Oestrogenic Activity in Girls with Signs of Precocious Puberty as Exposure Biomarker to Endocrine Disrupting Chemicals: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaires
Data Processing
2.3. Chemiluminescence Immunoassay (E2 Concentrations)
2.4. Gene Reporter Luciferase Assay (Oestrogenic Activity)
2.5. Data Analysis and Statistical Analysis
3. Results and Discussion
3.1. Questionnaire Results
3.1.1. General Characteristics
3.1.2. General Characteristics: Comparison between C and P
3.1.3. EDC Exposure
3.1.4. EDC Exposure: Comparison between C and P
3.2. Results of Laboratory Analyses
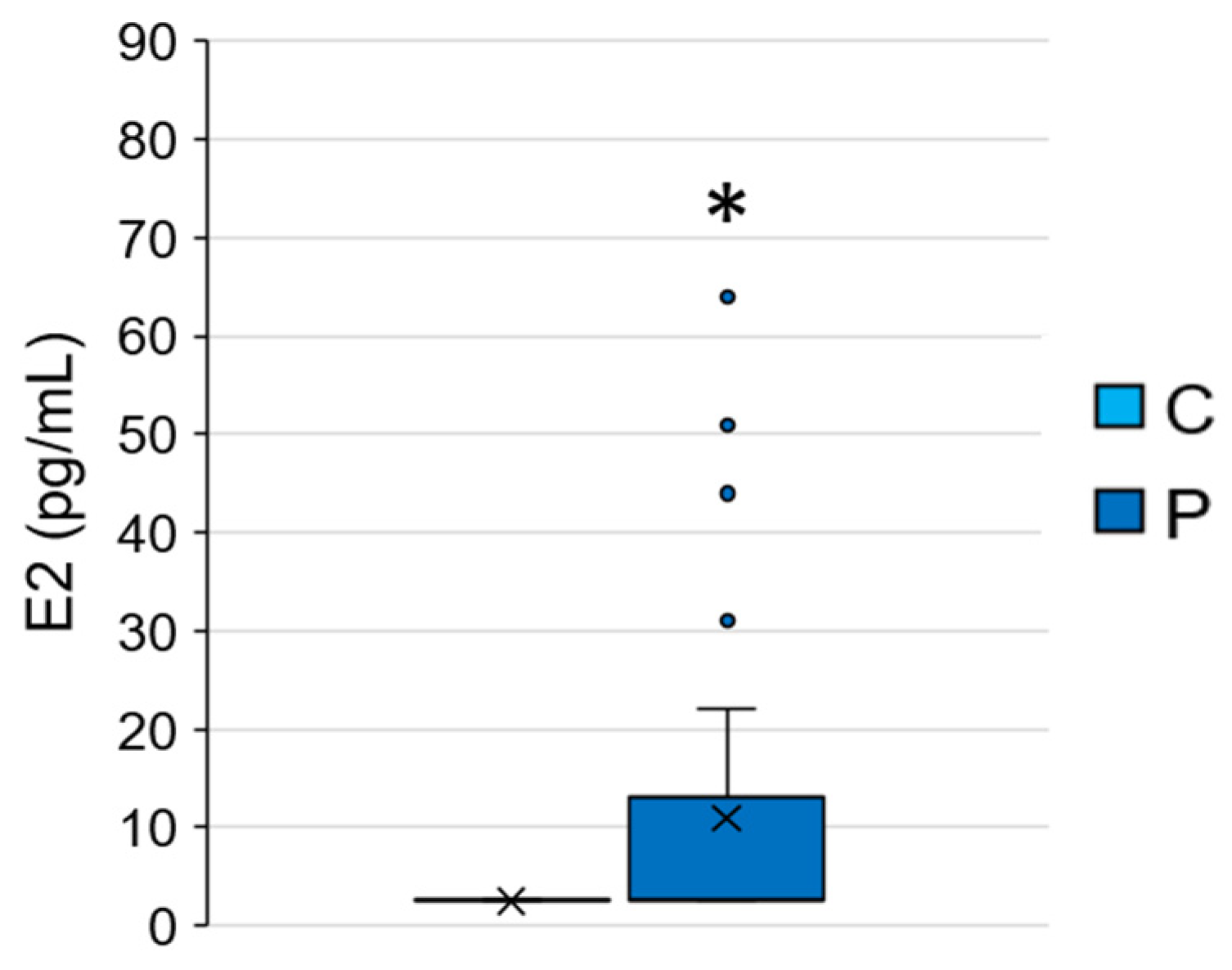
3.2.1. E2 Concentrations
3.2.2. EEQ Concentrations
3.2.3. Relationship between EDC Exposure (Low/High) and EEQ
3.2.4. Relationship between Living Environment (Rural/Urban) and EEQ
3.3. Study Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheuiche, A.V.; da Silveira, L.G.; de Paula, L.C.P.; Lucena, I.R.S.; Silveiro, S.P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr. 2021, 180, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Chioma, L.; Bizzarri, C.; Verzani, M.; Fava, D.; Salerno, M.; Capalbo, D.; Guzzetti, C.; Penta, L.; Di Luigi, L.; di Iorgi, N.; et al. Sedentary lifestyle and precocious puberty in girls during the COVID-19 pandemic: An Italian experience. Endocr. Connect. 2022, 11, e210650. [Google Scholar] [CrossRef]
- Kiess, W.; Hoppmann, J.; Gesing, J.; Penke, M.; Körner, A.; Kratzsch, J.; Pfaeffle, R. Puberty–genes, environment and clinical issues. J. Pediatr. Endocrinol. Metab. 2016, 29, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Argente, J. Central precocious puberty, functional and tumor-related. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101262. [Google Scholar] [CrossRef]
- Valsamakis, G.; Arapaki, A.; Balafoutas, D.; Charmandari, E.; Vlahos, N.F. Diet-induced hypothalamic inflammation, phoenixin, and subsequent precocious puberty. Nutrients 2021, 13, 3460. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.P.; Kaiser, U.B. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Berberoğlu, M. Precocious Puberty and Normal Variant Puberty: Definition, etiology, diagnosis and current management-Review. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 164–174. [Google Scholar] [CrossRef]
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary axis and puberty. Mol. Cell. Endocrinol. 2021, 520, 111094. [Google Scholar] [CrossRef]
- Buluş, A.D.; Aşci, A.; Erkekoglu, P.; Balci, A.; Andiran, N.; Koçer-Gümüşel, B. The evaluation of possible role of endocrine disruptors in central and peripheral precocious puberty. Toxicol. Mech. Methods 2016, 26, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Latronico, A.C.; Brito, V.N.; Carel, J.C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef]
- Gonc, E.N.; Kandemir, N. Body composition in sexual precocity. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, J.; Zhang, X.; Lu, Y.; Miao, J.; Xue, H. Obesity is a risk factor for central precocious puberty: A case-control study. BMC Pediatr. 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wu, M.; Li, Y.L.; Rao, R.; Li, S.; Cen, Q.; Wu, H.; Lv, L.; Huang, M.; Ge, Y.P.; et al. Physical deviation and precocious puberty among school-aged children in Leshan City: An investigative study. J. Int. Med. Res. 2020, 48, 300060520939672. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Magenes, V.C.; Pascuzzi, M.C.; Rossi, V.; Sangiorgio, A.; Bosetti, A.; Zuccotti, G.; Mameli, C. The role of pediatric nutrition as a modifiable risk factor for precocious puberty. Life 2021, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef]
- Schilirò, T.; Pignata, C.; Rovere, R.; Fea, E.; Gilli, G. The endocrine disrupting activity of surface waters and of wastewater treatment plant effluents in relation to chlorination. Chemosphere 2009, 75, 335–340. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence and risk assessment of multiclass endocrine disrupting compounds in an urban tropical river and a proposed risk management and monitoring framework. Sci. Total Environ. 2019, 671, 431–442. [Google Scholar] [CrossRef]
- Zheng, R.H.; Fang, C.; Hong, F.K.; Kuang, W.M.; Lin, C.; Jiang, Y.L.; Chen, J.C.; Zhang, Y.S.; Bo, J. Developing and applying a classification system for ranking the biological effects of endocrine disrupting chemicals on male rockfish Sebastiscus marmoratus in the Maowei Sea, China. Mar. Pollut. Bull. 2021, 163, 111931. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Tap water contamination: Multiclass endocrine disrupting compounds in different housing types in an urban settlement. Chemosphere 2021, 264, 128488. [Google Scholar] [CrossRef]
- Darbre, P.D. Overview of air pollution and endocrine disorders. Int. J. Gen. Med. 2018, 11, 191–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plunk, E.C.; Richards, S.M. Endocrine-disrupting air pollutants and their effects on the hypothalamus-pituitary-gonadal axis. Int. J. Mol. Sci. 2020, 21, 9191. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Perovich, L.J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Teil, M.J.; Moreau-Guigon, E.; Blanchard, M.; Alliot, F.; Gasperi, J.; Cladière, M.; Mandin, C.; Moukhtar, S.; Chevreuil, M. Endocrine disrupting compounds in gaseous and particulate outdoor air phases according to environmental factors. Chemosphere 2016, 146, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Pozo, L.; Gómez-Regalado, M.D.C.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental endocrine-disrupting chemical exposure: Role in non-communicable diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef]
- Rose, M.; Bennett, D.H.; Bergman, A.K.E.; Fängström, B.; Pessah, I.N.; Hertz-Picciotto, I. PRDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environ. Sci. Technol. 2010, 44, 2648–2653. [Google Scholar] [CrossRef] [Green Version]
- Gea, M.; Toso, A.; Schilirò, T. Estrogenic activity of biological samples as a biomarker. Sci. Total Environ. 2020, 740, 140050. [Google Scholar] [CrossRef]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. Br. Med. J. 2007, 335, 194–197. [Google Scholar] [CrossRef] [Green Version]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. Br. Med. J. 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Sonneveld, E.; Jansen, H.J.; Riteco, J.A.C.; Brouwer, A.; van der Burg, B. Development of androgen- and estrogen-responsive bioassays members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol. Sci. 2005, 83, 136–148. [Google Scholar] [CrossRef]
- Schilirò, T.; Porfido, A.; Spina, F.; Varese, G.C.; Gilli, G. Oestrogenic activity of a textile industrial wastewater treatment plant effluent evaluated by the E-screen test and MELN gene-reporter luciferase assay. Sci. Total Environ. 2012, 432, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Vermeirssen, E.L.M.; Buchinger, S.; Behr, M.; Magdeburg, A.; Oehlmann, J. Deriving bio-equivalents from in vitro bioassays: Assessment of existing uncertainties and strategies to improve accuracy and reporting. Environ. Toxicol. Chem. 2013, 32, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Lauria, L.; Spinelli, A.; Buoncristiano, M.; Nardone, P. Decline of childhood overweight and obesity in Italy from 2008 to 2016: Results from 5 rounds of the population-based surveillance system. BMC Public Health 2019, 19, 618. [Google Scholar] [CrossRef] [PubMed]
- Castiello, F.; Freire, C. Exposure to non-persistent pesticides and puberty timing: A systematic review of the epidemiological evidence. Eur. J. Endocrinol. 2021, 184, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jung, H.W.; Lee, Y.J.; Lee, Y.A. Early-life exposure to endocrine-disrupting chemicals and pubertal development in girls. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, B.; Cheng, J.; Chen, D.; Wu, Y.; Luo, Q.; Zhou, L. Obesity-related genetic polymorphisms are associated with the risk of early puberty in Han Chinese girls. Clin. Endocrinol. 2022, 96, 319–327. [Google Scholar] [CrossRef]
- Bulcao Macedo, D.; Nahime Brito, V.; Latronico, A.C. New causes of central precocious puberty: The role of genetic factors. Neuroendocrinology 2014, 100, 1–8. [Google Scholar] [CrossRef]
- Roberts, S.A.; Kaiser, U.B. Genetics in endocrinology genetic etiologies of central precocious puberty and the role of imprinted genes. Eur. J. Endocrinol. 2020, 183, R107–R117. [Google Scholar] [CrossRef]
- Varimo, T.; Iivonen, A.P.; Känsäkoski, J.; Wehkalampi, K.; Hero, M.; Vaaralahti, K.; Miettinen, P.J.; Niedziela, M.; Raivio, T. Familial central precocious puberty: Two novel MKRN3 mutations. Pediatr. Res. 2021, 90, 431–435. [Google Scholar] [CrossRef]
- Pamplona-Silva, M.T.; Mazzeo, D.E.C.; Bianchi, J.; Marin-Morales, M.A. Estrogenic compounds: Chemical characteristics, detection methods, biological and environmental effects. Water Air Soil Pollut. 2018, 229, 144. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Alqahtani, S.S.; Alshahrani, S. Diet: A source of endocrine disruptors. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.Y.; Lin, Y.S.; Yen, C.H.; Miaw, C.L.; Chen, T.C.; Wu, M.C.; Hsieh, C.Y. Identification, contribution, and estrogenic activity of potential EDCs in a river receiving concentrated livestock effluent in Southern Taiwan. Sci. Total Environ. 2018, 636, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US national health and nutrition examination survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef] [PubMed]
- Lorber, M.; Schecter, A.; Paepke, O.; Shropshire, W.; Christensen, K.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 2015, 77, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melough, M.M.; Maffini, M.V.; Otten, J.J.; Sathyanarayana, S. Diet quality and exposure to endocrine-disrupting chemicals among US adults. Environ. Res. 2022, 211, 113049. [Google Scholar] [CrossRef] [PubMed]
- Steele, E.M.; Khandpur, N.; da Costa Louzada, M.L.; Monteiro, C.A. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS ONE 2020, 15, e0236738. [Google Scholar] [CrossRef]
- Wagner, M.; Oehlmann, J. Endocrine disruptors in bottled mineral water: Estrogenic activity in the E- Screen. J. Steroid Biochem. Mol. Biol. 2011, 127, 128–135. [Google Scholar] [CrossRef]
- Duan, X.; Shen, G.; Yang, H.; Tian, J.; Wei, F.; Gong, J.; Zhang, J. Dietary intake of polycyclica aromatic hydrocarbons (PAHs) and associated cancer risk in a cohort of Chinese urban adults: Inter- and intra-individual variability. Chemosphere 2016, 144, 2469–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Kim, S.; Lee, E.; Cheong, H.-K.; Chang, S.-S.; Kang, D.; Choi, Y.; Lee, S.-M.; Jang, J.-Y. Sources of polycyclic aromatic hydrocarbon exposure in non-occupationally exposed Koreans. Environ. Mol. Mutagen. 2003, 42, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Franssen, D.; Svingen, T.; Lopez Rodriguez, D.; Van Duursen, M.; Boberg, J.; Parent, A.S. A putative adverse outcome pathway network for disrupted female pubertal onset to improve testing and regulation of endocrine disrupting chemicals. Neuroendocrinology 2022, 112, 101–114. [Google Scholar] [CrossRef]
- Jansen, E.C.; Marín, C.; Mora-Plazas, M.; Villamor, E. Higher childhood red meat intake frequency is associated with earlier age at menarche. J. Nutr. 2016, 146, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, Y.; Zhang, Y.; Sun, W.; Jiang, Y.; Song, Y.; Zhu, Q.; Mei, H.; Wang, X.; Liu, S.; et al. Association between dietary patterns and precocious puberty in children: A population-based study. Int. J. Endocrinol. 2018, 2018, 4528704. [Google Scholar] [CrossRef] [Green Version]
- Eugster, E.A. Update on precocious puberty in girls. J. Pediatr. Adolesc. Gynecol. 2019, 32, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, P.; Bloch, C. Evaluation and referral of children with signs of early puberty. Pediatrics 2016, 137, e20153732. [Google Scholar] [CrossRef] [Green Version]
- Maione, L.; Bouvattier, C.; Kaiser, U.B. Central precocious puberty: Recent advances in understanding the aetiology and in the clinical approach. Clin. Endocrinol. 2021, 95, 542–555. [Google Scholar] [CrossRef]
- Zhou, F.; Jin, Z.; Zhu, L.; Huang, F.; Ye, A.; Hou, C. A preliminary study on the relationship between environmental endocrine disruptors and precocious puberty in girls. J. Pediatr. Endocrinol. Metab. 2022, 35, 989–997. [Google Scholar] [CrossRef]
- Janfaza, M.; Sherman, T.I.; Larmore, K.A.; Brown-Dawson, J.; Klein, K.O. Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: A 10 year experience. J. Pediatr. Endocrinol. Metab. 2006, 19, 901–909. [Google Scholar] [CrossRef]
- Klein, K.O.; Mericq, V.; Brown-Dawson, J.M.; Larmore, K.A.; Cabezas, P.; Cortinez, A. Estrogen levels in girls with premature thelarche compared with normal prepubertal girls as determined by an ultrasensitive recombinant cell bioassay. J. Pediatr. 1999, 134, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.O.; Baron, J.; Colli, M.J.; McDonnell, D.P.; Cutler, G.B. Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J. Clin. Investig. 1994, 94, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Larmore, K.A.; O’Connor, D.; Sherman, T.I.; Funanage, V.L.; Hassink, S.G.; Klein, K.O. Leptin and estradiol as related to change in pubertal status and body weight. Med. Sci. Monit. 2002, 8, 206–211. [Google Scholar]
- Mesa Valencia, D.C.; Mericq, V.; Corvalán, C.; Pereira, A. Obesity and related metabolic biomarkers and its association with serum levels of estrogen in pre-pubertal chilean girls. Endocr. Res. 2020, 45, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Gaspari, L.; Servant, N.; Philibert, P.; Sultan, C. Increased serum estrogenic bioactivity in girls with premature thelarche: A marker of environmental pollutant exposure? Gynecol. Endocrinol. 2013, 29, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Servant, N.; Térouanne, B.; Balaguer, P.; Nicolas, J.C.; Sultan, C. A new recombinant cell bioassay for ultrasensitive determination of serum estrogenic bioactivity in children. J. Clin. Endocrinol. Metab. 2002, 87, 791–797. [Google Scholar] [CrossRef]
- Pereira, A.; Corvalán, C.; Uauy, R.; Klein, K.O.; Mericq, V. Ultrasensitive estrogen levels at 7 years of age predict earlier thelarche: Evidence from girls of the growth and obesity Chilean cohort. Eur. J. Endocrinol. 2015, 173, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.A.; Heinrichs, C.; Larmore, K.A.; Craen, M.; Brown-Dawson, J.; Shaywitz, S.; Ross, J.; Oeter Klein, K. Estradiol levels in girls with Turner’s syndrome compared to normal prepubertal girls as determined by an ultrasensitive assay. J. Pediatr. Endocrinol. Metab. 2003, 16, 91–96. [Google Scholar] [CrossRef]
- Gaspari, L.; Paris, F.; Jeandel, C.; Sultan, C. Peripheral precocious puberty in a 4-month-old girl: Role of pesticides? Gynecol. Endocrinol. 2011, 27, 721–724. [Google Scholar] [CrossRef]
- Maurício, R.; Dias, R.; Ribeiro, V.; Fernandes, S.; Vicente, A.C.; Pinto, M.I.; Noronha, J.P.; Amaral, L.; Coelho, P.; Mano, A.P. 17α-Ethinylestradiol and 17β-estradiol removal from a secondary urban wastewater using an RBC treatment system. Environ. Monit. Assess. 2018, 190, 320. [Google Scholar] [CrossRef]
- Liu, B.; Xue, Z.; Zhu, X.; Jia, C. Long-term trends (1990–2014), health risks, and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the U.S. Environ. Pollut. 2017, 220, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Kim, H.S.; Park, H.; Ye, S.; Lee, D.; Ha, E.H. Does exposure to PM10 decrease age at menarche? Environ. Int. 2018, 117, 16–21. [Google Scholar] [CrossRef] [PubMed]
- McGuinn, L.A.; Voss, R.W.; Laurent, C.A.; Greenspan, L.C.; Kushi, L.H.; Windham, G.C. Residential proximity to traffic and female pubertal development. Environ. Int. 2016, 94, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Wronka, I.; Kliś, K. Effect of air pollution on age at menarche in polish females, born 1993–1998. Sci. Rep. 2022, 12, 4820. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Busch, A.S.; Eckert-Lind, C.; Koch, T.; Hickey, M.; Juul, A. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998 to 2017. JAMA Netw. Open 2020, 3, e2015665. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, A.; Jung, M.K.; Kim, K.E.; Suh, J.; Chae, H.W.; Kim, D.H.; Ha, S.; Seo, G.H.; Kim, H.S. Incidence and prevalence of central precocious puberty in Korea: An epidemiologic study based on a national database. J. Pediatr. 2019, 208, 221–228. [Google Scholar] [CrossRef]
- Stagi, S.; De Masi, S.; Bencini, E.; Losi, S.; Paci, S.; Parpagnoli, M.; Ricci, F.; Ciofi, D.; Azzari, C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital. J. Pediatr. 2020, 46, 165. [Google Scholar] [CrossRef] [PubMed]



| C (%) | P (%) | Chi-Square Test (p) | |
|---|---|---|---|
| Use of disposable plastic containers | 40.0 | 6.7 | 0.011 |
| Storage of warm foods in plastic containers | 90.0 | 33.3 | 0.002 |
| Consumption of packaged meat/vegetables | 80.0 | 43.3 | 0.044 |
| Use of candles/incense/air fresheners | 80.0 | 16.7 | <0.001 |
| Frequency | C (%) | P (%) | Chi-Square Test (p) | |
|---|---|---|---|---|
| Consumption of sheep meat | Never | 0.0 | 23.3 | 0.047 |
| Annually | 0.0 | 20.0 | ||
| Monthly | 12.5 | 23.3 | ||
| Weekly | 87.5 | 33.4 | ||
| Consumption of pork meat | Never | 0.0 | 13.3 | 0.022 |
| Annually | 0.0 | 0.0 | ||
| Monthly | 10.0 | 46.7 | ||
| Weekly | 90.0 | 40.0 | ||
| Consumption of other meat * | Never | 0.0 | 60.7 | <0.001 |
| Annually | 0.0 | 7.2 | ||
| Monthly | 0.0 | 21.4 | ||
| Weekly | 100.0 | 10.7 | ||
| Consumption of canned foods | Never | 50.0 | 13.3 | 0.017 |
| Annually | 12.5 | 0.0 | ||
| Monthly | 12.5 | 50.0 | ||
| Weekly | 25.0 | 36.7 | ||
| Consumption of water in glass bottles | Never | 25.0 | 70.0 | 0.041 |
| Annually | 0.0 | 0.0 | ||
| Monthly | 0.0 | 3.3 | ||
| Weekly | 75.0 | 26.7 | ||
| Consumption of water from public water sources | Never | 14.3 | 82.8 | 0.002 |
| Annually | 14.3 | 3.4 | ||
| Monthly | 0.0 | 0.0 | ||
| Weekly | 71.4 | 13.8 | ||
| Consumption of fried food | Never | 0.0 | 3.3 | 0.011 |
| Annually | 0.0 | 23.3 | ||
| Monthly | 10.0 | 43.4 | ||
| Weekly | 90.0 | 30.0 | ||
| Consumption of grilled food | Never | 0.0 | 3.3 | 0.006 |
| Annually | 0.0 | 23.3 | ||
| Monthly | 10.0 | 46.7 | ||
| Weekly | 90.0 | 26.7 |
| Reference | Study Population (n) | EEQ (Mean ± SD, pg/mL) | |
|---|---|---|---|
| Pre-pubertal girls | Present study | 10 | 21.03 ± 21.41 |
| Klein et al., 1994 [62] | 21 | 0.6 ± 0.6 | |
| Klein et al., 1999 [61] | 15 | 0.9 ± 1.0 | |
| Paris et al., 2002 [66] | 18 | 3.5 ± 2.2 | |
| Larmore et al., 2002 [63] | 12 a | 0.3 ± 0.4 | |
| Larmore et al., 2002 [63] | 12 b | 0.6 ± 1.3 | |
| Wilson et al., 2003 [68] | 34 | 3.4 ± 2.9 | |
| Janfaza et al., 2006 [60] | 147 | 1.6 ± 2.6 | |
| Pereira et al., 2015 [67] | 91 | 3.6 ± 2.3 | |
| Mesa Valencia et al., 2019 [64] | 107 | 3.6 ± 2.3 | |
| PP girls | Present study | 30 | 15.14 ± 13.24 |
| Klein et al., 1999 [61] | 20 | 2.3 ± 1.2 | |
| Larmore et al., 2002 [63] | 12 | 2.2 ± 2.7 | |
| Gaspari et al., 2011 [69] | 1 | 13.5 ± 1.0 | |
| Paris et al., 2013 [65] | 15 | 8.4 ± 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gea, M.; Toso, A.; Bentivegna, G.N.; Buganza, R.; Abrigo, E.; De Sanctis, L.; Schilirò, T. Oestrogenic Activity in Girls with Signs of Precocious Puberty as Exposure Biomarker to Endocrine Disrupting Chemicals: A Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 14. https://doi.org/10.3390/ijerph20010014
Gea M, Toso A, Bentivegna GN, Buganza R, Abrigo E, De Sanctis L, Schilirò T. Oestrogenic Activity in Girls with Signs of Precocious Puberty as Exposure Biomarker to Endocrine Disrupting Chemicals: A Pilot Study. International Journal of Environmental Research and Public Health. 2023; 20(1):14. https://doi.org/10.3390/ijerph20010014
Chicago/Turabian StyleGea, Marta, Anna Toso, Giuseppe Nicolò Bentivegna, Raffaele Buganza, Enrica Abrigo, Luisa De Sanctis, and Tiziana Schilirò. 2023. "Oestrogenic Activity in Girls with Signs of Precocious Puberty as Exposure Biomarker to Endocrine Disrupting Chemicals: A Pilot Study" International Journal of Environmental Research and Public Health 20, no. 1: 14. https://doi.org/10.3390/ijerph20010014
APA StyleGea, M., Toso, A., Bentivegna, G. N., Buganza, R., Abrigo, E., De Sanctis, L., & Schilirò, T. (2023). Oestrogenic Activity in Girls with Signs of Precocious Puberty as Exposure Biomarker to Endocrine Disrupting Chemicals: A Pilot Study. International Journal of Environmental Research and Public Health, 20(1), 14. https://doi.org/10.3390/ijerph20010014









