Circadian Clock Desynchronization and Insulin Resistance
Abstract
1. Introduction
2. Impact of Central and Peripheral Clocks Misalignment
3. Impact of Circadian Secretory Profiles
4. Role of the Microbiome
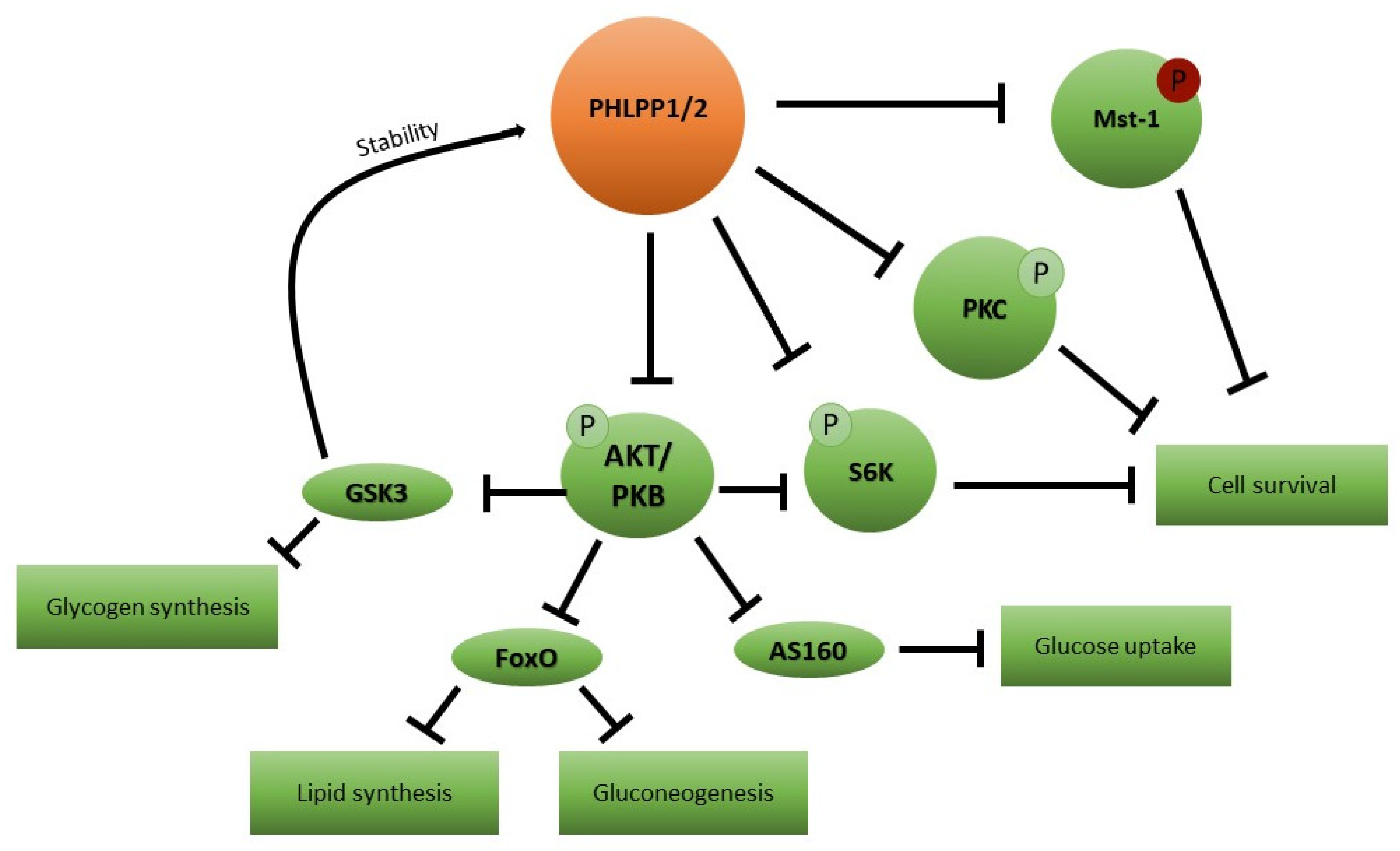
5. Candidate Mediator Molecules
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- White, M.F.; Kahn, C.R. Insulin action at a molecular level-100 years of progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.P. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes 1995, 44, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Hribal, M.L.; Mancuso, E.; Spiga, R.; Mannino, G.C.; Fiorentino, T.V.; Andreozzi, F.; Sesti, G. PHLPP phosphatases as a therapeutic target in insulin resistance related diseases. Expert Opin. Ther. Targets 2016, 20, 663–675. [Google Scholar] [CrossRef]
- Neves, A.R.; Albuquerque, T.; Quintela, T.; Costa, D. Circadian rhythm and disease: Relationship, new insights, and future perspectives. J. Cell. Physiol. 2022, 237, 3239–3256. [Google Scholar] [CrossRef]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lann, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Z. RBP4 functions as a hepatokine in the regulation of glucose metabolism by the circadian clock in mice. Diabetologia 2016, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, R.; Ohta, Y. Metabolic state switches between morning and evening in association with circadian clock in people without diabetes. J. Diabetes Investig. 2022, 13, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Skinner, N.J.; Rizwan, M.Z. Chronic Light Cycle Disruption Alters Central Insulin and Leptin Signaling as well as Metabolic Markers in Male Mice. Endocrinology 2019, 13, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.E.; Wopereis, S.; Kalsbeek, A. The Circadian Clock, Shift Work, and Tissue-Specific Insulin Resistance. Endocrinology 2020, 161, bqaa180. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, A.; Martchenko, S.E.; Biancolin, A.D.; Brubaker, P.L. Circadian Rhythms and the Gastrointestinal Tract: Relationship to Metabolism and Gut Hormones. Endocrinology 2020, 161, bqaa167. [Google Scholar] [CrossRef]
- Pan, X.; Terada, T.; Okuda, M.; Inui, K. The diurnal rhythm of the intestinal transporters SGLT1 and PEPT1 is regulated by the feeding conditions in rats. J. Nutr. 2004, 134, 2211–2215. [Google Scholar] [CrossRef]
- Pan, X.; Hussain, M.M. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J. Biol. Chem. 2007, 282, 24707–24717. [Google Scholar] [CrossRef]
- Rhoads, D.B.; Rosenbaum, D.H.; Unsal, H.; Isselbacher, K.J.; Levitsky, L.L. Circadian periodicity of intestinal Na+/glucose cotransporter 1 mRNA levels is transcriptionally regulated. J. Biol. Chem. 1998, 273, 9510–9516. [Google Scholar] [CrossRef]
- McCommis, K.S.; Butler, A.A. The Importance of Keeping Time in the Liver. Endocrinology 2021, 162, bqaa230. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.-F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced Oxidative Stress and its Role in Diabetes Mellitus Related Cardiovascular Diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterle, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, R.; Krook, A.; Schrauwen, P.; Hesselink, M.K.C. Obesity, Diurnal Regulation of Peripheral Glucose Metabolism: Potential Effects of Exercise Timing. Obesity 2020, 28, S38–S45. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Sinha, J.; Bahrami-Nejad, Z.; Teruel, M.N. The circadian clock mediates daily bursts of cell differentiation by periodically restricting cell-differentiation commitment. Proc. Natl. Acad. Sci. USA 2022, 119, e2204470119. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Lin, Y.; Lee, J.; Paul, A.; Yechoor, V.; Figueiro, M.; Ma, K. Chronic circadian shift leads to adipose tissue inflammation and fibrosis. Mol. Cell. Endocrinol. 2021, 521, 111110. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Navez, B.; Brichard, S.M. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat. Commun. 2021, 12, 2388. [Google Scholar] [CrossRef] [PubMed]
- Perelis, M.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian transcription from beta cell function to diabetes pathophysiology. J. Biol. Rhythms 2016, 31, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.H.; Hussein, B.; Botezzelli, J.B.; Wang, S.; Zhang, J.A.; Noursadeghi, N.; Jessen, N.; Rodrigues, B.; Timmons, J.A.; Johnson, J.D. Human and mouse muscle transcriptomic analyses identify insulin receptor mRNA downregulation in hyperinsulinemia-associated insulin resistance. FASEB J. 2022, 36, e22088. [Google Scholar] [CrossRef]
- Petrenko, V.; Dibner, C. Circadian orchestration of insulin and glucagon release. Cell Cycle 2017, 16, 1141–1142. [Google Scholar] [CrossRef]
- Lamia, K.A.; Papp, S.J.; Yu, R.T.; Barish, G.D.; Uhlenhaut, N.H.; Jonker, J.W.; Downes, M.; Evans, R.M. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 2011, 480, 552–556. [Google Scholar] [CrossRef]
- Noguchi, T.; Ikeda, M.; Ohmiya, Y.; Nakajima, Y. A dual-color luciferase assay system reveals circadian resetting of cultured fibroblasts by co-cultured adrenal glands. PLoS ONE 2012, 7, e37093. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.A.; Sherlock, M.; Ghatercole, L.L.; Lavery, G.G.; Lenaghan, C.; Bujalska, I.J.; Laber, D.; Yu, A.; Convey, G.; Mayers, R.; et al. 11beta-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid-induced insulin resistance in skeletal muscle. Diabetes 2009, 58, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Pivonello, C.; Simeoli, C.; Di Gennaro, G.; Venneri, M.A.; Sciarra, F.; Ferrigno, R.; de Angelis, C.; Sbardella, E.; De Martino, M.C.; et al. Cortisol circadian rhythm and insulin resistance in muscle: Effect of dosing and timing of hydrocortisone exposure on insulin sensitivity in synchronized muscle cells. Neuroendocrinology 2021, 111, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Laermans, J.; Vancleef, L.; Tack, J.; Depoortere, I. Role of the clock gene Bmal1 and the gastric ghrelin-secreting cell in the circadian regulation of the ghrelin-GOAT system. Sci. Rep. 2015, 5, 16748. [Google Scholar] [CrossRef]
- Tschop, M.; Weyer, C.; Tataranno, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef]
- Lindqvist, A.; Shcherbina, L.; Prasad, R.B.; Miskelly, M.G.; Abels, M.; Martinez-Lopez, J.A.; Fred, R.G.; Nergard, B.J.; Hedenbro, J.; Groop, L.; et al. Ghrelin suppresses insulin secretion in human islets and type 2 diabetes patients have diminished islet ghrelin cell number and lower plasma ghrelin levels. Mol. Cell. Endocrinol. 2020, 511, 1110835. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, Z.; He, L.; Yang, M.; Liu, D.; Gu, H.F.; Liu, H.; Zu, Z.; Zheng, H.; Li, L.; et al. Gut ghrelin regulates hepatic glucose production and insulin signaling via a gut-brain-liver pathway. Cell Commun. Signal. 2019, 17, 8. [Google Scholar] [CrossRef]
- Tripathy, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef]
- Heyde, I.; Begemann, K.; Oster, H. Contribution of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology 2021, 162, bqab009. [Google Scholar] [CrossRef]
- Heo, Y.J.; Choi, S.-E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Visfatin induces inflammation and insulin resistance via the NF-kB and STAT3 signaling pathways in hepatocytes. J. Diabetes Res. 2019, 2019, 4021623. [Google Scholar] [CrossRef]
- Lin, Z.; Tian, H.; Lam, K.S.L.; Lin, S.; Hoo, R.C.L.; Konishi, M.; Itoh, N.; Wang, Y.; Bornstein, S.R.; Xu, A.; et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013, 17, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Abellan, P.; Gomez-Santos, C.; Madrid, J.A.; Milagro, F.I.; Campion, J.; Martinez, J.A.; Ordovas, J.M.; Garaulet, M. Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology 2010, 151, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Graham, T.E. Serum retinol-binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Pan, S.; Xu, P.; Xue, T.; Wang, J.; Guo, Y.; Jia, L.; Qiao, X.; Li, L.; Zhai, Y. Melatonin orchestrates lipid homesostasis through the hepatointestinal circadian clock and microbiota during constant light exposure. Cells 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Owino, S.; Sánchez-Bretaño, A. Nocturnal activation of melatonin receptor type 1 signaling modulates diurnal insulin sensitivity via regulation of PI3K activity. J. Pineal Res. 2018, 64, e12462. [Google Scholar] [CrossRef]
- Alvarez, Y.; Glotfelty, L.G.; Blank, N.; Dohnalova, N.; Thaiss, C.A. The Microbiome as a Circadian Coordinator of Metabolism. Endocrinology 2020, 161, bqaa059. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Rácz, B.; Dušková, M. Links between the circadian rhythm, obesity and the microbiome. Physiol. Res. 2018, 67, S409–S420. [Google Scholar] [CrossRef] [PubMed]
- Vidović, T.; Ewald, C.Y. Longevity-Promoting Pathways and Transcription Factors Respond to and Control Extracellular Matrix Dynamics During Aging and Disease. Front. Aging 2022, 3, 935220. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kang, X.; Liu, J.; Li, H.; Ma, Z.; Jin, X.; Qian, Z.; Xie, T.; Qin, N.; Feng, D.; et al. Clock Gene Bmal1 Modulates Human Cartilage Gene Expression by Crosstalk with Sirt1. Endocrinology 2016, 157, 3096–3107. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Yan, M.; Huang, R.; Wang, Y.; He, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zhang, F.; et al. CLOCK and BMAL1 Regulate Muscle Insulin Sensitivity via SIRT1 in Male Mice. Endocrinology 2016, 157, 2259–2269. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Y.; Zhang, F.; Xia, Y.; Liu, J.; Huang, R.; Wang, Y.; Hu, Y.; Wu, J.; Dai, C.; et al. CLOCK/BMAL1 Regulates Circadian Change of Mouse Hepatic Insulin Sensitivity by SIRT1. Hepatology 2014, 59, 2196–2206. [Google Scholar] [CrossRef]
- Raciti, G.A.; Iadicicco, C.; Ulianich, L.; Vind, B.F.; Gaster, M.; Andreozzi, F.; Longo, M.; Teperino, R.; Ungaro, P.; Di Jeso, B.; et al. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia 2010, 53, 955–965. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Procopio, T.; Mancuso, E.; Arcidiacono, G.P.; Andreozzi, F.; Arturi, F.; Sciacqua, A.; Perticone, F.; Hribal, M.L.; Sesti, G. SRT1720 counteracts glucosamine-induced endoplasmic reticulum stress and endothelial dysfunction. Cardiovasc. Res. 2015, 107, 295–306. [Google Scholar] [CrossRef]
- Yang, Z.; Kim, H.; Ali, A.; Zheng, Z.; Zhang, K. Interaction between stress responses and circadian metabolism in metabolic disease. Liver Res. 2017, 1, 156–162. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Giles, A.; Nakamura, K.; Lee, J.W.; Hou, X.; Donmez, G.; Li, J.; Luo, Z.; Walsh, K.; et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011, 25, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Furnari, F.; Newton, A.C. PHLPP: A phosphatase that directly dephosphorylates Akt promotes apoptosis and suppresses tumor growth. Cell 2005, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Okada, M.; Takano, A.; Nagai, K. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999, 458, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, F.; Procopio, C.; Greco, A.; Mannino, G.C.; Miele, C.; Raciti, G.A.; Iadicicco, C.; Beguinot, F.; Pontiroli, A.E.; Hribal, M.L.; et al. Increased levels of the Akt-specific phosphatase PH domain leucine-rich repeat protein phosphatase (PHLPP)-1 in obese participants are associated with insulin resistance. Diabetologia 2011, 54, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Hribal, M.L.; Mancuso, E.; Arcidiacono, G.P.; Greco, A.; Musca, D.; Procopio, T.; Ruffo, M.; Sesti, G. The Phosphatase PHLPP2 Plays a Key Role in the Regulation of Pancreatic Beta-Cell Survival. Int. J. Endocrinol. 2020, 2020, 1027386. [Google Scholar] [CrossRef]
- Cozzone, D.; Frojdo, S.; Disse, E.; Debard, M.; Laville, M.; Pirola, L.; Vidal, H. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia 2008, 51, 512–521. [Google Scholar] [CrossRef]
- Brognard, J.; Sierecki, E.; Gao, T.; Newton, A.C. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol. Cell 2007, 25, 917–931. [Google Scholar] [CrossRef]
- Masubuchi, S.; Gao, T.; O’Neill, A.; Eckel-Mahan, K.; Newton, A.C.; Sassone-Corsi, P. Protein phosphatase PHLPP1 controls the light-induced resetting of the circadian clock. Proc. Natl. Acad. Sci. USA 2010, 107, 1642–1647. [Google Scholar] [CrossRef]
- Kriebs, A. Insulin receptor sets liver clock. Nat. Rev. Endocrinol. 2022, 18, 393. [Google Scholar] [CrossRef]
| Organ/System | CR Regulated Function | Direct Effect When CR Is Altered | Output |
|---|---|---|---|
| GI tract | Motility | Altered transit time | Impaired insulin signaling due to gluco/lipo toxicity |
| Nutrient digestion and absorption | Increased/decreased nutrient flux | Impaired insulin signaling due to gluco/lipo toxicity | |
| Incretin release | Altered insulin secretion | Hypo/Hyperinsulinemia, InsR downregulation | |
| Liver | Nutrient utilization | Altered FA and glucose flux | Impaired insulin signaling due to gluco/lipo toxicity |
| Skeletal muscle | Response to physical activity | Impaired energy consumption | Reduced insulin sensitizing potential of physical activity |
| Adipose tissue | Differentiation | Altered fat depots | Impaired insulin signaling due to inflammation |
| Secretion of inflammatory factors | Inflammation | Impaired insulin signaling due to inflammation | |
| Resistin release | Altered food intake and energy expenditure | Impaired insulin signaling due to gluco/lipo toxicity | |
| Visfatin release | Increased inflammation | Impaired insulin signaling due to inflammation | |
| Adiponectin release | Altered energy metabolism | Impaired insulin signaling due to gluco/lipo toxicity | |
| Resistin release | Increased proinflammatory cytokines secretion | Impaired insulin signaling due to inflammation | |
| Liver/adipose tissue | RBP4 release | Time-dependent activation of JAK/STAT pathway | Impaired insulin signaling due to JAK/STAT induced genes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, F.; De Vito, F.; Cassano, V.; Fiorentino, T.V.; Sciacqua, A.; Hribal, M.L. Circadian Clock Desynchronization and Insulin Resistance. Int. J. Environ. Res. Public Health 2023, 20, 29. https://doi.org/10.3390/ijerph20010029
Catalano F, De Vito F, Cassano V, Fiorentino TV, Sciacqua A, Hribal ML. Circadian Clock Desynchronization and Insulin Resistance. International Journal of Environmental Research and Public Health. 2023; 20(1):29. https://doi.org/10.3390/ijerph20010029
Chicago/Turabian StyleCatalano, Federica, Francesca De Vito, Velia Cassano, Teresa Vanessa Fiorentino, Angela Sciacqua, and Marta Letizia Hribal. 2023. "Circadian Clock Desynchronization and Insulin Resistance" International Journal of Environmental Research and Public Health 20, no. 1: 29. https://doi.org/10.3390/ijerph20010029
APA StyleCatalano, F., De Vito, F., Cassano, V., Fiorentino, T. V., Sciacqua, A., & Hribal, M. L. (2023). Circadian Clock Desynchronization and Insulin Resistance. International Journal of Environmental Research and Public Health, 20(1), 29. https://doi.org/10.3390/ijerph20010029







