Impact of Mineralocorticoid Receptor Gene NR3C2 on the Prediction of Functional Classification of Left Ventricular Remodeling and Arrhythmia after Acute Myocardial Infarction
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Blood Samples
2.3. Genetic Analysis
2.4. Echocardiographic Data Acquisition
2.5. Statistical Analysis
3. Results
3.1. Baseline Patients’ Characteristics
3.2. Baseline Echocardiography Characteristics
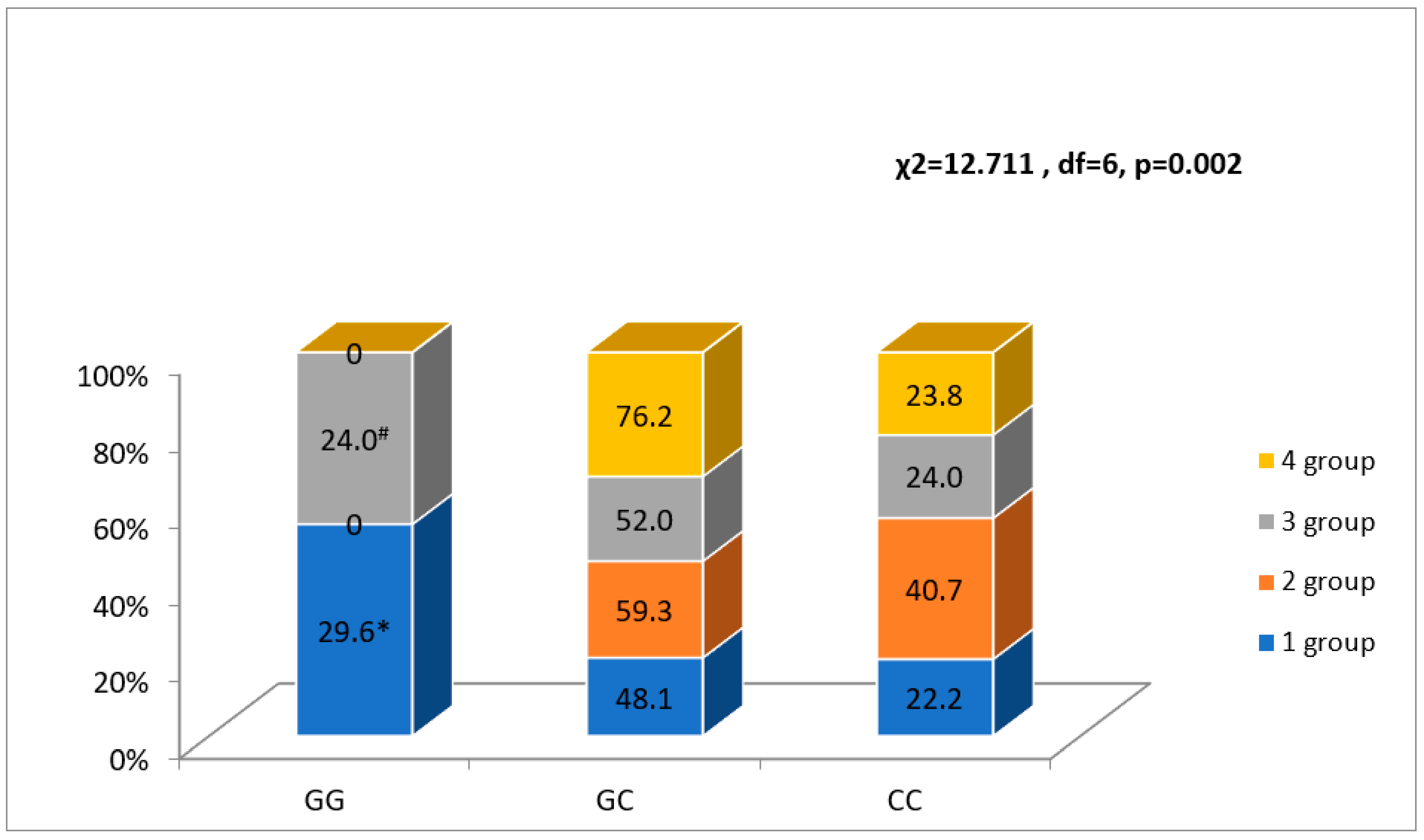
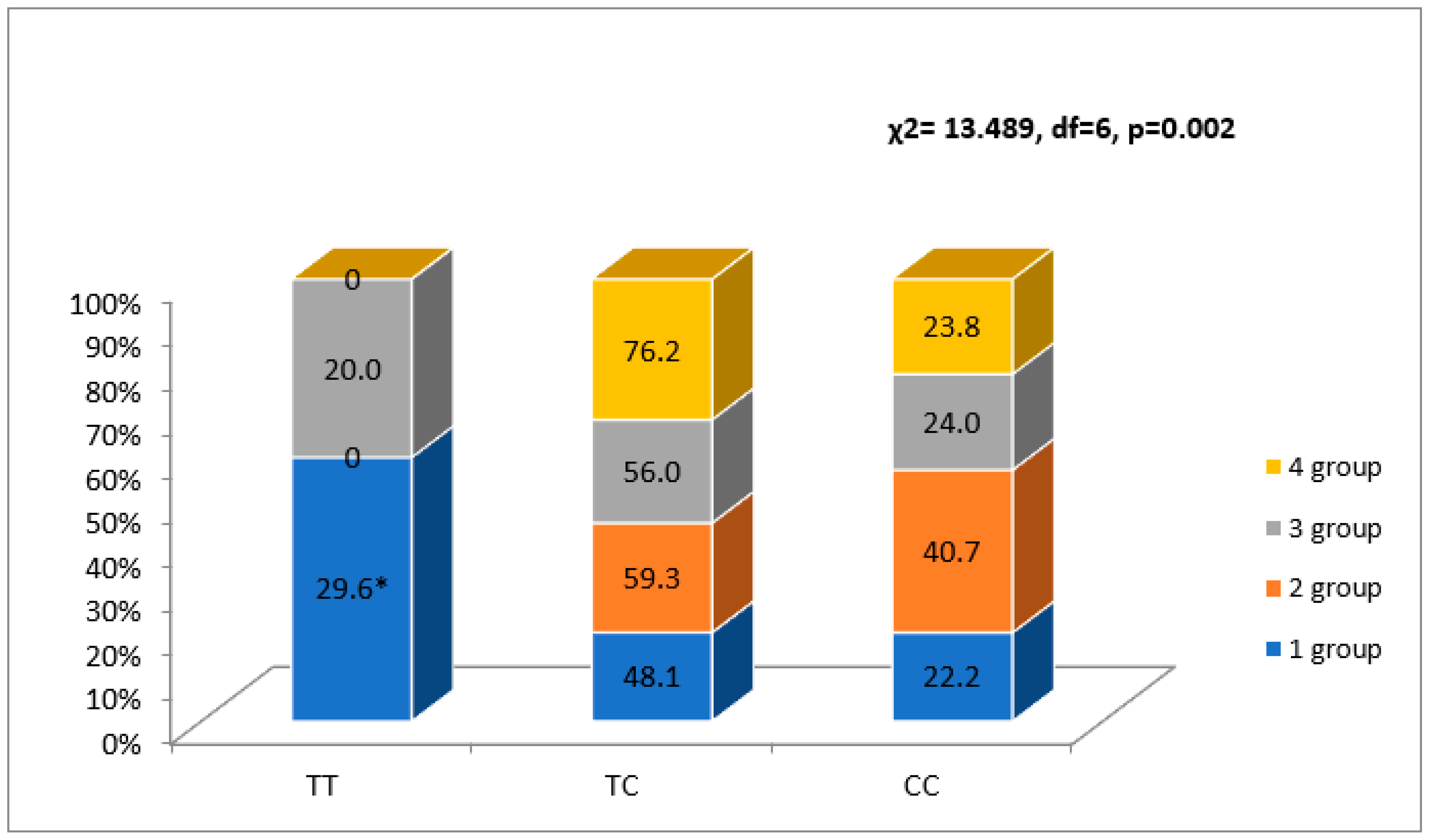
3.3. NR3C2 Genotype Associations with Four FLVR Groups
3.4. NR3C2 Gene Polymorphisms and Serum Cortisol Association with Rhythm and Conduction Disorders
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMI | acute myocardial infarction |
| ACE | angiotensin converting enzyme |
| AF | atrial fibrillation |
| AFL | atrial flutter |
| ARB | angiotensin receptor blockers |
| AVB | atrioventricular block |
| CRP | C-reactive protein |
| EDV1 | end-diastolic volume during the first 72 h of hospitalization for AMI |
| E’ average1 | E’ average1 during the first 72 h of hospitalization for AMI |
| EDV2 | end-diastolic volume after 6-month follow-up |
| EF1 | ejection fraction during the first 72 h of hospitalization for AMI |
| EF2 | ejection fraction after 6-month follow-up |
| ESV1 | end-systolic volume during the first 72 h of hospitalization for AMI |
| ESV2 | end-systolic volume after 6-month follow-up |
| FLVR | functional left ventricular remodeling |
| GR | glucocorticoid receptor |
| HAVB | high-grade atrioventricular block |
| Hb | hemoglobin |
| HDL | high density lipoprotein |
| HPA | hypothalamic-pituitary-adrenal axis |
| LDL | low density lipoprotein |
| LV | left ventricular |
| M | mean |
| Mitral A wave velocity1 | mitral A wave velocity during the first 72 h of hospitalization for AMI |
| MM1 | myocardial mass during the first 72 h of hospitalization for AMI |
| MR | mineralocorticoid receptor |
| MRA | mineralocorticoid receptor antagonists |
| MV | mitral valve |
| n | number |
| NSTEMI | non-ST segment elevation myocardial infarction |
| PCI | percutaneous coronary intervention |
| SD | standard deviation |
| STEMI | ST-segment elevation myocardial infarction |
| SWT1 | septal wall thickness during the first 72 h of hospitalization for AMI |
| PCI | percutaneous coronary intervention |
| PWT1 | posterior wall thickness during the first 72 h of hospitalization for AMI |
| TG | triglyceride |
| VA | ventricular arrhythmia |
| VF | ventricular fibrillation |
| VFL | ventricular flutter |
| VT | ventricular tachycardia |
| WBC | white blood cell |
References
- Xu, X.; Bao, H.; Strait, K.M.; Edmondson, D.E.; Davidson, K.W.; Beltrame, J.F.; Bueno, H.; Lin, H.; Dreyer, R.P.; Brush, J.E.; et al. Perceived Stress After Acute Myocardial Infarction: A Comparison Between Young and Middle-Aged Women Versus Men. Psychosom. Med. 2017, 79, 50–58. [Google Scholar] [CrossRef]
- Perez de la Hoz, R.A.; Swieszkowski, S.P.; Cintora, F.M.; Aladio, J.M.; Papini, C.M.; Matsudo, M.; Scazziota, A.S. Neuroendocrine System Regulatory Mechanisms: Acute Coronary Syndrome and Stress Hyperglycaemia. Eur. Cardiol. Rev. 2018, 13, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cruz-Topete, D.; He, B.; Foley, J.F.; Myers, P.H.; Xu, X.; Gomez-Sanchez, C.E.; Chambon, P.; Willis, M.S.; Cidlowski, J.A. Cardiomyocyte Glucocorticoid and Mineralocorticoid Receptors Directly and Antagonistically Regulate Heart Disease in Mice. Sci. Signal. 2019, 12, 9685. [Google Scholar] [CrossRef] [PubMed]
- Mihailidou, A.S.; Loan Le, T.Y.; Mardini, M.; Funder, J.W. Glucocorticoids Activate Cardiac Mineralocorticoid Receptors During Experimental Myocardial Infarction. Hypertension 2009, 54, 1306–1312. [Google Scholar] [CrossRef]
- Bienvenu, L.A.; Reichelt, M.E.; Delbridge, L.M.D.; Young, M.J. Mineralocorticoid Receptors and the Heart, Multiple Cell Types and Multiple Mechanisms: A Focus on the Cardiomyocyte. Clin. Sci. 2013, 125, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Buonafine, M.; Bonnard, B.; Jaisser, F. Mineralocorticoid Receptor and Cardiovascular Disease. Am. J. Hypertens. 2018, 31, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Rossier, M.F. The Cardiac Mineralocorticoid Receptor (MR): A Therapeutic Target Against Ventricular Arrhythmias. Front. Endocrinol. 2021, 12, 694758. [Google Scholar] [CrossRef]
- Bui, A.; Waks, J. Risk Stratification of Sudden Cardiac Death After Acute Myocardial Infarction. J. Innov. Card. Rhythm Manag. 2018, 9, 3035–3049. [Google Scholar] [CrossRef]
- Yalta, K.; Yilmaz, M.B.; Yalta, T.; Palabiyik, O.; Taylan, G.; Zorkun, C. Late Versus Early Myocardial Remodeling After Acute Myocardial Infarction: A Comparative Review on Mechanistic Insights and Clinical Implications. J. Cardiovasc. Pharmacol. Ther. 2019, 25, 15–26. [Google Scholar] [CrossRef]
- Parkash, R.; MacIntyre, C.; Dorian, P. Predicting Sudden Cardiac Death After Myocardial Infarction. Circ. Arrhythmia Electrophysiol. 2021, 14, e009422. [Google Scholar] [CrossRef]
- Di Fabio, F.; Gottlieb, B.; Beitel, L.K.; Trifiro, M.; NR3C2 (Nuclear Receptor Subfamily 3, Group C, Member 2). Atlas of Genetics and Cytogenetics in Oncology and Haematology. Available online: https://worldwidescience.org/topicpages/m/mthfr+gene+mutation.html (accessed on 1 April 2011).
- ter Heegde, F.; De Rijk, R.H.; Vinkers, C.H. The Brain Mineralocorticoid Receptor and Stress Resilience. Psychoneuroendocrinology 2015, 52, 92–110. [Google Scholar] [CrossRef] [PubMed]
- De Rijk, R.H.; Wüst, S.; Meijer, O.C.; Zennaro, M.-C.; Federenko, I.S.; Hellhammer, D.H.; Giacchetti, G.; Vreugdenhil, E.; Zitman, F.G.; de Kloet, E.R. A Common Polymorphism in the Mineralocorticoid Receptor Modulates Stress Responsiveness. J. Clin. Endocrinol. Metab. 2006, 91, 5083–5089. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, R.; Williamson, D.E.; Hariri, A.R. Mineralocorticoid Receptor Iso/Val (rs5522) Genotype Moderates the Association Between Previous Childhood Emotional Neglect and Amygdala Reactivity. Am. J. Psychiatry 2012, 169, 515–522. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Chimed, S.; van der Bijl, P.; Lustosa, R.; Fortuni, F.; Montero-Cabezas, J.M.; Ajmone Marsan, N.; Gersh, B.J.; Delgado, V.; Bax, J.J. Functional Classification of Left Ventricular Remodelling: Prognostic Relevance in Myocardial Infarction. ESC Heart Fail. 2022, 9, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; FitzGerald, G.; Yan, A.T.; Brieger, D.; Fox, K.A.A.; López-Sendón, J.; Yan, R.T.; Eagle, K.A.; Steg, P.G.; Budaj, A.; et al. High-grade atrioventricular block in acute coronary syndromes: Insights from the Global Registry of Acute Coronary Events. Eur. Heart J. 2014, 36, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Plieger, T.; Felten, A.; Splittgerber, H.; Duke, É.; Reuter, M. The Role of Genetic Variation in the Glucocorticoid Receptor (NR3C1) and Mineralocorticoid Receptor (NR3C2) in the Association between Cortisol Response and Cognition under Acute Stress. Psychoneuroendocrinology 2018, 87, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.A.; Kaplan, R.C.; Swett, K.; Balfour, P.; Kansal, M.M.; Talavera, G.A.; Perreira, K.; Blaha, M.J.; Benjamin, E.J.; Robertson, R.; et al. Smoking Intensity and Duration Is Associated with Cardiac Structure and Function: The ECHOcardiographic Study of Hispanics/Latinos. Open Heart BMJ 2017, 4, e000614. [Google Scholar] [CrossRef]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological Ventricular Remodeling. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef]
- de Albuquerque, F.N.; Brandão, A.A.; da Silva, D.A.; Mourilhe-Rocha, R.; Duque, G.S.; Gondar, A.F.P.; de Almeida Neves, L.M.; Bittencourt, M.I.; Pozzan, R.; de Albuquerque, D.C. Angiotensin-Converting Enzyme Genetic Polymorphism: Its Impact on Cardiac Remodeling. Arq. Bras. De Cardiol. 2013, 102, 70–79. [Google Scholar] [CrossRef]
- Zaliaduonyte-Peksiene, D.; Simonyte, S.; Lesauskaitė, V.; Vaskelyte, J.; Gustiene, O.; Mizariene, V.; Jurkevicius, R.; Jariene, G.; Tamosiunas, A.; Zaliunas, R. Left Ventricular Remodelling after Acute Myocardial Infarction: Impact of Clinical, Echocardiographic Parameters and Polymorphism of Angiotensinogen Gene. J. Renin-Angiotensin-Aldosterone Syst. 2013, 15, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.V.; Fontana, V.; de Faria, A.P.C.; Modolo, R.; Barbaro, N.R.; Sabbatini, A.R.; Peres, H.; Biagi, C.; Silva, P.S.; Lopes, P.C.; et al. Association of Mineralocorticoid Receptor Polymorphism I180V With Left Ventricular Hypertrophy in Resistant Hypertension. Am. J. Hypertens. 2015, 29, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.M.; Wertz, S.L.; Pollard, C.M.; Desimine, V.L.; Maning, J.; McCrink, K.A.; Lymperopoulos, A. Novel Insights into the Crosstalk between Mineralocorticoid Receptor and G Protein-Coupled Receptors in Heart Adverse Remodeling and Disease. Int. J. Mol. Sci. 2018, 19, 3764. [Google Scholar] [CrossRef]
- Zipes, D.P.; Camm, A.J.; Borggrefe, M.; Buxton, A.E.; Chaitman, B.; Fromer, M.; Gregoratos, G.; Klein, G.; Moss, A.J.; Myerburg, R.J.; et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2006, 8, 746–837. [Google Scholar] [CrossRef]
- Schmitt, J.; Duray, G.; Gersh, B.J.; Hohnloser, S.H. Atrial Fibrillation in Acute Myocardial Infarction: A Systematic Review of the Incidence, Clinical Features and Prognostic Implications. Eur. Heart J. 2008, 30, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Vukmirović, M.; Bošković, A.; Tomašević Vukmirović, I.; Vujadinovic, R.; Fatić, N.; Bukumirić, Z.; Vukmirović, F. Predictions and Outcomes of Atrial Fibrillation in the Patients with Acute Myocardial Infarction. Open Med. 2017, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Stamboul, K.; Fauchier, L.; Gudjoncik, A.; Buffet, P.; Garnier, F.; Lorgis, L.; Beer, J.C.; Touzery, C.; Cottin, Y. New Insights into Symptomatic or Silent Atrial Fibrillation Complicating Acute Myocardial Infarction. Arch. Cardiovasc. Dis. 2015, 108, 598–605. [Google Scholar] [CrossRef][Green Version]
- Fukunami, M. Transient Atrial Fibrillation During Acute Myocardial Infarction Is a Predictor of Poor Outcomes. Circ. J. 2016, 80, 1534–1536. [Google Scholar] [CrossRef]
- Pitt, B. 707-4 The Randomized Aldactone Evaluation Study (RALES): Parallel Dose Finding Trial. J. Am. Coll. Cardiol. 1995, 25, 45A. [Google Scholar] [CrossRef][Green Version]
- Pitt, B.; Williams, G.; Remme, W.; Martinez, F.; Lopez-Sendon, J.; Zannad, F.; Neaton, J.; Roniker, B.; Hurley, S.; Burns, D.; et al. The EPHESUS Trial: Eplerenone in Patients with Heart Failure Due to Systolic Dysfunction Complicating Acute Myocardial Infarction. Cardiovasc. Drugs Ther. 2001, 15, 79–87. [Google Scholar] [CrossRef]

| Baseline Characteristics | Group 1 n= 54 | Group 2 n = 27 | Group 3 n = 25 | Group 4 n = 21 | p-Value |
|---|---|---|---|---|---|
| Male n, (%) | 36 (66.7) | 23 (85.2) | 17 (68.0) | 15 (71.4) | NS |
| Diabetes mellitus n, (%) | 7 (13.0) | 1 (3.7) | 5 (20.0) | 3 (14.3) | NS |
| Hypertension n, (%) | 51 (94.4) | 25 (92.6) | 23 (92.0) | 17 (81.0) | NS |
| Dyslipidemia n, (%) | 51 (94.4) | 25 (92.6) | 23 (92.0) | 19 (90.5) | NS |
| Family history of heart attacks n, (%) | 2 (3.7) | 3 (11.1) | 5 (20.0) | 4 (19.0) | NS |
| Smoking n, (%) | 13 (24.2) a | 13 (48.1) a,b | 6 (24.3) a | 14 (67.3) b | 0.002 |
| Previous stroke n, (%) | 4 (7.4) | 2 (7.4) | 2 (8.0) | 5 (23.8) | NS |
| STEMI/NSTEMI n, (%) | 43 (79.6)/11 (20.4) | 24 (88.9)/3 (11.1) | 20 (80.0)/5 (20.0) | 19 (90.5)/2 (9.5) | NS |
| Dual/triple antiplatelet therapy n, (%) | 51 (94.4)/3 (5.6) | 27 (100)/0 (0) | 22 (88.0)/3 (12.0) | 19 (90.5)/2 (9.5) | NS |
| ACE inhibitors or ARB n, (%) | 50 (92.6) | 24 (88.9) | 21 (84.0) | 20 (95.2) | NS |
| Beta-blockers n, (%) | 48 (88.9) | 24 (88.9) | 20 (80.0) | 20 (95.2) | NS |
| Statins n, (%) | 53 (98.1) | 27 (100) | 25 (100) | 21 (100) | NS |
| MRA n, (%) | 4 (7.4) a | 8 (29.6) b,c | 4 (16.7) a,c | 11 (55.0) b | 0.001 |
| Age, years M (SD) | 65.0 (9.4) a | 58.4 (10.3) b | 64.6 (11.1) a,b | 57.1 (12.3) b | 0.006 |
| Troponin, μg/L median [IQR] | 0.56 [0.07–7.3] | 0.52 [0.06–5.1] | 0.9 [0.08–10.4] | 0.9 [0.21–8.1] | NS |
| Hbg, g/L mean (SD) | 143.0 (13.9) | 147.0 (20.7) | 143.4 (13.5) | 138 (13.7) | NS |
| WBC, 109/L median [IQR] | 9.3 [8.4–12.7] | 8.7 [7.1–11.1] | 8.8 [7.3–10.0] | 10.8 [7.7–13.9] | NS |
| CRP, g/L median [IQR] | 5.0 [5.0–19.3] | 5.0 [2.2 –10.4] | 5.0 [4.8–27.7] | 6.2 [4.2–36.0] | NS |
| Total cholesterol, mmol/L mean (SD) | 5.3 (1.1) | 5.4 (1.3) | 5.5 (1.1) | 5.3 (1.0) | NS |
| HDL, mmol/L median [IQR] | 1.2 [1.0–1.5] | 1.2 [0.97–1.5] | 1.3 [1.1–1.5] | 1.1 [0.96–1.3] | NS |
| LDL, mmol/L median [IQR] | 3.5 (1.0) | 3.6 (1.1) | 3.7 (0.9) | 3.5 (0.9) | NS |
| TG, mmol/L median [IQR] | 1.2 [0.8–1.7] | 1.3 [0.9–1.6] | 1.2 [0.9–1.5] | 1.4 [1.0–2.1] | NS |
| Cortisol, nmol/L median [IQR] | 726 [490–1019] | 599.5 [423.8–832.7] | 561.4 [406.6–880.2] | 580.4 [368.8–1359.6] | NS |
| Glucose, mmol/L median [IQR] | 6.8 [6.0–8.7] | 5.7 [5.3–7.0] | 6.3 [5.2–7.7] | 6.9 [6.2–9.1] | 0.028 |
| Echocardiography Characteristics | Group 1 n= 54 | Group 2 n = 27 | Group 3 n = 25 | Group 4 n = 21 | Unadjusted OR | p-Value |
|---|---|---|---|---|---|---|
| LVEDV1, mL, median [IQR] | 88.0 [77.9–103.6] | 112.8 [91.1–135.4] | 77.3 [61.2–94.4] | 88.3 [70.5–108.5] | 0.996 [0.985–1.007] | NS |
| LVEDV2, mL, median [IQR] | 83.3 [66.2–97.0] | 101.2 [83.9–123.1] | 111.5 [93.0–132.6] | 132.1 [104.6–170.6] | 1.038 [1.026–1.051] | 0.001 |
| LVESV1, mL, median [IQR] | 54.8 [46.1–62.4] | 57.5 [46.7–68.0] | 45.7 [36.9–61.0] | 51.2 [30.2–67.6] | 0.985 [0.967–1.003] | NS |
| LVESV2, mL, median [IQR] | 39.9 [31.0–51.1] | 55.5 [49.2–66.4] | 58.0 [45.5–75.8] | 84.5 [48.5–103.3] | 1.056 [1.038–1.074] | 0.001 |
| Global longitudinal strain1, median [IQR] | −12.9 [−15.3–(−10.1)] | −15.7 [−17.9–(−11.9)] | −13.0 [−15.3–(−11.3)] | −13.2 [−16.3–(−9.3)] | 0.978 [0.909–1.053] | NS |
| LVEF1, %, median [IQR] | 41.0 [33.8–45.1] | 48.6 [44.2–53.5] | 40.4 [34.4 –46.7] | 47.7 [40.2–55.0] | 1.044 [1.005–1.084] | 0.025 |
| LVEF2, %, median [IQR] | 50.1 [45.0–56.7] | 42.9 [37.7–46.7] | 48.3 [41.3–52.8] | 39.9 [32.4–46.3] | 0.921 [0.886–0.958] | 0.001 |
| LV SWT1, mm, median [IQR] | 12 [11–13] | 11.5 [10.7–12.0] | 12.00 [11.00–14.3] | 11.0 [10.0–12.8] | 0.968 [0.810–1.157] | NS |
| LV PWT1, mm, median [IQR] | 11 [10–12] | 11 [10–12] | 11.0 [10.0–12.0] | 10.0 [10.0–11.1] | 0.986 [0.761–1.277] | NS |
| LV MM1, median [IQR] | 186.0 [167.9–227.2] | 213.1 [173.9–226.8] | 206.7 [187.0–253.5] | 199.8 [158.0–232.8] | 1.007 [1.000–1.013] | 0.051 |
| MMI1, median [IQR] | 98.8 [86.0–111.8] | 103.0 [92.0–109.4] | 109.6 [98.8–118.9] | 104.7 [84.0–120.9] | 1.011 [0.996–1.026] | NS |
| Relative wall thickness1, median [IQR] | 0.46 [0.43–0.49] | 0.43 [0.39–0.47] | 0.44 [0.39–0.52] | 0.42 [0.38–0.51] | 1.003 [0.929–1.082] | NS |
| Mitral E velocity1 (cm/s), median [IQR] | 66 [56–82] | 60.0 [50.5–77.0] | 58.5 [48.8–86.3] | 58.0 [55.0–79.0] | 0.992 [0.975–1.010] | NS |
| Mitral A velocity1 (cm/s), median [IQR] | 76 [63–90] | 72.0 [51.0–86.0] | 71.5 [55.5–83.5] | 67.0 [55.9–78.0] | 0.983 [0.967–0.999] | 0.038 |
| Mitral E/A ratio1, median [IQR] | 0.79 [0.66–1.18] | 0.76 [0.63–1.42] | 0.87 [0.60–1.45] | 0.86 [0.74–1.29] | 1.376 [0.644–2.939] | NS |
| LV S’ average1, cm/s, median [IQR] | 8.2 [6.7–9.4] | 9.0 [7.5–11.6] | 8.4 [7.0–9.9] | 7.7 [6.6–9.6] | 1.044 [0.902–1.209] | NS |
| LV E’ average1, cm/s, median [IQR] | 6.6 [5.5–7.6] | 7.8 [7.0–9.3] | 6.8 [5.7–8.6] | 6.7 [6.1–8.3] | 1.231 [1.019–1.487] | 0.031 |
| MV anulus motion average1, mm, median [IQR] | 11.1 [9.0–12.3] | 11.6 [10.2–12.6] | 10.6 [8.7–12.7] | 10.0 [9.2–11.6] | 0.997 [0.849–1.172] | NS |
| TV annulus motion1, mm, [IQR] | 23 [18–25] | 24 [20–25] | 21 [19–25] | 22 [17–25] | 0.992 [0.928–1.061] | NS |
| RV S’1, mm, [IQR] | 13.0 [11.5–15.0] | 13.7 [11.8–15.0] | 12.4 [11.1–15.0] | 13.0 [10.7–14.2] | 0.929 [0.835–1.034] | NS |
| LA diameter1, mm, [IQR] | 40 [37–44] | 40 [36.0–42.0] | 41 [38–44] | 38 [35–42] | 0.986 [0.931–1.045] | NS |
| Rhythm and Conduction Disorders | Serum Cortisol, nmol/L | p-Value |
|---|---|---|
| VA/no VA | 1028 (324)/718 (365) | 0.001 |
| AF/AFL/no AF/AFL | 981 (351)/700 (359) | 0.001 |
| HAVB/no HAVB | 856 (389)/728 (367) | NS |
| NR3C2 Gene Polymorphisms | Rhythm and Conduction Disorders | ||||||
|---|---|---|---|---|---|---|---|
| VA/No VA n = 19/282 | p-Value | AF/AFL/No AF/AFL n = 40/261 | p-Value | HAVB/No HAVB n = 23/278 | p-Value | ||
| rs2070950, n (%) | GG | 2 (10.5)/46 (16.3) | NS | 9 (22.5)/39 (15.0) | NS | 0 (0.0)/48 (17.3) | NS |
| GC | 10 (52.5)/148 (52.6) | 18 (45.0)/140 (53.5) | 15 (65.2)/143 (51.4) | ||||
| CC | 7 (36.8)/88 (31.2) | 13 (32.5)/82 (31.5) | 8 (34.8)/87 (31.3) | ||||
| G allele | 14 (37.0)/240 (43.0) | NS | 36 (45.0)/218 (42.0) | NS | 15 (33.0)/239 (43.0) | NS | |
| C allele | 24 (63.0)/324 (57.0) | 44 (55.0)/304 (58.0) | 31 (67.0)/317 (57.0) | ||||
| rs4635799, n (%) | TT | 2 (10.5)/47 (16.7) | NS | 9 (22.5)/40 (15.4) | NS | 0 (0.0) a/49 (17.6) b | NS |
| TC | 10 (52.6)/147 (52.1) | 18 (45.0)/139 (53.1) | 15 (65.2)/142 (51.1) | ||||
| CC | 7 (36.8)/88 (31.2) | 13 (32.5)/82 (31.5) | 8 (34.8)/87 (31.3) | ||||
| T allele | 14 (37.0)/241 (43.0) | NS | 36 (45.0)/219 (42.0) | NS | 15 (33.0)/240 (43.0) | NS | |
| C allele | 24 (63.0)/323 (57.0) | 44 (55.0)/303 (58.0) | 31 (67.0)/316 (57.0) | ||||
| rs5522, n (%) | TT | 19 (100)/223 (79.1) | NS | 39 (97.5) a/203 (77.7) b | 0.014 | 21 (91.3)/221 (79.5) | NS |
| TC | 0 (0.0)/54 (19.1) | 1 (2.5) a/53 (20.4) b | 2 (8.7)/52 (18.7) | ||||
| CC | 0 (0.0)/5 (1.8) | 0 (0.0)/5 (1.9) | 0 (0.0)/5 (1.8) | ||||
| T allele | 38 (100.0)/500 (89.0) | 0.028 | 79 (99.0)/459 (88.0) | 0.003 | 44 (96.0)/494 (89.0) | NS | |
| C allele | 0 (0) a/64 (11.0) b | 1 (1.0) a/63 (12.0) b | 2 (4.0)/62 (11.0) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braukyliene, R.; Aldujeli, A.; Haq, A.; Maciulevicius, L.; Jankauskaite, D.; Jurenas, M.; Unikas, R.; Zabiela, V.; Lesauskaite, V.; Simonyte, S.; et al. Impact of Mineralocorticoid Receptor Gene NR3C2 on the Prediction of Functional Classification of Left Ventricular Remodeling and Arrhythmia after Acute Myocardial Infarction. Int. J. Environ. Res. Public Health 2023, 20, 12. https://doi.org/10.3390/ijerph20010012
Braukyliene R, Aldujeli A, Haq A, Maciulevicius L, Jankauskaite D, Jurenas M, Unikas R, Zabiela V, Lesauskaite V, Simonyte S, et al. Impact of Mineralocorticoid Receptor Gene NR3C2 on the Prediction of Functional Classification of Left Ventricular Remodeling and Arrhythmia after Acute Myocardial Infarction. International Journal of Environmental Research and Public Health. 2023; 20(1):12. https://doi.org/10.3390/ijerph20010012
Chicago/Turabian StyleBraukyliene, Rima, Ali Aldujeli, Ayman Haq, Laurynas Maciulevicius, Darija Jankauskaite, Martynas Jurenas, Ramunas Unikas, Vytautas Zabiela, Vaiva Lesauskaite, Sandrita Simonyte, and et al. 2023. "Impact of Mineralocorticoid Receptor Gene NR3C2 on the Prediction of Functional Classification of Left Ventricular Remodeling and Arrhythmia after Acute Myocardial Infarction" International Journal of Environmental Research and Public Health 20, no. 1: 12. https://doi.org/10.3390/ijerph20010012
APA StyleBraukyliene, R., Aldujeli, A., Haq, A., Maciulevicius, L., Jankauskaite, D., Jurenas, M., Unikas, R., Zabiela, V., Lesauskaite, V., Simonyte, S., & Zaliaduonytė, D. (2023). Impact of Mineralocorticoid Receptor Gene NR3C2 on the Prediction of Functional Classification of Left Ventricular Remodeling and Arrhythmia after Acute Myocardial Infarction. International Journal of Environmental Research and Public Health, 20(1), 12. https://doi.org/10.3390/ijerph20010012







