Comprehensive Dental Treatment under General Anesthesia Improves Mastication Capability in Children with Early Childhood Caries—A One-Year Follow-Up Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
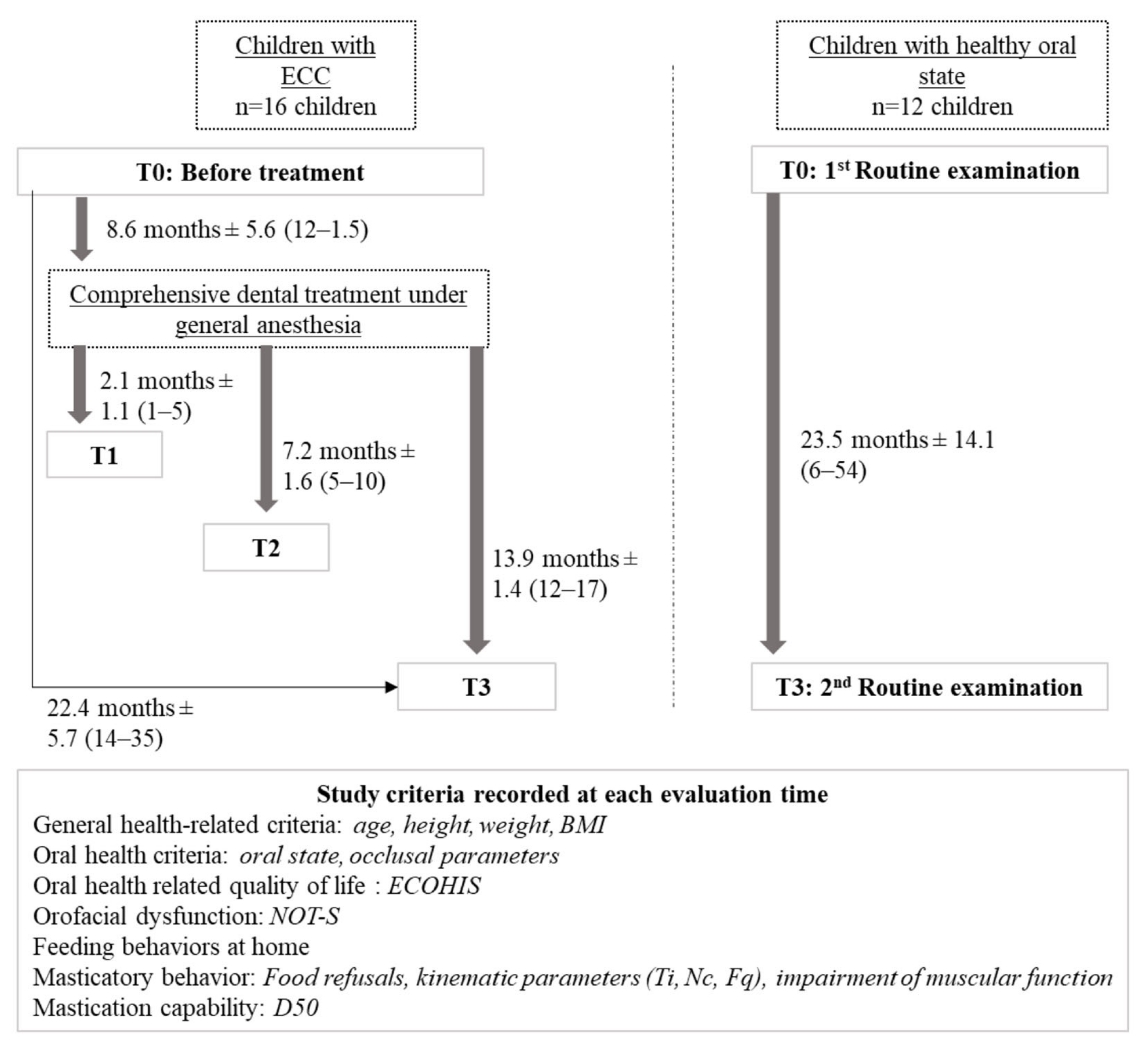
2.2. Participants
2.3. Experimental Design and Data Collection
2.4. Study Evaluation Criteria
2.4.1. General Health-Related Criteria
2.4.2. Oral Health Criteria
2.4.3. Oral Health-Related Quality of Life
2.4.4. Orofacial Dysfunction Frequency
2.4.5. Feeding Habits at Home
2.4.6. Masticatory Behavior
Food Refusals during Mastication Tests
Kinematic Parameters
- -
- Chewing time (Ti, seconds): time between the moment the food was placed into the mouth until the last food bolus manipulation, just before complete swallowing;
- -
- Number of masticatory cycles (Nc): number of chewing strokes during the chewing time period, with or without lip closure, corresponding to biting movements (tongue and perioral muscle manipulation movements were not counted);
- -
- Chewing frequency (Fq = Nc/Ti): calculated ratio between the number of masticatory cycles and chewing time.
Impairment of Muscular Function
2.4.7. Mastication Capability
2.5. Statistical Analysis
2.5.1. Evolution between Evaluation Times for Each Group of Children
2.5.2. Comparison between Both Groups of Children at the Different Evaluation Times
2.5.3. Impact of Posterior Teeth Extractions in Children with ECC
3. Results
3.1. General Health-Related Characteristics
3.1.1. Age, Weight and Height
3.1.2. Body Mass Index
3.2. Oral Health Criteria
3.3. Oral Health-Related Quality of Life (ECOHIS)
3.4. Orofacial Dysfunction (NOT-S)
3.5. Feeding Habits at Home
3.6. Masticatory Behavior
3.6.1. Food Refusals during the Mastication Tests
3.6.2. Kinematic Parameters
3.6.3. Impairment of Muscular Function
3.7. Masticatory Capability
3.8. Impact of Posterior Teeth Extractions on Chewing Frequency and D50 Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Academy of Pediatric Dentistry Policy on Early Childhood Caries (ECC): Classifications, Consequences, and Preventive Strategies. Pediatr. Dent. 2016, 38, 52–54. [PubMed]
- American Academy of Pediatric Dentistry Policy on Early Childhood Caries (ECC): Classifications, Consequences, and Preventive Strategies. Pediatr. Dent. 2008, 30, 40–43. [PubMed]
- Uribe, S.E.; Innes, N.; Maldupa, I. The Global Prevalence of Early Childhood Caries: A Systematic Review with Meta-Analysis Using the WHO Diagnostic Criteria. Int J. Paediatr. Dent. 2021, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. GBD 2015 Oral Health Collaborators Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, S.; Colares, V.; Pinkham, J. The Psychosocial Effects of Severe Caries in 4-Year-Old Children in Recife, Pernambuco, Brazil. Cad. Saude Publica 2005, 21, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Zaror, C.; Matamala-Santander, A.; Ferrer, M.; Rivera-Mendoza, F.; Espinoza-Espinoza, G.; Martínez-Zapata, M.J. Impact of Early Childhood Caries on Oral Health-Related Quality of Life: A Systematic Review and Meta-Analysis. Int. J. Dent. Hyg. 2022, 20, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.E.; Abou El Fadl, R.K.; Thabet, R.A.; Helmi, M.; Kamal, S.H. Iron Deficiency Anaemia and Early Childhood Caries: A Cross-Sectional Study. Aust. Dent. J. 2021, 66 (Suppl. S1), S27–S36. [Google Scholar] [CrossRef]
- Schroth, R.J.; Levi, J.A.; Sellers, E.A.; Friel, J.; Kliewer, E.; Moffatt, M.E.K. Vitamin D Status of Children with Severe Early Childhood Caries: A Case-Control Study. BMC Pediatr. 2013, 13, 174. [Google Scholar] [CrossRef]
- Schroth, R.J.; Levi, J.; Kliewer, E.; Friel, J.; Moffatt, M.E.K. Association between Iron Status, Iron Deficiency Anaemia, and Severe Early Childhood Caries: A Case-Control Study. BMC Pediatr. 2013, 13, 22. [Google Scholar] [CrossRef]
- Seminario, A.L.; Jumani, K.; Velan, E.; Scott, J.M.; Latimer, J.; Schroth, R.J. Suboptimal Serum Vitamin D Associated with Early Childhood Caries in Special Health Care Needs Children. J. Dent. Child. (Chic.) 2018, 85, 93–101. [Google Scholar]
- Easwaran, H.N.; Annadurai, A.; Muthu, M.S.; Sharma, A.; Patil, S.S.; Jayakumar, P.; Jagadeesan, A.; Nagarajan, U.; Pasupathy, U.; Wadgave, U. Early Childhood Caries and Iron Deficiency Anaemia: A Systematic Review and Meta-Analysis. Caries Res. 2022, 56, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Vania, A.; Parisella, V.; Capasso, F.; Di Tanna, G.L.; Vestri, A.; Ferrari, M.; Polimeni, A. Early Childhood Caries Underweight or Overweight, That Is the Question. Eur. J. Paediatr. Dent. 2011, 12, 231–235. [Google Scholar] [PubMed]
- Davidson, K.; Schroth, R.J.; Levi, J.A.; Yaffe, A.B.; Mittermuller, B.-A.; Sellers, E.A.C. Higher Body Mass Index Associated with Severe Early Childhood Caries. BMC Pediatr. 2016, 16, 137. [Google Scholar] [CrossRef]
- Kennedy, T.; Rodd, C.; Daymont, C.; Grant, C.G.; Mittermuller, B.-A.; Pierce, A.; Moffatt, M.E.K.; Schroth, R.J. The Association of Body Mass Index and Severe Early Childhood Caries in Young Children in Winnipeg, Manitoba: A Cross-Sectional Study. Int. J. Paediatr. Dent. 2020, 30, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Alantali, K.; Al-Halabi, M.; Hussein, I.; El-Tatari, A.; Hassan, A.; Kowash, M. Changes in Preschool Children’s Oral Health-Related Quality of Life Following Restorative Dental General Anaesthesia. Br. Dent. J. 2020, 229, 670–676. [Google Scholar] [CrossRef]
- Linas, N.; Peyron, M.-A.; Eschevins, C.; Hennequin, M.; Nicolas, E.; Collado, V. Natural Food Mastication Capability in Preschool Children According to Their Oral Condition: A Preliminary Study. J. Texture Stud. 2020, 51, 755–765. [Google Scholar] [CrossRef]
- Linas, N.; Peyron, M.-A.; Hennequin, M.; Eschevins, C.; Nicolas, E.; Delfosse, C.; Collado, V. Masticatory Behavior for Different Solid Foods in Preschool Children According to Their Oral State. J. Texture Stud. 2019, 50, 224–236. [Google Scholar] [CrossRef]
- Laguna, L.; Chen, J. The Eating Capability: Constituents and Assessments. Food Qual. Prefer. 2016, 48, 345–358. [Google Scholar] [CrossRef]
- Oubenyahya, H.; Bouhabba, N. General Anesthesia in the Management of Early Childhood Caries: An Overview. J. Dent. Anesth. Pain Med. 2019, 19, 313–322. [Google Scholar] [CrossRef]
- Liu, F.; Yang, K.; Wang, P.; Wu, T.; Li, J.; Guo, Q. Trends, Characteristics, and Success Rates of Treatment for Severe Early Childhood Caries Under General Anesthesia: A Retrospective Study in Northwest China. J. Clin. Pediatr. Dent. 2021, 45, 278–283. [Google Scholar] [CrossRef]
- Collado, V.; Pichot, H.; Delfosse, C.; Eschevins, C.; Nicolas, E.; Hennequin, M. Impact of Early Childhood Caries and Its Treatment under General Anesthesia on Orofacial Function and Quality of Life: A Prospective Comparative Study. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e333–e341. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, L.; Qin, D.; He, S.; Wang, J. Effects of Dental General Anaesthesia Treatment on Early Childhood Caries: A Prospective Cohort Study in China. BMJ Open 2019, 9, e028931. [Google Scholar] [CrossRef] [PubMed]
- Boukhobza, S.; Stamm, T.; Glatthor, J.; Meißner, N.; Bekes, K. Changes in Oral Health-Related Quality of Life among Austrian Preschool Children Following Dental Treatment under General Anaesthesia. Clin. Oral Investig. 2021, 25, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Salerno, C.; Sangianantoni, G.; Caruso, S.; Ingenito, A.; Cantile, T. The Effect of Dental Treatment under General Anesthesia on Quality of Life and Growth and Blood Chemistry Parameters in Uncooperative Pediatric Patients with Compromised Oral Health: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 4407. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, J.; Bansal, K.; Chopra, R. Effect of Comprehensive Dental Rehabilitation on Growth Parameters in Pediatric Patients with Severe Early Childhood Caries. Int. J. Clin. Pediatr. Dent. 2016, 9, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Sangianantoni, S.; Mitrano, R.L.; Ingenito, A.; Alcidi, B.; Cantile, T. Assessing Changes in Oral Health-Related Quality of Life and Body Growth in 3-5 Years Old Children Following Dental Treatment under General Anaesthesia Due to Severe Dental Caries. Eur. J. Paediatr. Dent. 2019, 20, 214–218. [Google Scholar] [CrossRef]
- Khong, J.S.Y.; Goh, A.T.; Sim, Y.F.; Lai, B.W.P.; Forde, C.G.; Hong, C.H.L. Masticatory Function after Comprehensive Dental Treatment in Children with Severe Early Childhood Caries. Int. J. Paediatr. Dent. 2022, 32, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Van der Bilt, A. Assessment of Mastication with Implications for Oral Rehabilitation: A Review. J. Oral Rehabil. 2011, 38, 754–780. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body Mass Index Cut Offs to Define Thinness in Children and Adolescents: International Survey. BMJ 2007, 335, 194. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health Surveys: Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013; ISBN 978 92 4 154864 9. [Google Scholar]
- Shoaib, L.; Deery, C.; Ricketts, D.N.J.; Nugent, Z.J. Validity and Reproducibility of ICDAS II in Primary Teeth. Caries Res. 2009, 43, 442–448. [Google Scholar] [CrossRef]
- Monse, B.; Heinrich-Weltzien, R.; Benzian, H.; Holmgren, C.; van Palenstein Helderman, W. PUFA--an Index of Clinical Consequences of Untreated Dental Caries. Community Dent. Oral Epidemiol. 2010, 38, 77–82. [Google Scholar] [CrossRef]
- Marquezin, M.C.S.; Kobayashi, F.Y.; Montes, A.B.M.; Gavião, M.B.D.; Castelo, P.M. Assessment of Masticatory Performance, Bite Force, Orthodontic Treatment Need and Orofacial Dysfunction in Children and Adolescents. Arch. Oral Biol. 2013, 58, 286–292. [Google Scholar] [CrossRef]
- Brook, P.H.; Shaw, W.C. The Development of an Index of Orthodontic Treatment Priority. Eur J. Orthod. 1989, 11, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Veronneau, J.; Allison, P.J. Validation of a French Language Version of the Early Childhood Oral Health Impact Scale (ECOHIS). Health Qual. Life Outcomes 2008, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Bergendal, B.; McAllister, A.; Sjögreen, L.; Asten, P. Development and Evaluation of a Comprehensive Screening for Orofacial Dysfunction. Swed. Dent. J. 2007, 31, 75–84. [Google Scholar]
- Castetbon, K.; Lafay, L.; Volatier, J.; Escalon, H.; Delamaire, C.; Chauliac, M.; Ledésert, B.; Hercberg, S. The French National Nutrition and Health Program (PNNS): Report of the studies and observed results. Cah. Nutr. Diét. 2011, 46, S11–S25. [Google Scholar] [CrossRef]
- Pires, S.C.; Giugliani, E.R.J.; Caramez da Silva, F. Influence of the Duration of Breastfeeding on Quality of Muscle Function during Mastication in Preschoolers: A Cohort Study. BMC Public Health 2012, 12, 934. [Google Scholar] [CrossRef]
- Gisel, E.G. Chewing Cycles in 2- to 8-Year-Old Normal Children: A Developmental Profile. Am. J. Occup. Ther. 1988, 42, 40–46. [Google Scholar] [CrossRef]
- Wilson, E.M.; Green, J.R.; Weismer, G. A Kinematic Description of the Temporal Characteristics of Jaw Motion for Early Chewing: Preliminary Findings. J. Speech Lang. Hear. Res. 2012, 55, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Green, J.R.; Moore, C.A.; Ruark, J.L.; Rodda, P.R.; Morvée, W.T.; VanWitzenburg, M.J. Development of Chewing in Children from 12 to 48 Months: Longitudinal Study of EMG Patterns. J. Neurophysiol. 1997, 77, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Almotairy, N.; Kumar, A.; Grigoriadis, A. Effect of Food Hardness on Chewing Behavior in Children. Clin. Oral Investig. 2021, 25, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Almotairy, N.; Kumar, A.; Trulsson, M.; Grigoriadis, A. Development of the Jaw Sensorimotor Control and Chewing—A Systematic Review. Physiol. Behav. 2018, 194, 456–465. [Google Scholar] [CrossRef]
- Limme, M. [The need of efficient chewing function in young children as prevention of dental malposition and malocclusion]. Arch. Pediatr. 2010, 17 (Suppl. S5), S213–S219. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Coulthard, H. Early Eating Behaviours and Food Acceptance Revisited: Breastfeeding and Introduction of Complementary Foods as Predictive of Food Acceptance. Curr. Obes. Rep. 2016, 5, 113–120. [Google Scholar] [CrossRef]
- Bott, T.S.; von Kalle, T.; Schilling, A.; Diez, O.H.; Besch, S.; Mehlig, U.; Hetjens, S.; Wessel, L.; Loff, S. Esophageal Diameters in Children Correlated to Body Weight. Eur. J. Pediatr. Surg. 2019, 29, 528–532. [Google Scholar] [CrossRef]
- Woda, A.; Nicolas, E.; Mishellany-Dutour, A.; Hennequin, M.; Mazille, M.-N.; Veyrune, J.-L.; Peyron, M.-A. The Masticatory Normative Indicator. J. Dent. Res. 2010, 89, 281–285. [Google Scholar] [CrossRef]
- Barbosa, T. de S.; Tureli, M.C. de M.; Nobre-dos-Santos, M.; Puppin-Rontani, R.M.; Gavião, M.B.D. The Relationship between Oral Conditions, Masticatory Performance and Oral Health-Related Quality of Life in Children. Arch. Oral Biol. 2013, 58, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Le Révérend, B.J.D.; Edelson, L.R.; Loret, C. Anatomical, Functional, Physiological and Behavioural Aspects of the Development of Mastication in Early Childhood. Br. J. Nutr. 2014, 111, 403–414. [Google Scholar] [CrossRef]
- Iodice, G.; Danzi, G.; Cimino, R.; Paduano, S.; Michelotti, A. Association between Posterior Crossbite, Skeletal, and Muscle Asymmetry: A Systematic Review. Eur. J. Orthod. 2016, 38, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, A.A.; Alkhuriaf, A.; Pani, S.C. An Evaluation of Bite Pattern in Children with Severe-Early Childhood Caries Before and After Complete Dental Rehabilitation. Pediatr. Dent. 2017, 39, 455–459. [Google Scholar] [PubMed]
- Liberali, R.; Kupek, E.; Assis, M.A.A. de Dietary Patterns and Childhood Obesity Risk: A Systematic Review. Child. Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Thang Le, V.N.; Kim, J.-G.; Yang, Y.-M.; Lee, D.-W. Risk Factors for Early Childhood Caries: An Umbrella Review. Pediatr. Dent. 2021, 43, 176–194. [Google Scholar] [PubMed]
- Vichayanrat, T.; Sudha, K.; Kumthanom, K.; Apisuttisin, J.; Uawatanasakul, N.; Ariyakieatsakul, Y. What Factors Influence Mothers’ Behavior Regarding Control of Their Children’s Sugary Snack Intake?: An Application of the Theory of Planned Behavior. Int. Dent. J. 2018, 68, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, A.; Simmonds, M.; Owen, C.G.; Woolacott, N. Childhood Obesity as a Predictor of Morbidity in Adulthood: A Systematic Review and Meta-Analysis. Obes. Rev. 2016, 17, 56–67. [Google Scholar] [CrossRef] [PubMed]


| Criteria | Group with ECC | Group with HOS | Statistical Differences between Groups of Children | ||
|---|---|---|---|---|---|
| (16 Children) | (12 Children) | ||||
| Evaluation Times | Mean ± SD (Min–Max) | Evaluation Times | Mean ± SD (Min–Max) | Student’s t-Test | |
| Age (months) | T0 | 58.6 ± 8.3 (47–71) | T0 | 58.4 ± 8.3 (47–71) | NS |
| T1 | 69.3 ± 9.3 (53–82) | ||||
| T2 | 74.6 ± 9.0 (60–86) | ||||
| T3 | 80.9 ± 9.5 (66–94) | T3 | 81.7 ± 16.0 (60–115) | NS | |
| Weight (kg) | T0 | 17.3 ± 2.2 (13.3–23.3) | T0 | 19.1 ± 2.4 (15–22.5) | NS |
| T1 | 20.0 ± 3.2 (13.5–25.2) | ||||
| T2 | 21.7 ± 4.2 (14.5–31.4) | ||||
| T3 | 23.5 ± 4.9 (15–31) | T3 | 24.0 ± 6.3 (15–35) | NS | |
| Height (cm) | T0 | 106.0 ± 5.6 (96.7–116) | T0 | 110 ± 5.4 (104–120) | |
| T1 | 112.1 ± 6.7 (102–122) | ||||
| T2 | 115.9 ± 6.0 (104.5–125.5) | ||||
| T3 | 118.0 ± 6.5 (106–129) | T3 | 121.6 ± 10.6 (104–138) | NS | |
| Criteria | Group with ECC | Group with HOS | Differences between Groups | ||
|---|---|---|---|---|---|
| (16 Children) | (12 Children) | ||||
| Evaluation Time | Mean ± SD | Evaluation Time | Mean ± SD | Student’s t-Test | |
| (Min–Max) | (Min–Max) | ||||
| dmft total score | T0 | 12.3 ± 4.6 (3–19) | T0 | 0 | p ≤ 0.001 |
| - decayed | 11.7 ± 4.4 (3–19) | ||||
| - missing | 0.2 ± 0.5 (0–2) | ||||
| - filled | 0.4 ± 1.2 (0–4) | ||||
| PUFA total score | T0 | 7.6 ± 4.4 (1–16) | T0 | 0 | p ≤ 0.001 |
| - Visible pulp (p) | 6.6 ± 4.1 (1–15) | ||||
| - Ulceration (u) | 0 | ||||
| - Fistula (f) | 0.3 ± 0.4 (0–1) | ||||
| - Abscess (a) | 0 | ||||
| Treatment performed during GA: | |||||
| Tooth extractions | 3.5 ± 2.71 (0–8) | 0 | p ≤ 0.001 | ||
| - Incisor or canine | 2.19 ± 2.10 (0–6) | ||||
| - Molar | 1.31 ± 1.35 (0–4) | ||||
| Conservative treatment | 8.19 ± 2.66 (4–13) | 0 | p ≤ 0.001 | ||
| - Coronal restauration | 2.63 ± 2.0 (0–6) | ||||
| - Stainless-steel crown | 0.06 ± 0.25 (0–1) | ||||
| - Pulpotomy and coronal restauration | 0.94 ± 1.48 (0–4) | ||||
| - Pulpotomy and stainless-steel crown | 4.31 ± 1.82 (2–7) | ||||
| - Pulpectomy and coronal restauration | 0.13 ± 0.34 (0–1) | ||||
| - Pulpectomy and stainless-steel crown | 0.13 ± 0.34 (0–1) | ||||
| Selective grinding of canine(s) | 1 ± 1.79 (0–4) | 0 | p ≤ 0.05 | ||
| Number of 1st permanent molar | T0 | 0 | T0 | 0 | |
| T1 | 1.31 ± 1.62 (0–4) | ||||
| T2 | 2.06 ± 1.88 (0–4) | ||||
| T3 | 2.94 ± 1.48 (0–4) | T3 | 1.83 ± 1.99 (0–4) | NS | |
| Orofacial dysmorphologies: | n | n | Fisher’s exact test | ||
| - Dento-maxillary disharmony | T0 | 0 | T0 | 0 | NS |
| - Eruption or number of anomalies | 0 | 0 | NS | ||
| - Sagittal dimension abnormality | 1 | 3 | NS | ||
| - Transverse dimension abnormality | 3 | 0 | NS | ||
| - Vertical dimension abnormality | 3 | 5 | NS | ||
| Tested Food | Refusals Reasons | Group with ECC (16) | Group with HOS (12) | Differences between Groups of Children | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T0 | T3 | Fisher’s Exact Test | |||
| n Children | n Children | T0 | T3 | ||||||
| CAR | Don’t like | 1 | 1 | 3 | 3 | 1 | 1 | ||
| Don’t know/Never tried | 1 | 0 | 1 | 0 | 0 | 0 | |||
| Painful | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Too difficult to eat | 4 | 2 | 1 | 1 | 0 | 0 | |||
| Total | 6 | 3 | 5 | 4 | 1 | 1 | p ≤ 0.05 | NS | |
| CHS | Don’t like | 3 | 2 | 3 | 3 | 2 | 1 | ||
| Don’t know/Never tried | 0 | 0 | 1 | 0 | 0 | 0 | |||
| Painful | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Too difficult to eat | 2 | 1 | 0 | 0 | 0 | 0 | |||
| Total | 5 | 3 | 4 | 3 | 2 | 1 | NS | NS | |
| CER | Don’t like | 1 | 1 | 1 | 1 | 0 | 0 | ||
| Don’t know/Never tried | 0 | 0 | 1 | 0 | 0 | 0 | |||
| Painful | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Too difficult to eat | 2 | 0 | 0 | 0 | 0 | 0 | |||
| Total | 3 | 1 | 2 | 1 | 0 | 0 | p ≤ 0.05 NS | ||
| Tested Food | Kinematic Parameters | Group with ECC | Group with HOS | Differences between Groups | ||||
|---|---|---|---|---|---|---|---|---|
| Study Time | Number of Children | Mean ± SD | Study Time | Number of Children | Mean ± SD | Student’s t-Test | ||
| CAR | Ti (s) | T0 | 10 | 49.05 ± 35.68 | T0 | 11 | 33.0 ± 12.95 | NS |
| T1 | 13 | 46.58 ± 31.12 | ||||||
| T2 | 11 | 31.06 ± 12.93 | ||||||
| T3 | 12 | 28.08 ± 11.12 * | T3 | 11 | 21.32 ± 5.04 $$$ | p ≤ 0.05 | ||
| Nc (n) | T0 | 10 | 44.2 ± 20.17 | T0 | 11 | 44.5 ± 13.31 | NS | |
| T1 | 13 | 53.77 ± 26.50 | ||||||
| T2 | 11 | 43.55 ± 18.20 | ||||||
| T3 | 12 | 38.38 ± 15.14 | T3 | 11 | 34.18 ± 7.30 $$ | NS | ||
| Fq (n/s) | T0 | 10 | 1.05 ± 0.31 | T0 | 11 | 1.43 ± 0.30 | p ≤ 0.001 | |
| T1 | 13 | 1.25 ± 0.28 | ||||||
| T2 | 11 | 1.43 ± 0.33 *** | ||||||
| T3 | 12 | 1.39 ± 0.22 *** | T3 | 11 | 1.64 ± 0.28 $ | p ≤ 0.01 | ||
| CHS | Ti (s) | T0 | 11 | 21.36 ± 8.10 | T0 | 10 | 19.65 ± 6.48 | NS |
| T1 | 13 | 22.27 ± 16.66 | ||||||
| T2 | 12 | 15.36 ± 5.94 | ||||||
| T3 | 13 | 14.08 ± 5.63 * | T3 | 11 | 16.41 ± 5.24 | NS | ||
| Nc (n) | T0 | 11 | 23.09 ± 7.49 | T0 | 10 | 24.1 ± 6.21 | NS | |
| T1 | 13 | 21.73 ± 9.91 | ||||||
| T2 | 12 | 20.56 ± 8.55 | ||||||
| T3 | 13 | 19.12 ± 6.94 | T3 | 11 | 23.5 ± 6.15 | p ≤ 0.05 | ||
| Fq (n/s) | T0 | 11 | 1.13 ± 0.22 | T0 | 10 | 1.36 ± 0.26 | p ≤ 0.01 | |
| T1 | 13 | 1.15 ± 0.39 | ||||||
| T2 | 12 | 1.35 ± 0.26 * | ||||||
| T3 | 13 | 1.39 ± 0.21 ** | T3 | 11 | 1.48 ± 0.30 | NS | ||
| CER | Ti (s) | T0 | 13 | 28.04 ± 9.85 | T0 | 12 | 20.38 ± 5.56 | p ≤ 0.01 |
| T1 | 15 | 29.9 ± 9.65 | ||||||
| T2 | 14 | 23.57 ± 6.22 | ||||||
| T3 | 15 | 20.43 ± 7.38 ** | T3 | 12 | 19.29 ± 6.80 | NS | ||
| Nc (n) | T0 | 13 | 25.04 ± 6.27 | T0 | 12 | 26.25 ± 6.84 | NS | |
| T1 | 15 | 28.33 ± 9.73 | ||||||
| T2 | 14 | 28.36 ± 9.60 | ||||||
| T3 | 15 | 25.87 ± 10.69 | T3 | 12 | 26.58 ± 6.61 | NS | ||
| Fq (n/s) | T0 | 13 | 0.95 ± 0.25 | T0 | 12 | 1.30 ± 0.20 | p ≤ 0.001 | |
| T1 | 15 | 0.99 ± 0.28 | ||||||
| T2 | 14 | 1.20 ± 0.23 *** | ||||||
| T3 | 15 | 1.27 ± 0.21 *** | T3 | 12 | 1.45 ± 0.30 | p ≤ 0.05 | ||
| Parameter | Food | Evaluation Time | No Posterior Extractions | ≥1 Posterior Extraction | ||
|---|---|---|---|---|---|---|
| Number of Children | Mean ± SD | Number of Children | Mean ± SD | |||
| Chewing frequency (Fq) | CAR | T0 | 5 | 1.01 ± 0.34 | 5 | 1.10 ± 0.29 |
| T3 | 6 | 1.36 ± 0.25 | 6 | 1.41 ± 0.20 | ||
| CHS | T0 | 4 | 1.12 ± 0.15 | 7 | 1.13 ± 0.26 | |
| T3 | 4 | 1.42 ± 0.32 | 9 | 1.38 ± 0.14 | ||
| CER | T0 | 5 | 0.99 ± 0.19 | 8 | 0.93 ± 0.28 | |
| T3 | 5 | 1.18 ± 0.18 | 10 | 1.32 ± 0.22 | ||
| D50 (mm) | CAR | T0 | 5 | 4.39 ± 0.85 | 5 | 4.49 ± 1.26 |
| T3 | 6 | 2.75 ± 0.44 | 6 | 4.21 ± 0.77 *** | ||
| CHS | T0 | 4 | 4.68 ± 1.33 | 7 | 4.98 ± 1.58 | |
| T3 | 4 | 3.22 ± 0.56 | 9 | 4.48 ± 1.30 ** | ||
| CER | T0 | 5 | 1.01 ± 0.18 | 8 | 1.25 ± 0.24 * | |
| T3 | 5 | 1.12 ± 0.19 | 10 | 1.12 ± 0.26 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linas, N.; Peyron, M.-A.; Cousson, P.-Y.; Decerle, N.; Hennequin, M.; Eschevins, C.; Nicolas, E.; Collado, V. Comprehensive Dental Treatment under General Anesthesia Improves Mastication Capability in Children with Early Childhood Caries—A One-Year Follow-Up Study. Int. J. Environ. Res. Public Health 2023, 20, 677. https://doi.org/10.3390/ijerph20010677
Linas N, Peyron M-A, Cousson P-Y, Decerle N, Hennequin M, Eschevins C, Nicolas E, Collado V. Comprehensive Dental Treatment under General Anesthesia Improves Mastication Capability in Children with Early Childhood Caries—A One-Year Follow-Up Study. International Journal of Environmental Research and Public Health. 2023; 20(1):677. https://doi.org/10.3390/ijerph20010677
Chicago/Turabian StyleLinas, Natacha, Marie-Agnès Peyron, Pierre-Yves Cousson, Nicolas Decerle, Martine Hennequin, Caroline Eschevins, Emmanuel Nicolas, and Valérie Collado. 2023. "Comprehensive Dental Treatment under General Anesthesia Improves Mastication Capability in Children with Early Childhood Caries—A One-Year Follow-Up Study" International Journal of Environmental Research and Public Health 20, no. 1: 677. https://doi.org/10.3390/ijerph20010677
APA StyleLinas, N., Peyron, M.-A., Cousson, P.-Y., Decerle, N., Hennequin, M., Eschevins, C., Nicolas, E., & Collado, V. (2023). Comprehensive Dental Treatment under General Anesthesia Improves Mastication Capability in Children with Early Childhood Caries—A One-Year Follow-Up Study. International Journal of Environmental Research and Public Health, 20(1), 677. https://doi.org/10.3390/ijerph20010677






