68) | - | - |
| Identification–Subjective intensity | 6.95 (1.42) | 6.75 (1.46) | 6.03 (2.39) | - | - |
| Total score | 30.11 (4.83) | 30.51 (4.39) | 25.77 (8.46) | - | - |
| Olfactory Unrelated COVID-19 Symtoms | COV-without (n = 29) | COV-with (n = 59) | chi | p |
|---|---|---|---|---|
| Asymptomatic | 9 | - | - | - |
| Headache | 10 | 33 | 1.421 | 0.234 |
| Muscle pain | 9 | 29 | 1.923 | 0.166 |
| Fatigue | 11 | 28 | 0.552 | 0.457 |
| Abdominal pain | 1 | 13 | 3.727 | 0.053 |
| Diarrhea | 7 | 19 | 0.281 | 0.595 |
| Respiratory issues | 8 | 12 | 0.242 | 0.623 |
| Chest oppression | 4 | 10 | 0.005 | 0.944 |
| Short of breath | 8 | 12 | 0.242 | 0.623 |
| Intense cough (dry or mucous) | 7 | 25 | 2.061 | 0.151 |
| Fever | 3 | 7 | 0.001 | 0.999 |
| Pharyngitis | 5 | 6 | 0.36 | 0.548 |
| Loss of appetite | 4 | 22 | 4.082 | 0.043 * |
| Pneumonia | 0 | 6 | - | - |
| Olfactory related COVID-19 symptoms | ||||
| Olfactory loss or decrease | - | 59 | ||
| Changes in food taste | - | 43 | ||
| Stuffy nose | - | 21 | ||
| Parosmia | - | 26 | ||
| Phantosmia | - | 7 |
| Olfactory Measure | Factor or Covariate | Mean Squared | df | F | p |
|---|---|---|---|---|---|
| Threshold | Age | 72.2 | 1 | 8.762 | 0.003 ** |
| Smoking | 7.798 | 1 | 0.946 | 0.332 | |
| COVID | 29.992 | 2 | 3.64 | 0.028 * | |
| COVID × Age | 18.788 | 2 | 2.28 | 0.106 | |
| COVID × Smoking | 2.713 | 2 | 0.329 | 0.72 | |
| Residuals | 8.24 | 154 | |||
| Discrimination | Age | 26.259 | 1 | 3.721 | 0.055 |
| Smoking | 18.737 | 1 | 2.655 | 0.105 | |
| COVID | 25.48 | 2 | 3.611 | 0.029 * | |
| COVID × Age | 2.845 | 2 | 0.403 | 0.669 | |
| COVID × Smoking | 8.74 | 2 | 1.239 | 0.292 | |
| Residuals | 7.057 | 154 | |||
| Identification–Free recall | Age | 25.058 | 1 | 5.41 | 0.021 * |
| Smoking | 9.609 | 1 | 2.074 | 0.152 | |
| COVID | 15.303 | 2 | 3.307 | 0.039 * | |
| COVID × Age | 1.068 | 2 | 0.231 | 0.794 | |
| COVID × Smoking | 2.957 | 2 | 0.638 | 0.529 | |
| Residuals | 4.632 | 154 | |||
| Identification-Recognition | Age | 0.277 | 1 | 0.034 | 0.853 |
| Smoking | 79.556 | 1 | 9.869 | 0.002 ** | |
| COVID | 54.908 | 2 | 6.811 | 0.001 ** | |
| COVID × Age | 0.136 | 2 | 0.017 | 0.983 | |
| COVID × Smoking | 1.834 | 2 | 0.227 | 0.797 | |
| Residuals | 8.062 | 154 | |||
| Identification–Subjective intensity | Age | 23.914 | 1 | 7.606 | 0.006 ** |
| Smoking | 10.192 | 1 | 3.242 | 0.073 | |
| COVID | 12.155 | 2 | 3.866 | 0.023 * | |
| COVID × Age | 0.468 | 2 | 0.149 | 0.861 | |
| COVID × Smoking | 2.387 | 2 | 0.759 | 0.469 | |
| Residuals | 3.144 | 154 | |||
| Total | Age | 200.155 | 1 | 5.281 | 0.011 * |
| Smoking | 257.297 | 1 | 6.789 | 0.022 * | |
| COVID | 312.041 | 2 | 8.233 | 0.0004 ** | |
| COVID × Age | 38.205 | 2 | 1.008 | 0.367 | |
| COVID × Smoking | 30.605 | 2 | 0.807 | 0.447 | |
| Residuals | 37.899 | 154 |
| COV-without-Symp | COV-with-Symp | |||
|---|---|---|---|---|
| Item | % | % | chi | p |
| 1 (Orange) | 93.1 | 84.5 | 1.196 | 0.274 |
| 2 (Leather) | 79.31 | 78.87 | 0.001 | 0.984 |
| 3 (Cinnamon) | 68.96 | 76.05 | 0.071 | 0.791 |
| 4 (Mint) | 100 | 95.77 | 0.878 | 0.349 |
| 5 (Banana) | 89.65 | 83.1 | 0.617 | 0.432 |
| 6 (Lemon) | 79.31 | 38.03 | 13.232 | <0.0001 ** |
| 7 (Liquorice) | 89.65 | 63.38 | 6.431 | 0.011 * |
| 8 (Solvent) | 75.86 | 53.52 | 3.923 | 0.047 * |
| 9 (Garlic) | 79.31 | 78.87 | 0.001 | 0.984 |
| 10 (Coffee) | 79.31 | 57.74 | 3.818 | 0.051 |
| 11 (Apple) | 65.51 | 49.29 | 1.915 | 0.166 |
| 12 (Clove) | 72.41 | 66.19 | 0.295 | 0.587 |
| 13 (Pineapple) | 75.86 | 63.38 | 1.284 | 0.257 |
| 14 (Rose) | 100 | 84.5 | 4.427 | 0.034 * |
| 15 (Anise) | 86.2 | 69.2 | 2.898 | 0.088 |
| 16 (Fish) | 93.11 | 92.95 | 0.002 | 0.963 |
| COV-without-Symp | COV-with-Symp | ||||
|---|---|---|---|---|---|
| Item | Mean (SD) | Mean (SD) | t | df | p |
| 1 (Orange) | 5.97 | 5.82 | 0.29 | 61.65 | 0.772 |
| 2 (Leather) | 5.24 | 4.91 | 0.68 | 57.48 | 0.497 |
| 3 (Cinnamon) | 6.04 | 5.24 | 1.26 | 52.43 | 0.213 |
| 4 (Mint) | 8.48 | 7.09 | 3.44 | 89.54 | 0.0008 ** |
| 5 (Banana) | 7.28 | 6.24 | 2.11 | 80.11 | 0.038 * |
| 6 (Lemon) | 5.45 | 4.64 | 1.65 | 59.59 | 0.104 |
| 7 (Liquorice) | 6.03 | 5.08 | 1.87 | 68.84 | 0.066 |
| 8 (Solvent) | 7.48 | 6.42 | 2.11 | 73.33 | 0.038 * |
| 9 (Garlic) | 8.17 | 7.26 | 2.06 | 88.64 | 0.042 * |
| 10 (Coffee) | 6.86 | 5.82 | 2.12 | 75.09 | 0.037 * |
| 11 (Apple) | 6.28 | 5.91 | 0.8 | 65.71 | 0.426 |
| 12 (Clove) | 6.97 | 6.32 | 1.3 | 67.25 | 0.197 |
| 13 (Pineapple) | 6.52 | 5.34 | 2.16 | 67.56 | 0.033 * |
| 14 (Rose) | 7.17 | 6.26 | 1.86 | 80 | 0.066 |
| 15 (Anise) | 5.83 | 5.08 | 1.31 | 61.81 | 0.195 |
| 16 (Fish) | 8.17 | 7.56 | 1.26 | 73.73 | 0.211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Losada, M.L.; Bouhaben, J.; Ruiz-Huerta, C.; Canto, M.V.; Delgado-Lima, A.H. Long-Lasting Olfactory Dysfunction in Hospital Workers Due to COVID-19: Prevalence, Clinical Characteristics, and Most Affected Odorants. Int. J. Environ. Res. Public Health 2022, 19, 5777. https://doi.org/10.3390/ijerph19095777
Delgado-Losada ML, Bouhaben J, Ruiz-Huerta C, Canto MV, Delgado-Lima AH. Long-Lasting Olfactory Dysfunction in Hospital Workers Due to COVID-19: Prevalence, Clinical Characteristics, and Most Affected Odorants. International Journal of Environmental Research and Public Health. 2022; 19(9):5777. https://doi.org/10.3390/ijerph19095777
Chicago/Turabian StyleDelgado-Losada, María Luisa, Jaime Bouhaben, Claudia Ruiz-Huerta, Marcelle V. Canto, and Alice Helena Delgado-Lima. 2022. "Long-Lasting Olfactory Dysfunction in Hospital Workers Due to COVID-19: Prevalence, Clinical Characteristics, and Most Affected Odorants" International Journal of Environmental Research and Public Health 19, no. 9: 5777. https://doi.org/10.3390/ijerph19095777
APA StyleDelgado-Losada, M. L., Bouhaben, J., Ruiz-Huerta, C., Canto, M. V., & Delgado-Lima, A. H. (2022). Long-Lasting Olfactory Dysfunction in Hospital Workers Due to COVID-19: Prevalence, Clinical Characteristics, and Most Affected Odorants. International Journal of Environmental Research and Public Health, 19(9), 5777. https://doi.org/10.3390/ijerph19095777