Performance of Bleach Method Sputum Smear Microscopy for the Diagnosis of Tuberculosis in a Highly Endemic District in Lima, Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Design
2.2. Participants
2.3. Techniques and Procedures
2.3.1. Sputum Samples Collection
2.3.2. Sputum Samples Treatment
2.3.3. Sputum Smears Staining and Microscopy
2.4. Statistical Analysis
2.5. Ethical Aspects
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

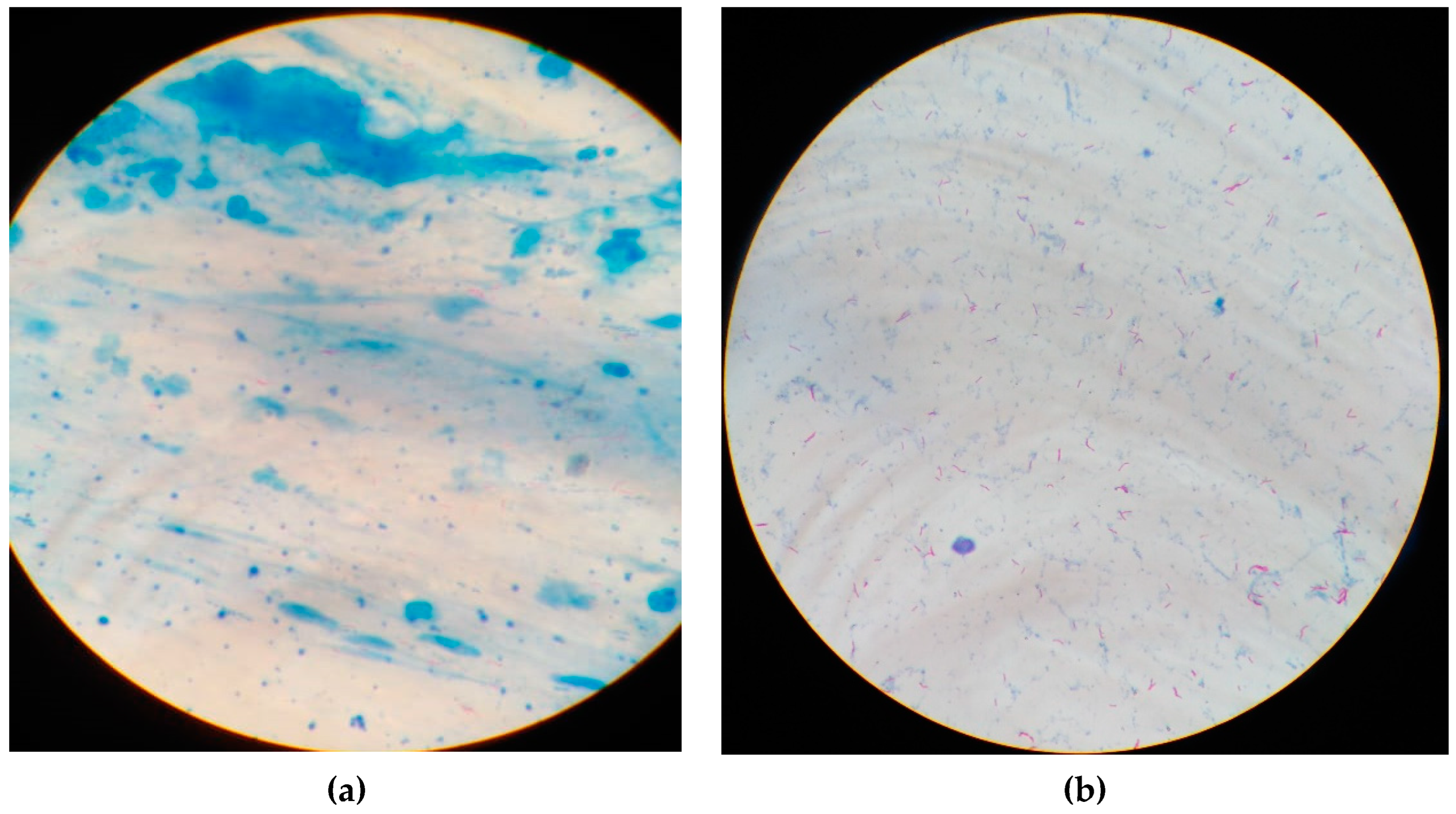

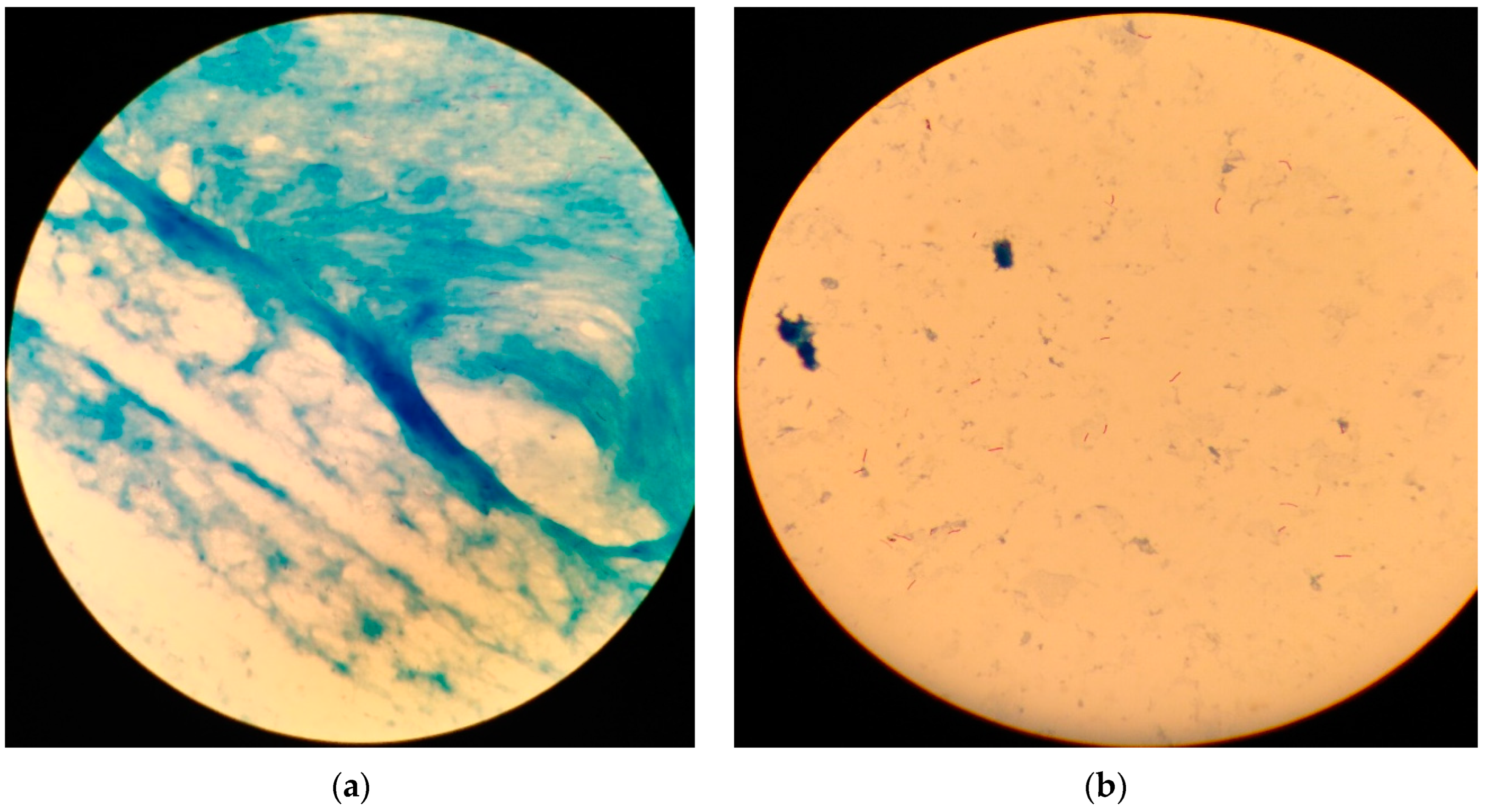
References
- WHO. Tuberculosis; World Health Organization: Geneva, Switzerland, 2022. Available online: https://www.who.int/health-topics/tuberculosis#tab=tab_1 (accessed on 20 October 2022).
- WHO. Millennium Development Goals (MDGs); World Health Organization: Geneva, Switzerland, 2018. Available online: http://www.who.int/topics/millennium_development_goals/es/ (accessed on 20 October 2022).
- PAHO. Tuberculosis en las Américas. Informe Regional 2020; Pan American Health Organization: Washington, DC, USA, 2021; Available online: https://www.paho.org/es/documentos/tuberculosis-americas-informe-regional-2020 (accessed on 21 October 2022).
- WHO. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 21 October 2022).
- MINSA. Dirección de Prevención y Control de Tuberculosis (DPCTB); MINSA: Lima, Peru, 2018. Available online: http://www.tuberculosis.minsa.gob.pe/ (accessed on 21 October 2022).
- Alarcón, V.; Alarcón, E.; Figueroa, C.; Mendoza-Ticona, A. Tuberculosis en el Perú: Situación epidemiológica, avances y desafíos para su control. RPMESP 2017, 34, 299–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahid, P.; Pai, M.; Hopewell, P.C. Advances in the diagnosis and treatment of tuberculosis. Proc. Am. Thorac. Soc. 2006, 3, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Saad, R., Jr.; Stirbulov, R. Pulmonary tuberculosis: Relationship between sputum bacilloscopy and radiological lesions. Rev. Inst. Med. Trop. São Paulo 2003, 45, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, P. Sputum smear microscopy in tuberculosis: Is it still relevant? Indian J. Med. Res. 2013, 137, 442–444. [Google Scholar]
- Mekonen, A.; Ayele, Y.; Berhan, Y.; Woldeyohannes, D.; Erku, W.; Sisay, S. Factors which contributed for low quality sputum smears for the detection of acid-fast bacilli (AFB) at selected health centers in Ethiopia: A quality control perspective. PLoS ONE. 2018, 13, e0198947. [Google Scholar] [CrossRef] [PubMed]
- Rawat, J.; Biswas, D.; Sindhwani, G.; Masih, V. An alternative 1-day smear microscopy protocol for the diagnosis of pulmonary tuberculosis. Respirology 2010, 15, 1127–1130. [Google Scholar] [CrossRef]
- Shah, R.R.; Dye, W.E. Use of dithiothreitol to replace n-acetyl-L-cysteine for routine sputum digestion-decontamination for the culture of mycobacteria. Am. Rev. Respir. Dis. 1966, 94, 454. [Google Scholar] [CrossRef]
- Hooja, S.; Pal, N.; Malhotra, B.; Goyal, S.; Kumar, V.; Vyas, L. Comparison of Ziehl Neelsen & Auramine O staining methods on direct and concentrated smears in clinical specimens. Indian J. Tuberc. 2011, 58, 72–76. [Google Scholar]
- Workineh, M.; Maru, M.; Seman, I.; Bezu, Z.; Negash, M.; Melku, M.; Gize, A.; Shibabaw, A. Agreement between Direct Fluorescent Microscopy and Ziehl-Neelsen Concentration Techniques in Detection of Pulmonary Tuberculosis in Northwest Ethiopia. Ethiop. J. Health Sci. 2017, 27, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Daley, P.; Michael, J.S.; S, K.; Latha, A.; Mathai, D.; John, K.R.; Pai, M. A pilot study of short-duration sputum pretreatment procedures for optimizing smear microscopy for tuberculosis. PLoS ONE 2009, 4, e5626. [Google Scholar] [CrossRef] [Green Version]
- Lawson, L.; Yassin, M.; Ramsay, A.; Emenyonu, E.; Thacher, T.; Davies, P.; Squire, S.; Cuevas, L. Short-term bleach digestion of sputum in the diagnosis of pulmonary tuberculosis in patients co-infected with HIV. Tuberculosis 2007, 87, 368–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MINSA. Boletín Epidemiológico del Perú; Ministerio de Salud: Lima, Peru, 2022.
- MINSA. Manuales de Capacitación para el Manejo de la Tuberculosis; Ministerio de Salud: Lima, Peru, 2017.
- Ongkhammy, S.; Amstutz, V.; Barennes, H.; Buisson, Y. The bleach method improves the detection of pulmonary tuberculosis in Laos. Comp. Study 2009, 13, 1124–1129. [Google Scholar]
- INS. Avances en el Diagnóstico y la Investigación de la Tuberculosis en el Perú; Instituto Nacional de Salud: Lima, Peru, 2017.
- INS. [Procedimientos para el Control de Calidad Externo de Baciloscopía para el Diagnóstico Bacteriológico de la Tuberculosis]; Instituto Nacional de Salud: Lima, Peru, 2014.
- Kim, S.Y.; Kim, Y.J.; Hwang, S.H.; Kim, H.H.; Lee, E.Y.; Chang, C.L. Evaluation of an acid-fast bacilli autostainer for concentrated sputum smears. Int. J. Tuberc. Lung Dis. 2008, 12, 39–43. [Google Scholar] [PubMed]
- Rasool, G.; Riaz, M.; Mahmood, Z.; Mohy-Ud-Din, R.; Akhtar, J.; Javed, I. Effects of household bleach on sputum smear microscopy to concentrate acid fast bacilli for the diagnosis of pulmonary tuberculosis. J. Biol. Regul. Homeost. Agents 2018, 32, 607–611. [Google Scholar]
- Chew, R.; Calderón, C.; Schumacher, S.G.; Sherman, J.M.; Caviedes, L.; Fuentes, P.; Coronel, J.; Valencia, T.; Hererra, B.; Zimic, M.; et al. Evaluation of bleach-sedimentation for sterilising and concentrating Mycobacterium tuberculosis in sputum specimens. BMC Infect. Dis. 2011, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Afsar, I.; Gunes, M.; Er, H.; Gamze Sener, A. Comparison of culture, microscopic smear and molecular methods in diagnosis of tuberculosis. Rev. Esp. Quimioter. 2018, 31, 435–438. [Google Scholar]
- Mizuno, K.; Chikamatsu, K.; Aono, A.; Azuma, Y.; Yamada, H.; Mitarai, S. Clinical evaluation of acid-fast smear examination with light emitting diode fluorescent microscopy. Kekkaku [Tuberculosis] 2009, 84, 627–629. [Google Scholar]
- Chaturvedi, S.; Raheja, P.; Thakur, R.; Singh, N.; Arora, V.K.; Suri, V.; Bhatia, A. A technically simple method for staining of acid-fast bacilli in cytology smears: An evaluation. Aust. J. Rural Health 2006, 14, 280–283. [Google Scholar] [CrossRef]
- Farnia, P.; Mohammadi, F.; Zarifi, Z.; Tabatabee, D.J.; Ganavi, J.; Ghazisaeedi, K.; Gheydi, M.; Bahadori, M.; Masjedi, M.; Velayati, A.A. Improving sensitivity of direct microscopy for detection of acid-fast bacilli in sputum: Use of chitin in mucus digestion. J. Clin. Microbiol. 2002, 40, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Mindolli, P.B.; Salmani, M.P.; Parandekar, P.K. Improved Diagnosis of Pulmonary Tuberculosis using bleach Microscopy Method. J. Clin. Diagn. Res. 2013, 7, 1336–1338. [Google Scholar] [CrossRef]
- Selvakumar, N.; Premkumar, M.; Kumar, V.; Gopi, P.G.; Sivagamasundari, S.; Prabhakaran, E.; Narayanan, P.R. Phenol ammonium sulphate basic fuchsin staining of sputum in pot for the detection of acid-fast bacilli. Indian J. Med. Res. 2008, 128, 194–197. [Google Scholar] [PubMed]
- Cattamanchi, A.; Davis, J.L.; Worodria, W.; Boon, S.D.; Yoo, S.; Matovu, J.; Kiidha, J.; Nankya, F.; Kyeyune, R.; Byanyima, P.; et al. Sensitivity and specificity of fluorescence microscopy for diagnosing pulmonary tuberculosis in a high HIV prevalence setting. Int. J. Tuberc. Lung Dis. 2009, 13, 1130–1136. [Google Scholar] [PubMed]
- Khatun, Z.; Kamal, M.; Roy, C.K.; Sultana, T.; Rahman, M.Q.; Azad, M.B.A.S.; Ahmed, A.N.N. Usefulness of light emitting diode (LED) fluorescent microscopy as a tool for rapid and effective method for the diagnosis of pulmonary tuberculosis. Bangladesh Med. Res. Counc. Bull. 2011, 37, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, M.; Ramsay, A.; Githui, W.; Gagnidze, L.; Varaine, F.; Guerin, P.J. Bleach sedimentation: An opportunity to optimize smear microscopy for tuberculosis diagnosis in settings of high prevalence of HIV. Clin. Infect. Dis. 2008, 46, 1710–1716. [Google Scholar] [CrossRef] [Green Version]
- Cattamanchi, A.; Davis, J.L.; Pai, M.; Huang, L.; Hopewell, P.C.; Steingart, K.R. Does bleach processing increase the accuracy of sputum smear microscopy for diagnosing pulmonary tuberculosis? J. Clin. Microbiol. 2010, 48, 2433–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, N.; Choi, S.M.; Lee, J.; Park, Y.S.; Lee, C.H.; Lee, S.M.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Yim, J.J. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS ONE 2013, 8, e77456. [Google Scholar] [CrossRef]
- Moure, R.; Muñoz, L.; Torres, M.; Santin, M.; Martín, R.; Alcaide, F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J. Clin. Microbiol. 2011, 49, 1137–1139. [Google Scholar] [CrossRef] [Green Version]
- Fradejas, I.; Ontañón, B.; Muñoz-Gallego, I.; Ramírez-Vela, M.J.; López-Roa, P. The value of xpert MTB/RIF-generated CT values for predicting the smear status of patients with pulmonary tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2018, 13, 9–12. [Google Scholar] [CrossRef]
- Tadesse, M.; Aragaw, D.; Rigouts, L.; Abebe, G. Increased detection of smear-negative pulmonary tuberculosis by GeneXpert MTB/RIF® assay after bleach concentration. Int. J. Mycobacteriol. 2016, 5, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, J.L.O.; Araújo, A.A.; Silva, L.O.; Coutinho, I.O.; Lima, J.F.C.; Leal, N.C.; Almeida, A.M.P. Bleach-concentrated direct sputum smear microscopy procedure for diagnosis of pulmonary tuberculosis in poverty areas. JBPML 2021, 57, e2292021. [Google Scholar] [CrossRef]
- Mendelson, M. Diagnosing tuberculosis in HIV-infected patients: Challenges and future prospects. Br. Med. Bull. 2007, 81–82, 149–165. [Google Scholar] [CrossRef] [PubMed]
| Bleach-Treated SSM | Direct SSM | |||||
|---|---|---|---|---|---|---|
| Neg | +/− | + | ++ | +++ | Total | |
| Neg | 114 (97.4) | 0 | 0 | 0 | 0 | 114 |
| +/− | 0 | 1 | 3 | 0 | 0 | 4 |
| + | 0 | 5 | 9 | 2 | 1 | 17 |
| ++ | 3 | 2 | 9 | 6 | 2 | 22 |
| +++ | 0 | 0 | 2 | 8 | 20 | 30 |
| Total | 117 | 8 | 23 | 16 | 23 | 187 |
| SSM | Xpert MTB/RIF Test | |
|---|---|---|
| MTB-Negative (n = 114) | MTB-Positive (n = 73) | |
| Direct SSM | ||
| Negative | 114 (100.0%) | 3 (4.1%) |
| Positive | 0 (0.0%) | 70 (95.9%) |
| Bleach-treated SSM | ||
| Negative | 114 (100.0%) | 0 (0.0%) |
| Positive | 0 (0.0%) | 73 (100.0%) |
| Demographics | Median (Interquartile Range) | ap-Value | |
|---|---|---|---|
| Direct SSM | Bleach-Treated SSM | ||
| Sex | |||
| Male | 213 (29–301) | 286 (92–381) | <0.001 |
| Female | 119 (92–367) | 336 (142–453) | <0.001 |
| Age group | |||
| <18 years | --- | --- | --- |
| 18–40 years | 106 (26–312) | 286 (125–401) | <0.001 |
| >40 years | 297 (182–317) | 302 (96–367) | 0.768 |
| Total | 213 (71–312) | 286 (125–392) | <0.001 |
| SSM | AUC | SE | CI95 | p-Value a |
|---|---|---|---|---|
| Direct | 97.95% | 1.2% | 92.5–100.0% | 0.079 |
| Bleach method | 100.0% | 0.0% | 100.0–100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales-Rimache, J.; Nunayalle-Vargas, M.; Rueda-Torres, L.; Inolopú-Cucche, J. Performance of Bleach Method Sputum Smear Microscopy for the Diagnosis of Tuberculosis in a Highly Endemic District in Lima, Peru. Int. J. Environ. Res. Public Health 2023, 20, 135. https://doi.org/10.3390/ijerph20010135
Rosales-Rimache J, Nunayalle-Vargas M, Rueda-Torres L, Inolopú-Cucche J. Performance of Bleach Method Sputum Smear Microscopy for the Diagnosis of Tuberculosis in a Highly Endemic District in Lima, Peru. International Journal of Environmental Research and Public Health. 2023; 20(1):135. https://doi.org/10.3390/ijerph20010135
Chicago/Turabian StyleRosales-Rimache, Jaime, Magda Nunayalle-Vargas, Lenin Rueda-Torres, and Jorge Inolopú-Cucche. 2023. "Performance of Bleach Method Sputum Smear Microscopy for the Diagnosis of Tuberculosis in a Highly Endemic District in Lima, Peru" International Journal of Environmental Research and Public Health 20, no. 1: 135. https://doi.org/10.3390/ijerph20010135
APA StyleRosales-Rimache, J., Nunayalle-Vargas, M., Rueda-Torres, L., & Inolopú-Cucche, J. (2023). Performance of Bleach Method Sputum Smear Microscopy for the Diagnosis of Tuberculosis in a Highly Endemic District in Lima, Peru. International Journal of Environmental Research and Public Health, 20(1), 135. https://doi.org/10.3390/ijerph20010135






