Effects of Pesticide Intake on Gut Microbiota and Metabolites in Healthy Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Determination of Pesticide Exposure Biomarkers in Urine
2.3. Metabolite Analysis in Fecal Samples
2.4. Sample Collection, DNA Isolation, and V3–V4 16S rRNA Sequencing
2.5. Statistical Analysis
3. Results
3.1. Study Subjects and Metabolite Analyses
3.2. Correlations of Pesticide Exposure Markers and Fecal Metabolites
3.3. Effect of OP Exposure on the Composition of the Gut Microbiome and Community Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Pesticides Use. Global, Regional and Country Trends, 1990–2018. 2021. Available online: https://www.fao.org/3/cb3411en/cb3411en.pdf (accessed on 10 October 2022).
- Koureas, M.; Tsakalof, A.; Tsatsakis, A.; Hadjichristodoulou, C. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol. Lett. 2012, 210, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Bellinger, D.C.; Wright, R.O.; Weisskopf, M.G. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 2010, 125, e1270–e1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, M.F.; Chevrier, J.; Harley, K.G.; Kogut, K.; Vedar, M.; Calderon, N.; Trujillo, C.; Johnson, C.; Bradman, A.; Barr, D.B.; et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 2011, 119, 1189–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Alzaga, B.; Lacasaña, M.; Aguilar-Garduño, C.; Rodríguez-Barranco, M.; Ballester, F.; Rebagliato, M.; Hernández, A.F. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol. Lett. 2014, 230, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Mattila, T.; Santonen, T.; Andersen, H.R.; Katsonouri, A.; Szigeti, T.; Uhl, M.; Wąsowicz, W.; Lange, R.; Bocca, B.; Ruggieri, F.; et al. Scoping Review-The Association between Asthma and Environmental Chemicals. Int. J. Environ. Res. Public Health 2021, 18, 1323. [Google Scholar] [CrossRef]
- Wang, B.; Tsakiridis, E.E.; Zhang, S.; Llanos, A.; Desjardins, E.M.; Yabut, J.M.; Green, A.E.; Day, E.A.; Smith, B.K.; Lally, J.S.V.; et al. The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue. Nat. Commun. 2021, 12, 5163. [Google Scholar] [CrossRef]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Urinary Dialkyl Phosphate Concentrations and Lung Function Parameters in Adolescents and Adults: Results from the Canadian Health Measures Survey. Environ. Health Perspect. 2016, 124, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Yoshida, T.; Hanada, H.; Takeuchi, I.; et al. Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 65. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Fang, B.; Li, J.W.; Zhang, M.; Ren, F.Z.; Pang, G.F. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem. Toxicol. 2018, 111, 144–152. [Google Scholar] [CrossRef]
- Gao, B.; Bian, X.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Health Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Nasuti, C.; Coman, M.M.; Olek, R.A.; Fiorini, D.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Fedeli, D.; Gabbianelli, R. Changes on fecal microbiota in rats exposed to permethrin during postnatal development. Environ. Sci. Pollut. Res. Int. 2016, 23, 10930–10937. [Google Scholar] [CrossRef]
- Rueda-Ruzafa, L.; Cruz, F.; Roman, P.; Cardona, D. Gut microbiota and neurological effects of glyphosate. Neurotoxicology 2019, 75, 1–8. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, D.B.; Wong, L.Y.; Bravo, R.; Weerasekera, G.; Odetokun, M.; Restrepo, P.; Kim, D.G.; Fernandez, C.; Whitehead, R.D., Jr.; Perez, J.; et al. Urinary concentrations of dialkylphosphate metabolites of organophosphorus pesticides: National Health and Nutrition Examination Survey 1999–2004. Int. J. Environ. Res. Public Health 2011, 8, 3063–3098. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, J.; Saito, I.; Takaishi, A.; Nomura, H.; Inoue, M.; Osaka, A.; Sugiura, Y.; Hayashi, Y.; Wakusawa, S.; Ogi, H.; et al. A revised method for determination of dialkylphosphate levels in human urine by solid-phase extraction and liquid chromatography with tandem mass spectrometry: Application to human urine samples from Japanese children. Environ. Health Prev. Med. 2014, 19, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, G.; Gries, W. Simultaneous determination of pyrethroid and pyrethrin metabolites in human urine by gas chromatography-high resolution mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 814, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Hamada, R.; Saito, I.; Nakane, K.; Sawa, R.; Ukai, M.; Shibata, E.; Sato, M.; Kamijima, M.; Ueyama, J. Optimization and validation of a highly sensitive method for determining glyphosate in human urine by solid-phase extraction and liquid chromatography with tandem mass spectrometry: A methodological study. Environ. Health Prev. Med. 2020, 25, 83. [Google Scholar] [CrossRef]
- Ueyama, J.; Harada, K.H.; Koizumi, A.; Sugiura, Y.; Kondo, T.; Saito, I.; Kamijima, M. Temporal Levels of Urinary Neonicotinoid and Dialkylphosphate Concentrations in Japanese Women Between 1994 and 2011. Environ. Sci. Technol. 2015, 49, 14522–14528. [Google Scholar] [CrossRef]
- Ueyama, J.; Oda, M.; Hirayama, M.; Sugitate, K.; Sakui, N.; Hamada, R.; Ito, M.; Saito, I.; Ohno, K. Freeze-drying enables homogeneous and stable sample preparation for determination of fecal short-chain fatty acids. Anal. Biochem. 2020, 589, 113508. [Google Scholar] [CrossRef]
- Xiong, X.Z.S. Rapid and Simultaneous Quantification of Polyamines in Human Plasma by LC–MS/MS After Pre-column Derivatization with N-(9-Fluorenylmethoxycarbonyloxy)succinimide. Chromatographia 2016, 79, 561–570. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [Green Version]
- Bradman, A.; Castorina, R.; Barr, D.B.; Chevrier, J.; Harnly, M.E.; Eisen, E.A.; McKone, T.E.; Harley, K.; Holland, N.; Eskenazi, B. Determinants of organophosphorus pesticide urinary metabolite levels in young children living in an agricultural community. Int. J. Environ. Res. Public Health 2011, 8, 1061–1083. [Google Scholar] [CrossRef]
- Roca, M.; Miralles-Marco, A.; Ferré, J.; Pérez, R.; Yusà, V. Biomonitoring exposure assessment to contemporary pesticides in a school children population of Spain. Environ. Res. 2014, 131, 77–85. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Wang, G.; Han, R.; Xie, X. Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 2016, 153, 287–293. [Google Scholar] [CrossRef]
- The Ministry of Health, Labor and Welfare of Japan. Diazinon. 2019. Available online: https://www.mhlw.go.jp/con-481tent/11130500/000559402.pdf (accessed on 10 October 2022).
- Ueyama, J.; Kimata, A.; Kamijima, M.; Hamajima, N.; Ito, Y.; Suzuki, K.; Inoue, T.; Yamamoto, K.; Takagi, K.; Saito, I.; et al. Urinary excretion of 3-phenoxybenzoic acid in middle-aged and elderly general population of Japan. Environ. Res. 2009, 109, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mallek, A.Y.; Moharram, A.M.; Abdel-Kader, M.I.; Omar, S.A. Effect of soil treatment with the organophosphorus insecticide Profenfos on the fungal flora and some microbial activities. Microbiol. Res. 1994, 149, 167–171. [Google Scholar] [CrossRef]
- Nagaraju, R.; Joshi, A.K.R.; Vamadeva, S.G.; Rajini, P.S. Deregulation of hepatic lipid metabolism associated with insulin resistance in rats subjected to chronic monocrotophos exposure. J. Biochem. Mol. Toxicol. 2020, 34, e22506. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [Green Version]
- Holme, F.; Thompson, B.; Holte, S.; Vigoren, E.M.; Espinoza, N.; Ulrich, A.; Griffith, W.; Faustman, E.M. The role of diet in children’s exposure to organophosphate pesticides. Environ. Res. 2016, 147, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.C.; Cantonwine, D.E.; Anzalota Del Toro, L.V.; Calafat, A.M.; Valentin-Blasini, L.; Davis, M.D.; Montesano, M.A.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Distribution and determinants of urinary biomarkers of exposure to organophosphate insecticides in Puerto Rican pregnant women. Sci. Total Environ. 2015, 512–513, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Associations between dietary factors and urinary concentrations of organophosphate and pyrethroid metabolites in a Canadian general population. Int. J. Hyg. Environ. Health 2015, 218, 616–626. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kato, K.; Murakami, H.; Hosomi, K.; Tanisawa, K.; Nakagata, T.; Ohno, H.; Konishi, K.; Kawashima, H.; Chen, Y.A.; et al. Comprehensive analysis of gut microbiota of a healthy population and covariates affecting microbial variation in two large Japanese cohorts. BMC Microbiol. 2021, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Nguyen, S.M.; Yang, Y.; Xu, W.; Cai, H.; Wu, J.; Cai, Q.; Long, J.; Zheng, W.; Shu, X.O. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am. J. Clin. Nutr. 2021, 113, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.H.; Guo, L.J.; Huang, Z.Y.; Rao, J.N.; Tang, C.W. Roles of cellular polyamines in mucosal healing in the gastrointestinal tract. J. Physiol. Pharmacol. 2013, 64, 681–693. [Google Scholar]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M. Prevention of Atherosclerosis by the Induction of Microbial Polyamine Production in the Intestinal Lumen. Biol. Pharm. Bull. 2020, 43, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, R.; Bowyer, R.C.E.; El Balkhi, S.; Saint-Marcoux, F.; Gardere, A.; Ducarmon, Q.R.; Geelen, A.R.; Zwittink, R.D.; Tsoukalas, D.; Sarandi, E.; et al. Impacts of dietary exposure to pesticides on faecal microbiome metabolism in adult twins. Environ. Health 2022, 21, 46. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [Green Version]
- Lotti, C.; Rubert, J.; Fava, F.; Tuohy, K.; Mattivi, F.; Vrhovsek, U. Development of a fast and cost-effective gas chromatography-mass spectrometry method for the quantification of short-chain and medium-chain fatty acids in human biofluids. Anal. Bioanal. Chem. 2017, 409, 5555–5567. [Google Scholar] [CrossRef]
| Parameters | ||
|---|---|---|
| Subject [n] (male/female) | 36 (18/18) | |
| Age [years] | Mean ± SD (range) | 68.7 ± 9.9 (39–81) |
| BMI [%] | Mean ± SD | 22.6 ± 3.0 |
| Smoking [n] | Never | 19 |
| Ex | 7 | |
| Currently | 2 | |
| Natural birth [n] | Yes, No, No data | 28, 0, 8 |
| Breast milk [n] | Yes, No, No data | 27, 1, 8 |
| Stool frequency (/week) | Mean ± SD (range) median | 9.0 ± 5.6 (2–21) 7.0 |
| Fecal water content [%] | Mean ± SD (range) | 74 ± 7 (55–86) |
| Detection Rate (%) | Concentrations | |||
|---|---|---|---|---|
| Median | Mean ± SD | Range | ||
| Fecal metabolites | ||||
| Acetate (mg/g) | 100 | 15.1 | 16.1 ± 9.2 | 3.9–35.1 |
| Propionate (mg/g) | 100 | 4.9 | 5.4 ± 3.0 | 0.1–15.2 |
| Butyrate (mg/g) | 100 | 3.4 | 4.4 ± 3.2 | 0.1–14.2 |
| Valeric Acid (mg/g) | 97 | 0.69 | 0.73 ± 0.50 | <LOD–1.97 |
| Lactate (mg/g) | 100 | 0.46 | 0.80 ± 0.94 | 0.22–4.72 |
| Succinic acid (mg/g) | 100 | 0.52 | 1.43 ± 1.87 | 0.06–8.62 |
| Putrescine (μg/g) | 100 | 90 | 210 ± 262 | 5–846 |
| Spermidine (μg/g) | 100 | 281 | 352 ± 213 | 12–806 |
| Pesticide exposure markers | ||||
| DMAP (μmol/g cre) | 100 | 0.18 | 0.43 ± 0.94 | 0.07–5.76 |
| DEAP (μmol/g cre) | 100 | 0.06 | 0.18 ± 0.37 | 0.01–2.02 |
| DAP (μmol/g cre) | 100 | 0.25 | 0.61 ± 1.02 | 0.09–5.91 |
| 3PBA (μg/g cre) | 97 | 0.59 | 1.01 ± 2.25 | <LOD–14.02 |
| Glyphosate (μg/g cre) | 64 | 0.15 | 0.13 ± 0.14 | <LOD–0.43 |
| pH | 7.2 | 7.4 ± 0.9 | 4.9–9.0 | |
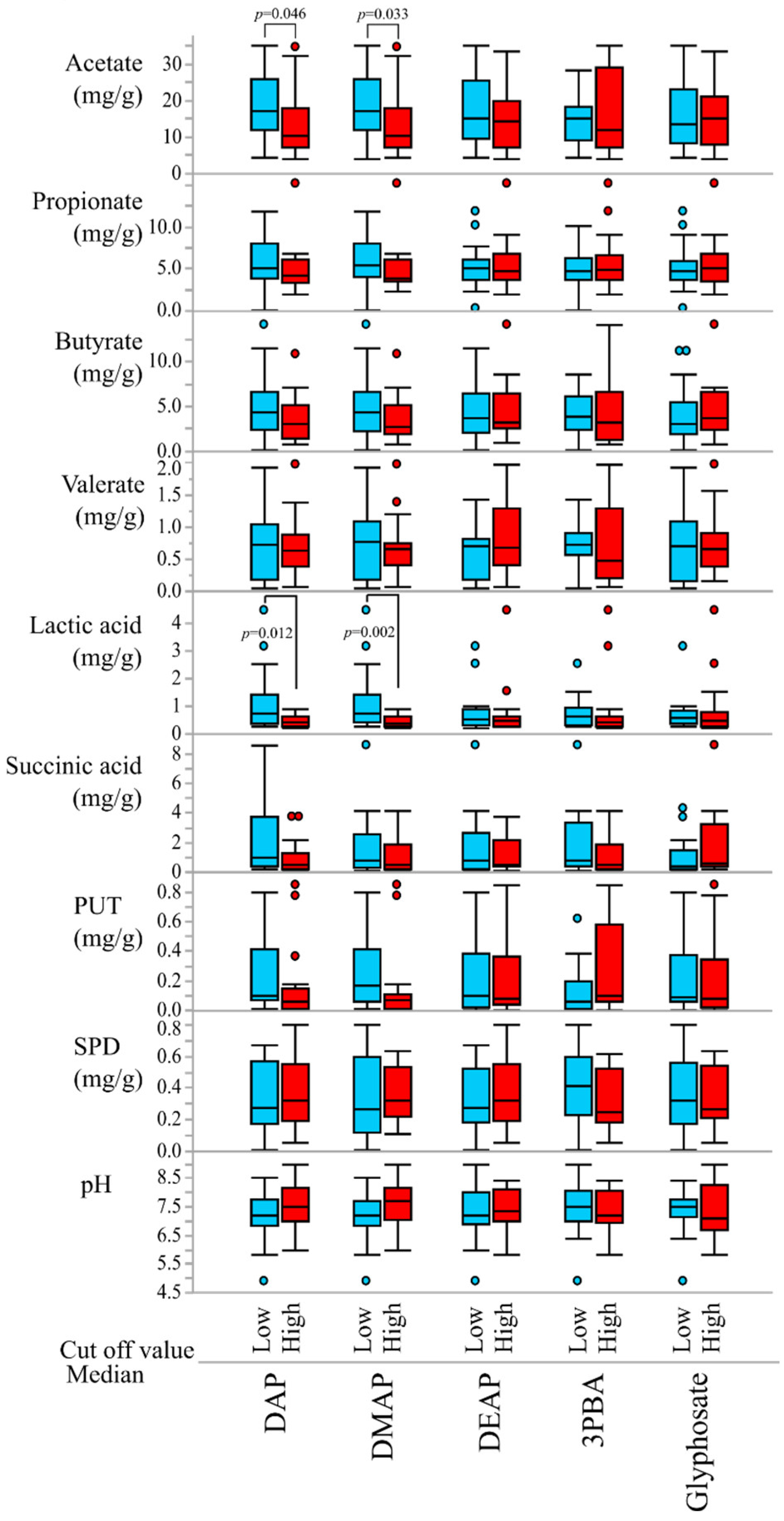
| Fecal Metabolite | Urinary Pesticide Markers | Correlation Coefficient | p-Value |
|---|---|---|---|
| Lactate | DMAP | −0.571 | 0.005 |
| Lactate | 3PBA | −0.477 | 0.022 |
| Lactate | DAP | −0.391 | 0.024 |
| SPD | 3PBA | −0.367 | 0.036 |
| Acetate | DAP | −0.345 | 0.043 |
| Acetate | Lactate | |
|---|---|---|
| R-square | 0.099 | 0.236 |
| Predictors | β, SE, t, p | β, SE, t, p |
| Constant Age Sex BMI Urinary pesticide markers DAP DMAP DEAP 3PBA Glyphosate | 18.96, 2.11, 8.98, <0.001 −6.23, 3.27, −1.91, 0.066 | 0.77, 1.44, 0.53, 0.600 −0.025, 0.015, −1.65, 0.110 0.092, 0.050, 1.82, 0.080 −1.10, 0.66, −1.68, 0.103 |
| Acetate | Lactate | |
|---|---|---|
| Adjusted R-squared | 0.751 | 0.920 |
| Predictors | β, SE, t, p | β, SE, t, p |
| Constant Age (years) Sex (male/female) BMI (%) Concentration of urinary pesticide markers DAP DMAP DEAP 3PBA Glyphosate Food intake frequency (/week) Rice Bread Pasta Potato Fish Meat Milk Fermented milk Beans Fermented beans Root vegetable Vegetable (others) Konjac Mushroom Seaweed Coffee Tea Supplement (Y/N) Beer can (350 mL) (/day) Other drinking (times/week) Smoking (n/p/c) Natural birth (Y/N) Breast milk (Y/N) | 37.47, 5.82, 6.44, <0.001 −23.98, 4.86, −4.93, 0.001 8.89, 3.48, 2.55, 0.020 9.44, 3.08, 3.06, 0.007 3.02, 1.04, 2.91, 0.009 6.82, 1.41, 4.85, <0.001 −13.56, 2.29, −5.92, <0.001 | −0.010, 0.447, −0.02, 0.981 −2.616, 0.493, −5.30, <0.001 0.876, 0.102, 8.61, <0.001 1.397, 0.154, 9.10, <0.001 −0.192, 0.156, −1.23, 0.239 −1.645, 0.188, −8.74, <0.001 0.025, 0.108, 0.23, 0.821 −0.218, 0.108, −2.02, 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueyama, J.; Hayashi, M.; Hirayama, M.; Nishiwaki, H.; Ito, M.; Saito, I.; Tsuboi, Y.; Isobe, T.; Ohno, K. Effects of Pesticide Intake on Gut Microbiota and Metabolites in Healthy Adults. Int. J. Environ. Res. Public Health 2023, 20, 213. https://doi.org/10.3390/ijerph20010213
Ueyama J, Hayashi M, Hirayama M, Nishiwaki H, Ito M, Saito I, Tsuboi Y, Isobe T, Ohno K. Effects of Pesticide Intake on Gut Microbiota and Metabolites in Healthy Adults. International Journal of Environmental Research and Public Health. 2023; 20(1):213. https://doi.org/10.3390/ijerph20010213
Chicago/Turabian StyleUeyama, Jun, Mai Hayashi, Masaaki Hirayama, Hiroshi Nishiwaki, Mikako Ito, Isao Saito, Yoshio Tsuboi, Tomohiko Isobe, and Kinji Ohno. 2023. "Effects of Pesticide Intake on Gut Microbiota and Metabolites in Healthy Adults" International Journal of Environmental Research and Public Health 20, no. 1: 213. https://doi.org/10.3390/ijerph20010213
APA StyleUeyama, J., Hayashi, M., Hirayama, M., Nishiwaki, H., Ito, M., Saito, I., Tsuboi, Y., Isobe, T., & Ohno, K. (2023). Effects of Pesticide Intake on Gut Microbiota and Metabolites in Healthy Adults. International Journal of Environmental Research and Public Health, 20(1), 213. https://doi.org/10.3390/ijerph20010213







