Preoperative Risk Factors for Conversion from Laparoscopic to Open Cholecystectomy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias and Quality Assessment
2.5. Statistical Analysis
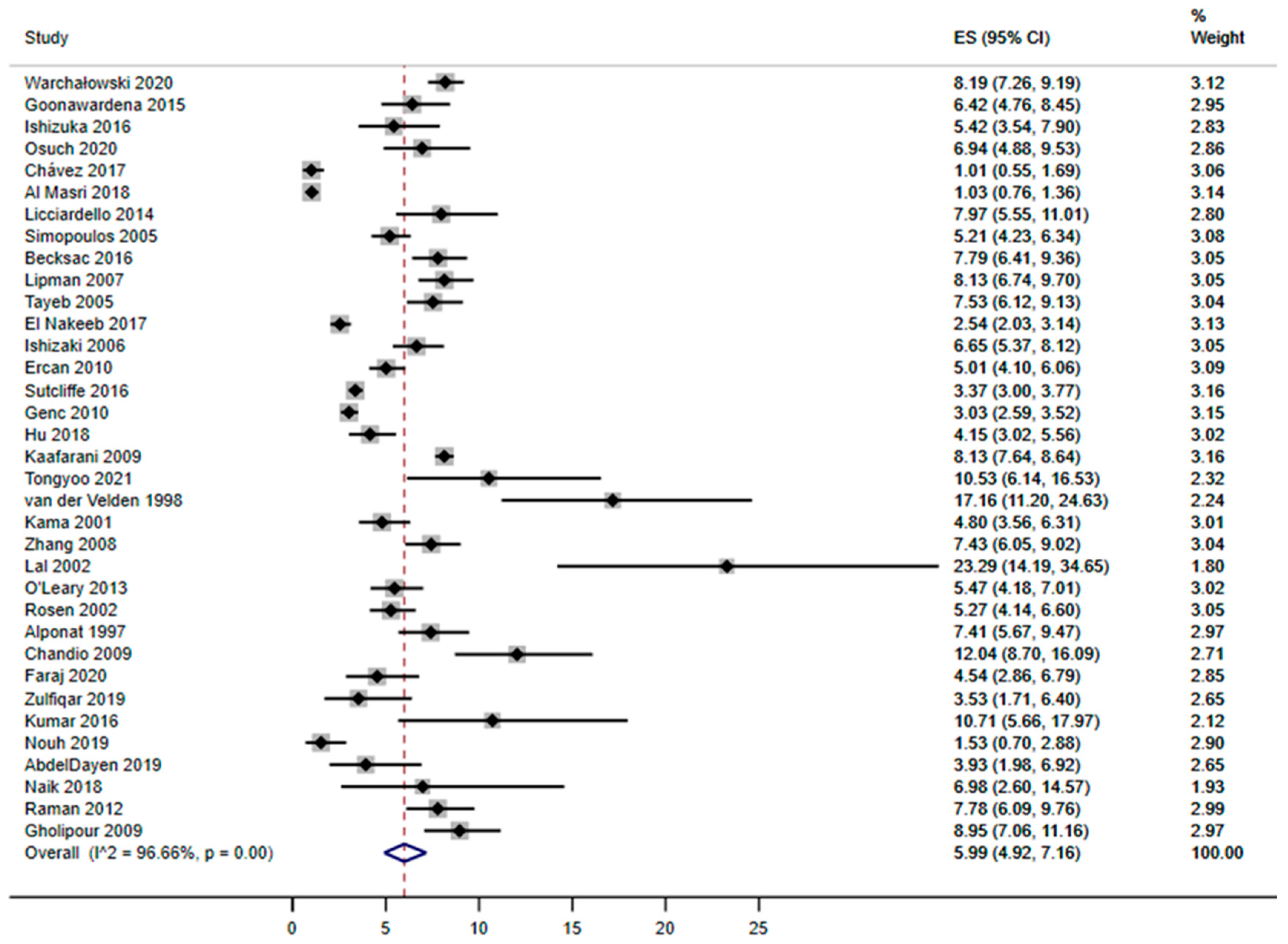
3. Results
3.1. Study Characteristics
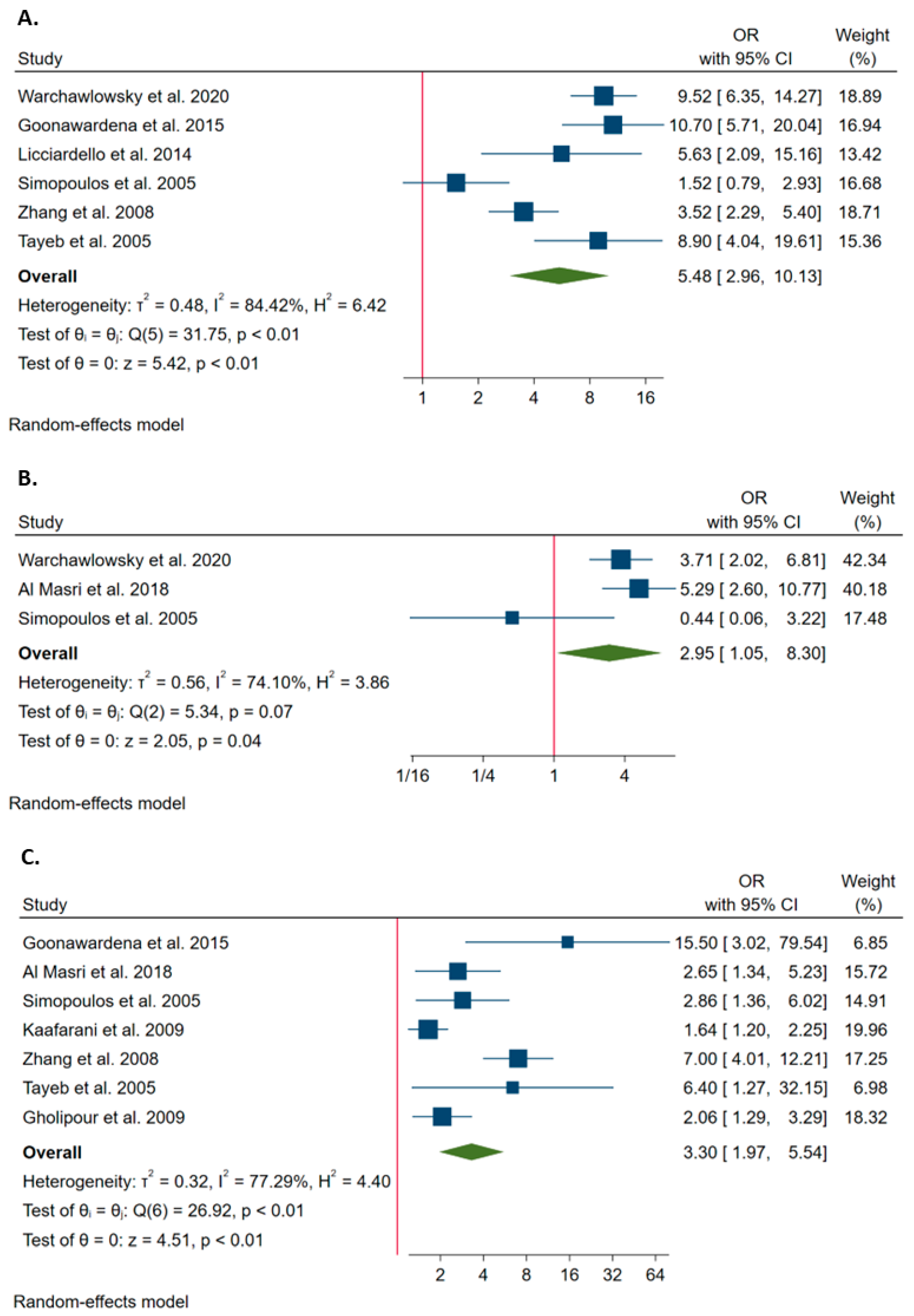
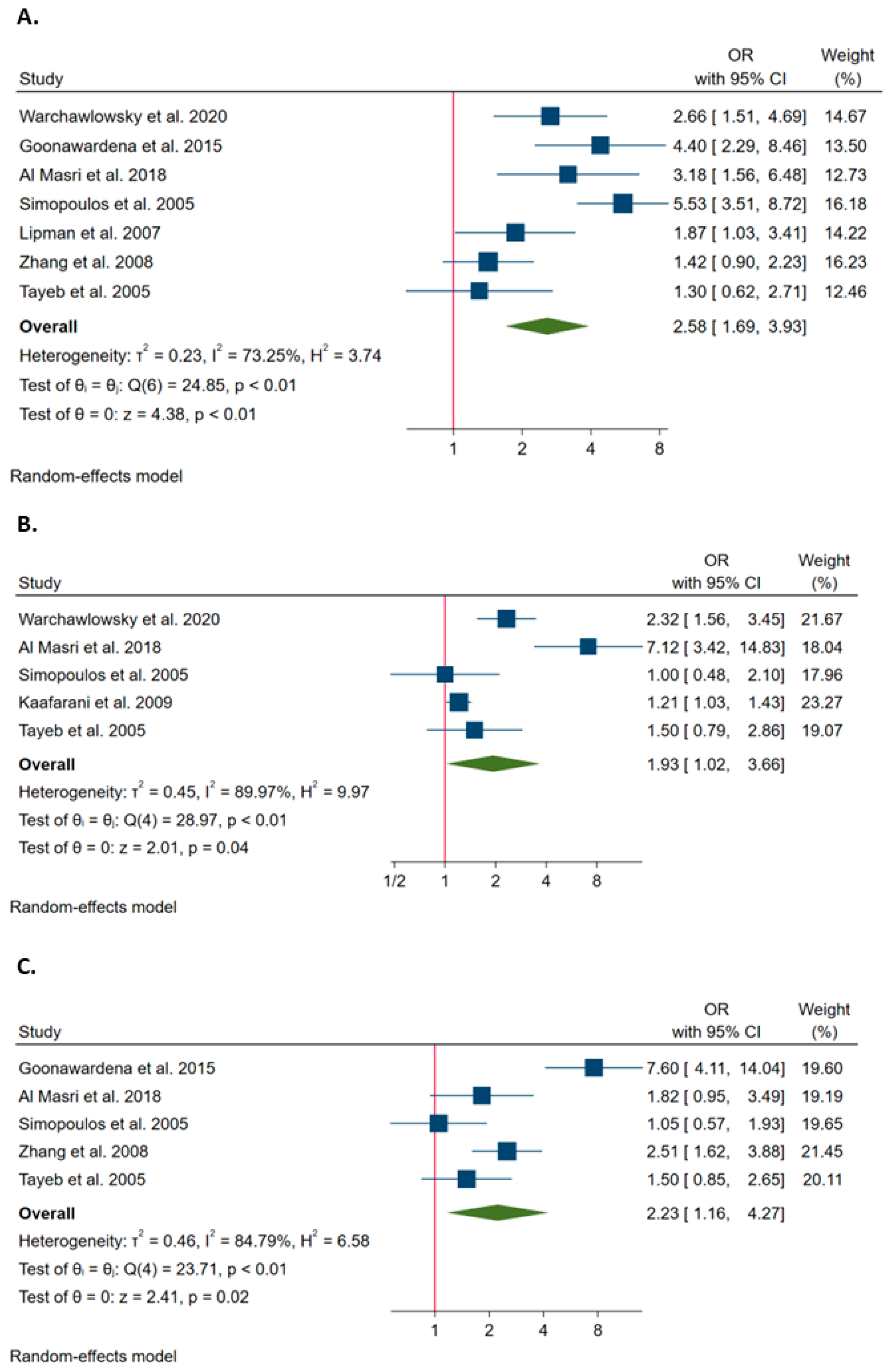
3.2. Risk Factors for Conversion from Laparoscopy to Open Surgery during Cholecystectomy
3.3. The Association of Clinical Factors with the Probability of Conversion
3.4. Quality of Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tongyoo, A.; Chotiyasilp, P.; Sriussadaporn, E.; Limpavitayaporn, P.; Mingmalairak, C. The pre-operative predictive model for difficult elective laparoscopic cholecystectomy: A modification. Asian J. Surg. 2021, 44, 656–661. [Google Scholar] [CrossRef] [PubMed]
- El Nakeeb, A.; Mahdy, Y.; Salem, A.; El Sorogy, M.; El Rafea, A.A.; El Dosoky, M.; Said, R.; Ellatif, M.A.; Alsayed, M.M.A. Open Cholecystectomy Has a Place in the Laparoscopic Era: A Retrospective Cohort Study. Indian J. Surg. 2017, 79, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Warchałowski, Ł.; Łuszczki, E.; Bartosiewicz, A.; Dereń, K.; Warchałowska, M.; Oleksy, Ł.; Stolarczyk, A.; Podlasek, R. The Analysis of Risk Factors in the Conversion from Laparoscopic to Open Cholecystectomy. Int. J. Environ. Res. Public Health 2020, 17, 7571. [Google Scholar] [CrossRef]
- Al Masri, S.; Shaib, Y.; Edelbi, M.; Tamim, H.; Jamali, F.; Batley, N.; Faraj, W.; Hallal, A. Predicting Conversion from Laparoscopic to Open Cholecystectomy: A Single Institution Retrospective Study. World J. Surg. 2018, 42, 2373–2382. [Google Scholar] [CrossRef]
- Chávez, K.V.; Márquez-González, H.; Aguirre, I.; Orellana, J.C. Prognostic risk factors for conversion in laparoscopic cholecystectomy. Updates Surg. 2018, 70, 67–72. [Google Scholar] [CrossRef]
- Hu, A.S.Y.; Menon, R.; Gunnarsson, R.; de Costa, A. Risk factors for conversion of laparoscopic cholecystectomy to open surgery–A systematic literature review of 30 studies. Am. J. Surg. 2017, 214, 920–930. [Google Scholar] [CrossRef]
- Kama, N.A.; Kologlu, M.; Doganay, M.; Reis, E.; Atli, M.; Dolapci, M. A risk score for conversion from laparoscopic to open cholecystectomy. Am. J. Surg. 2001, 181, 520–525. [Google Scholar] [CrossRef]
- Thesbjerg, S.E.; Harboe, K.M.; Bardram, L.; Rosenberg, J. Sex differences in laparoscopic cholecystectomy. Surg. Endosc. 2010, 24, 3068–3072. [Google Scholar] [CrossRef]
- van der Steeg, H.J.; Alexander, S.; Houterman, S.; Slooter, G.D.; Roumen, R.M. Risk factors for conversion during laparoscopic cholecystectomy–experiences from a general teaching hospital. Scand. J. Surg. 2011, 100, 169–173. [Google Scholar] [CrossRef]
- Angrisani, L.; Lorenzo, M.; De Palma, G.; Sivero, L.; Catanzano, C.; Tesauro, B.; Persico, G. Laparoscopic cholecystectomy in obese patients compared with nonobese patients. Surg. Laparosc. Endosc. 1995, 5, 197–201. [Google Scholar]
- Ammori, B.J.; Vezakis, A.; Davides, D.; Martin, I.G.; Larvin, M.; McMahon, M.J. Laparoscopic cholecystectomy in morbidly obese patients. Surg. Endosc. 2001, 15, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.; Delbene, R.; Ferreres, A. The feasibility of laparoscopic cholecystectomy in patients with previous abdominal surgery. HPB Surg. 1998, 10, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Karayiannakis, A.J.; Polychronidis, A.; Perente, S.; Botaitis, S.; Simopoulos, C. Laparoscopic cholecystectomy in patients with previous upper or lower abdominal surgery. Surg. Endosc. 2004, 18, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, M.; Aoki, H.; Kunitomo, T.; Mushiake, Y.; Yasuhara, I.; Taniguchi, F.; Arata, T.; Katsuda, K.; Tanakaya, K.; Takeuchi, H. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Cholecystectomy and the Usefulness of the 2013 Tokyo Guidelines. Acta Med. Okayama 2017, 71, 419–425. [Google Scholar] [CrossRef]
- Tang, B.; Cuschieri, A. Conversions during laparoscopic cholecystectomy: Risk factors and effects on patient outcome. J. Gastrointest. Surg. 2006, 10, 1081–1091. [Google Scholar] [CrossRef]
- Philip Rothman, J.; Burcharth, J.; Pommergaard, H.C.; Viereck, S.; Rosenberg, J. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Surgery–A Systematic Review and Meta-Analysis of Observational Studies. Dig. Surg. 2016, 33, 414–423. [Google Scholar] [CrossRef]
- Lipman, J.M.; Claridge, J.A.; Haridas, M.; Martin, M.D.; Yao, D.C.; Grimes, K.L.; Malangoni, M.A. Preoperative findings predict conversion from laparoscopic to open cholecystectomy. Surgery 2007, 142, 556–563. [Google Scholar] [CrossRef]
- Wells, G.A.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality If Nonrandomized Studies in Meta-Analyses. 2009. Available online: https://www.scienceopen.com/document?vid=54b48470-4655-4081-b5d4-e8ebe8d1792e (accessed on 1 November 2022).
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ishizuka, M.; Shibuya, N.; Shimoda, M.; Kato, M.; Aoki, T.; Kubota, K. Preoperative hypoalbuminemia is an independent risk factor for conversion from laparoscopic to open cholecystectomy in patients with cholecystolithiasis. Asian J. Endosc. Surg. 2016, 9, 275–280. [Google Scholar] [CrossRef]
- Licciardello, A.; Arena, M.; Nicosia, A.; Di Stefano, B.; Calì, G.; Arena, G.; Minutolo, V. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 60–68. [Google Scholar] [PubMed]
- Simopoulos, C.; Botaitis, S.; Polychronidis, A.; Tripsianis, G.; Karayiannakis, A.J. Risk factors for conversion of laparoscopic cholecystectomy to open cholecystectomy. Surg. Endosc. 2005, 19, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, C.; Fakhree, M.B.; Shalchi, R.A.; Abbasi, M. Prediction of conversion of laparoscopic cholecystectomy to open surgery with artificial neural networks. BMC Surg. 2009, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, M.; Raza, S.A.; Khan, M.R.; Azami, R. Conversion from laparoscopic to open cholecystectomy: Multivariate analysis of preoperative risk factors. J. Postgrad. Med. 2005, 51, 17–20. [Google Scholar] [PubMed]
- Hu, A.S.Y.; Donohue, P.O.; Gunnarsson, R.K.; de Costa, A. External validation of the Cairns Prediction Model (CPM) to predict conversion from laparoscopic to open cholecystectomy. Am. J. Surg. 2018, 216, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, J.J.; Berger, M.Y.; Bonjer, H.J.; Brakel, K.; Laméris, J.S. Can sonographic signs predict conversion of laparoscopic to open cholecystectomy? Surg. Endosc. 1998, 12, 1232–1235. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, J.M.; Wu, G.Z.; Luo, K.L.; Dong, Z.T. Risk factors affecting conversion in patients undergoing laparoscopic cholecystectomy. ANZ J. Surg. 2008, 78, 973–976. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Miwa, K.; Yoshimoto, J.; Sugo, H.; Kawasaki, S. Conversion of elective laparoscopic to open cholecystectomy between 1993 and 2004. Br. J. Surg. 2006, 93, 987–991. [Google Scholar] [CrossRef]
- Ercan, M.; Bostanci, E.B.; Teke, Z.; Karaman, K.; Dalgic, T.; Ulas, M.; Ozer, I.; Ozogul, Y.B.; Atalay, F.; Akoglu, M. Predictive factors for conversion to open surgery in patients undergoing elective laparoscopic cholecystectomy. J. Laparoendosc. Adv. Surg. Tech. A 2010, 20, 427–434. [Google Scholar] [CrossRef]
- Genc, V.; Sulaimanov, M.; Cipe, G.; Basceken, S.I.; Erverdi, N.; Gurel, M.; Aras, N.; Hazinedaroglu, S.M. What necessitates the conversion to open cholecystectomy? A retrospective analysis of 5164 consecutive laparoscopic operations. Clinics 2011, 66, 417–420. [Google Scholar] [CrossRef]
- O’Leary, D.P.; Myers, E.; Waldron, D.; Coffey, J.C. Beware the contracted gallbladder–Ultrasonic predictors of conversion. Surgeon 2013, 11, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.; Brody, F.; Ponsky, J. Predictive factors for conversion of laparoscopic cholecystectomy. Am. J. Surg. 2002, 184, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Alponat, A.; Kum, C.K.; Koh, B.C.; Rajnakova, A.; Goh, P.M. Predictive factors for conversion of laparoscopic cholecystectomy. World J. Surg. 1997, 21, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Chandio, A.; Timmons, S.; Majeed, A.; Twomey, A.; Aftab, F. Factors influencing the successful completion of laparoscopic cholecystectomy. JSLS 2009, 13, 581–586. [Google Scholar] [CrossRef]
- Raman, S.R.; Moradi, D.; Samaan, B.M.; Chaudhry, U.S.; Nagpal, K.; Cosgrove, J.M.; Farkas, D.T. The degree of gallbladder wall thickness and its impact on outcomes after laparoscopic cholecystectomy. Surg. Endosc. 2012, 26, 3174–3179. [Google Scholar] [CrossRef]
- Beksac, K.; Turhan, N.; Karaagaoglu, E.; Abbasoglu, O. Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Surgery: A New Predictive Statistical Model. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 693–696. [Google Scholar] [CrossRef]
- Zulfiqar, M.R.; Wasim, E. A retrospective analysis of incidence of transformation of laproscopic cholecystectomy into open cholecystectomy. Indo Am. J. Pharm. Sci. 2019, 6, 5231–5234. [Google Scholar]
- Nouh, T.A.; Abunayan, F.; Alsaadoun, A.S.; Alsehly, A.F.; Almanie, M.A.; Alhawassi, S.A. Factors Affecting Conversion from Laparoscopic Cholecystectomy to Open Cholecystectomy at a Tertiary Care Facility in Saudi Arabia: A Cross-Sectional Study. Int. Surg. 2019, 131, 98–101. [Google Scholar] [CrossRef]
- AbdelDayem, M.; Osgood, L.; Escofet, X.; Farag, M. A New Preoperative Scoring System to Predict Difficulty of Laparoscopic Cholecystectomy and Risk of Conversion to Open Surgery. Indian J. Surg. 2019, 82, 501–506. [Google Scholar] [CrossRef]
- Kama, N.A.; Doganay, M.; Dolapci, M.; Reis, E.; Atli, M.; Kologlu, M. Risk factors resulting in conversion of laparoscopic cholecystectomy to open surgery. Surg. Endosc. 2001, 15, 965–968. [Google Scholar] [CrossRef]
- Osuch, C.; Dolecki, M.; Rogula, W.P.; Łapiak, A.; Matyja, M.; Czerwińska, A.; Rubinkiewicz, M.; Matyja, A. Gender as a predictive factor in cholecystectomy–is it true or false? Folia Med. Cracov. 2020, 60, 97–107. [Google Scholar] [PubMed]
- Kaafarani, H.M.; Smith, T.S.; Neumayer, L.; Berger, D.H.; Depalma, R.G.; Itani, K.M. Trends, outcomes, and predictors of open and conversion to open cholecystectomy in Veterans Health Administration hospitals. Am. J. Surg. 2010, 200, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, R.P.; Hollyman, M.; Hodson, J.; Bonney, G.; Vohra, R.S.; Griffiths, E.A.; the CholeS study group, West Midlands Research Collaborative. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy: A validated risk score derived from a prospective U.K. database of 8820 patients. HPB 2016, 18, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Lal, P.; Agarwal, P.N.; Malik, V.K.; Chakravarti, A.L. A difficult laparoscopic cholecystectomy that requires conversion to open procedure can be predicted by preoperative ultrasonography. J. Soc. Laparoendosc. Surg. 2002, 6, 59–63. [Google Scholar]
- Faraj, F.I.; Da, A.; Hoaf, F.; Faruk, H.I.; Deari, A.; Halkawt, O. Laparoscopic Cholecystectomy to Open Cholecystectomy in Sulaymaniyah Teaching Hospital, Incidence and Risk Factors Assessment. Pak. J. Med. Health Sci. 2020, 14, 1244–1248. [Google Scholar]
- Kumar, N.S.B.; Zakkaria, M. Factors affecting conversion of laparoscopic cholecystectomy to open surgery in a tertiary hospital in south india. J. Evol. Med. Dent. Sci. 2016, 5, 256–261. [Google Scholar] [CrossRef]
- Naik, M.B.; Naik, M.G.; Sadhanala, N. Preoperative clinical and radiological assessment in predicting difficult laparoscopic cholecystectomy- a study at government general hospital, guntur. J. Evol. Med. Dent. Sci. 2018, 7, 2694–2698. [Google Scholar] [CrossRef]
- Goonawardena, J.; Gunnarsson, R.; de Costa, A. Predicting conversion from laparoscopic to open cholecystectomy presented as a probability nomogram based on preoperative patient risk factors. Am. J. Surg. 2015, 210, 492–500. [Google Scholar] [CrossRef]
- Bickel, A.; Rappaport, A.; Kanievski, V.; Vaksman, I.; Haj, M.; Geron, N.; Eitan, A. Laparoscopic management of acute cholecystitis. Prognostic factors for success. Surg. Endosc. 1996, 10, 1045–1049. [Google Scholar] [CrossRef]
- Zisman, A.; Gold-Deutch, R.; Zisman, E.; Negri, M.; Halpern, Z.; Lin, G.; Halevy, A. Is male gender a risk factor for conversion of laparoscopic into open cholecystectomy? Surg. Endosc. 1996, 10, 892–894. [Google Scholar] [CrossRef]
- Fried, G.M.; Barkun, J.S.; Sigman, H.H.; Joseph, L.; Clas, D.; Garzon, J.; Hinchey, E.J.; Meakins, J.L. Factors determining conversion to laparotomy in patients undergoing laparoscopic cholecystectomy. Am. J. Surg. 1994, 167, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.H.; Krailadsiri, W.; Incarbone, R.; Bremner, C.G.; Froes, E.; Ireland, A.P.; Crookes, P.; Ortega, A.E.; Anthone, G.A.; Stain, S.A. Reasons for conversion from laparoscopic to open cholecystectomy in an urban teaching hospital. Am. J. Surg. 1994, 168, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, J.R.; Gallinger, S.; Croxford, R.; Strasberg, S.M. Risk factors in elective laparoscopic cholecystectomy for conversion to open cholecystectomy. J. Am. Coll. Surg. 1994, 179, 696–704. [Google Scholar] [PubMed]
- Yang, T.F.; Guo, L.; Wang, Q. Evaluation of Preoperative Risk Factor for Converting Laparoscopic to Open Cholecystectomy: A Meta-Analysis. Hepatogastroenterology 2014, 61, 958–965. [Google Scholar] [PubMed]


| First Author and Publication Year | Country | Study Period | Study Design | Number of Patients | Age (Mean and SD) | Male (%) | Conversion Rate (%) |
|---|---|---|---|---|---|---|---|
| Warchałowski et al., 2020 [3] | Poland | 2008–2018 | Retrospective | 3213 | 65.7 ± 1.4 | 120 (45.6) | 263 (8.2) |
| Goonawardena et al., 2015 [49] | Australia | 2010–2012 | Prospective and retrospective | 732 | 56 ± 18 | 20(43) | 47 (6.4) |
| Ishizuka et al., 2016 [21] | Japan | 2000–2010 | Retrospective | 461 | NA | 231 (50.1) | 25 (5.4) |
| Osuch et al., 2020 [42] | Poland | 2013–2018 | Prospective | 504 | 54.8 ± 16.9 | 22 (62.9) | 35 (6.9) |
| Chávez et al., 2017 [5] | Mexico | 2009–2013 | Retrospective | 1386 | 42 ± 15 | 5 (35.7) | 14 (1) |
| Al Masri et al., 2018 [4] | Lebanon | 2000–2015 | Retrospective | 4668 | 68.35 ± 13.7 | 37 (80.4) | 48 (1.03) |
| Licciardello et al., 2014 [22] | Italy | 2011–2013 | Retrospective | 414 | 65.9 ± 13.9 | 14 (42.5) | 33 (7.9) |
| Simopoulos et al., 2005 [23] | Greece | 1992–2004 | Retrospective | 1804 | 52.66 ± 14.7 | 31 (33.0) | 94 (5.2) |
| Beksac et al., 2016 [37] | Turkey | 2006–2011 | Retrospective | 1335 | 61.9 ± 12.5 | 57 (54.8) | 104 (7.8) |
| Lipman et al., 2007 [17] | Ohio (USA) | 2000–2005 | Retrospective | 1377 | NA | 57 (50.9) | 112 (8.1) |
| Tayeb et al., 2005 [25] | Pakistan | 1997–2001 | Retrospective | 1249 | 44.2 ± 12.4 | 17 (23.3) | 94 (23.3) |
| El Nakeeb et al., 2017 [2] | Egypt | 2011–2016 | Retrospective | 3269 | 54 | 47 (56.6) | 83 (2.5) |
| Ishizaki et al., 2006 [29] | Japan | 1993–2004 | Retrospective | 1339 | 58 ± 13 | 57 (64) | 89 (7.5) |
| Ercan et al., 2010 [30] | Turkey | 2002–2007 | Retrospective | 2015 | 57.8 ± 13.0 | 60 (59.4) | 101 (5.0) |
| Sutcliffe et al., 2016 [44] | UK | 2014 | Prospective | 8820 | 51 ± 17 | 110 (49.8) | 297 (3.4) |
| Genc et al., 2010 [31] | Turkey | 1999–2010 | Retrospective | 5382 | 49.34 ± 9.9 | 84 (51.5) | 163 (3.2) |
| Hu et al., 2018 [26] | Australia | 2013–2016 | Retrospective | 1035 | 61 ± 15 | 24 (55.8) | 43 (4.2) |
| Kaafarani et al., 2009 [43] | USA | 2005–2008 | Prospective | 11,669 | 63.9 ± 12.4 | 903 (95.1) | 949 (8.1) |
| Tongyoo et al., 2021 [1] | Thailand | 2018–2019 | Prospective | 152 | 57.61 ± 13.8 | 9 (56.2) | 16 (10.5) |
| Van der Velden et al., 1998 [27] | Netherlands | 1993–1996 | Retrospective | 134 | 50.7 | NA | 23 (17.2) |
| Kama et al., 2001 [41] | Turkey | 1992–1999 | Prospective | 1000 | 43.8 | 22 (45.8) | 48 (4.8) |
| Zhang et al., 2008 [28] | China | 2005–2006 | Retrospective | 1265 | 60.0 ± 11.9 | 50 (53.2) | 94 (7.4) |
| Lal et al., 2002 [45] | India | 1999–2000 | Prospective | 73 | 35 | NA | 17 (23.3) |
| O’Leary et al., 2013 [32] | Ireland | 2000–2006 | Retrospective | 1061 | 43.3 | 31 (53.4) | 58 (5.5) |
| Rosen et al., 2002 [33] | USA | 1996–2000 | Retrospective | 1347 | 59.9 | 29 (41) | 71 (5.3) |
| Alponat et al., 1997 [34] | Singapore | 1990–1995 | Retrospective | 783 | NA | 22 (38) | 58 (7.4) |
| Chandio et al., 2009 [35] | Ireland | 2004–2006 | Retrospective | 324 | 61 | NA | 39 (12) |
| Faraj et al., 2020 [46] | Iraq | 2019 | Prospective | 485 | 52 ± 17.3 | 11 (50) | 22 (4.5) |
| Zulfiqar et al., 2019 [38] | Pakistan | 2017–2018 | Retrospective | 283 | NA | NA | 10 (3.5) |
| Kumar et al., 2016 [47] | India | 2013–2015 | Prospective | 112 | 43.4 | 5 (41.7) | 12 (10.7) |
| Nouh et al., 2019 [39] | Saudi Arabia | 2014–2015 | Retrospective | 589 | NA | 2 (32.2) | 9 (1.5) |
| AbdelDayen et al., 2019 [40] | UK | 2013–2014 | Retrospective | 280 | 49.9 | NA | 11 (3.9) |
| Naik et al., 2018 [48] | India | 2016–2017 | Prospective | 86 | 48.92 ± 11.9 | 2 (33.3) | 6 (6.9) |
| Raman et al., 2012 [36] | USA | 2006–2009 | Retrospective | 874 | NA | NA | 68 (7.8) |
| Gholipour et al., 2009 [24] | Iran | 1997–2004 | Retrospective | 793 | 48.9 ± 15 | NA | 71 (9) |
| First Author and Publication Year | Risk Factors for Conversion |
|---|---|
| Warchałowski et al., 2020 [3] | Gender, Age, Anatomical ambiguity, Admission Status, Acute Cholecystitis, Choledocholithiasis, Diabetes Mellitus, Hypertension, Heart Disease, Neurological Disease |
| Goonawardena et al., 2015 [49] | Gender, Age, Ethnicity, ASA > III, Acute Cholecystitis, Admission Status, BMI > 30, Obstructive Jaundice, Previous Upper Abdominal Surgery, Gallstone pancreatitis, Diabetes Mellitus, WBC, ALP, Bilirubin, Lipase, PCR, Choledocholithiasis, Cholecystitis, Gallbladder Wall width, Pericholecystic fluid, Impacted stone at the neck, Intrahepatic Duct dilatation |
| Ishizuka et al., 2016 [21] | Gender, Obesity, WBC, AST, ALT, ALP, Bilirubin, LDH, γ-GT, Albumin, PCR, Platelet count, Neutrophil ratio |
| Al Masri et al., 2018 [4] | Gender, Age, Admission Status, Obesity, Smoke, Previous Upper Abdominal Surgery, Diabetes Mellitus, Hypertension, Heart Disease, Dyslipidemia, Neurological Disease, Renal Disease, Lung Disease, WBC, AST, ALT, ALP, Bilirubin, Hemoglobin, γ-GT, Lipase, Platelet count, Choledocholithiasis, Gallbladder Wall width, Pericholecystic fluid, Impacted stone at the neck |
| Licciardello et al., 2014 [22] | Age, Acute Cholecystitis |
| Simopoulos et al., 2005 [23] | Gender, Age, Acute Cholecystitis, Presence of inflammation, Obesity, Previous Upper Abdominal Surgery, Diabetes Mellitus, Hypertension, Heart Disease |
| Lipman et al., 2007 [17] | Gender, Diabetes Mellitus, WBC, Bilirubin, Albumin, Pericholecystic fluid |
| Tayeb et al., 2005 [25] | Age, Acute Cholecystitis, Obesity, Obstructive jaundice, Previous Upper Abdominal Surgery, Gallstone pancreatitis, Tenderness in RUQ, Palpable Gallbladder, Diabetes Mellitus, Hypertension, WBC, ALP, Bilirubin, Gallbladder Wall with, Pericholecystic fluid, Dilatation of CBD, Multiple stones, US-Murphy sign |
| Kaafarani et al., 2009 [43] | Gender, Admission status, Previous Upper Abdominal Surgery, Hypertension, WBC, Albumin |
| Zhang et al., 2008 [28] | Gender, Age, Acute Cholecystitis, Previous Upper Abdominal Surgery, Diabetes Mellitus, Obesity, WBC, Gallbladder Wall width |
| Gholipour et al., 2009 [24] | Gender, Age, Admission Status, Smoke, Previous Upper Abdominal Surgery, WBC, ALP, Bilirubin, Gallbladder Wall width, Dilatation of CBD, Pericholecystic edema |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnano San Lio, R.; Barchitta, M.; Maugeri, A.; Quartarone, S.; Basile, G.; Agodi, A. Preoperative Risk Factors for Conversion from Laparoscopic to Open Cholecystectomy: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 408. https://doi.org/10.3390/ijerph20010408
Magnano San Lio R, Barchitta M, Maugeri A, Quartarone S, Basile G, Agodi A. Preoperative Risk Factors for Conversion from Laparoscopic to Open Cholecystectomy: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(1):408. https://doi.org/10.3390/ijerph20010408
Chicago/Turabian StyleMagnano San Lio, Roberta, Martina Barchitta, Andrea Maugeri, Serafino Quartarone, Guido Basile, and Antonella Agodi. 2023. "Preoperative Risk Factors for Conversion from Laparoscopic to Open Cholecystectomy: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 1: 408. https://doi.org/10.3390/ijerph20010408
APA StyleMagnano San Lio, R., Barchitta, M., Maugeri, A., Quartarone, S., Basile, G., & Agodi, A. (2023). Preoperative Risk Factors for Conversion from Laparoscopic to Open Cholecystectomy: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(1), 408. https://doi.org/10.3390/ijerph20010408










