Effect of Arbuscular Mycorrhiza Fungus Diversispora eburnea Inoculation on Lolium perenne and Amorpha fruticosa Growth, Cadmium Uptake, and Soil Cadmium Speciation in Cadmium-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation
2.2. Host Plants and AMF Inocula
2.3. Experimental Design
2.4. Harvest and Measurements
2.5. Calculation of Plant Cd Accumulation, Metal Tolerance Index, Transfer Factor, and Bioconcentration Factor
2.6. Statistical Analysis
3. Results
3.1. D. eburnea Colonization Rate
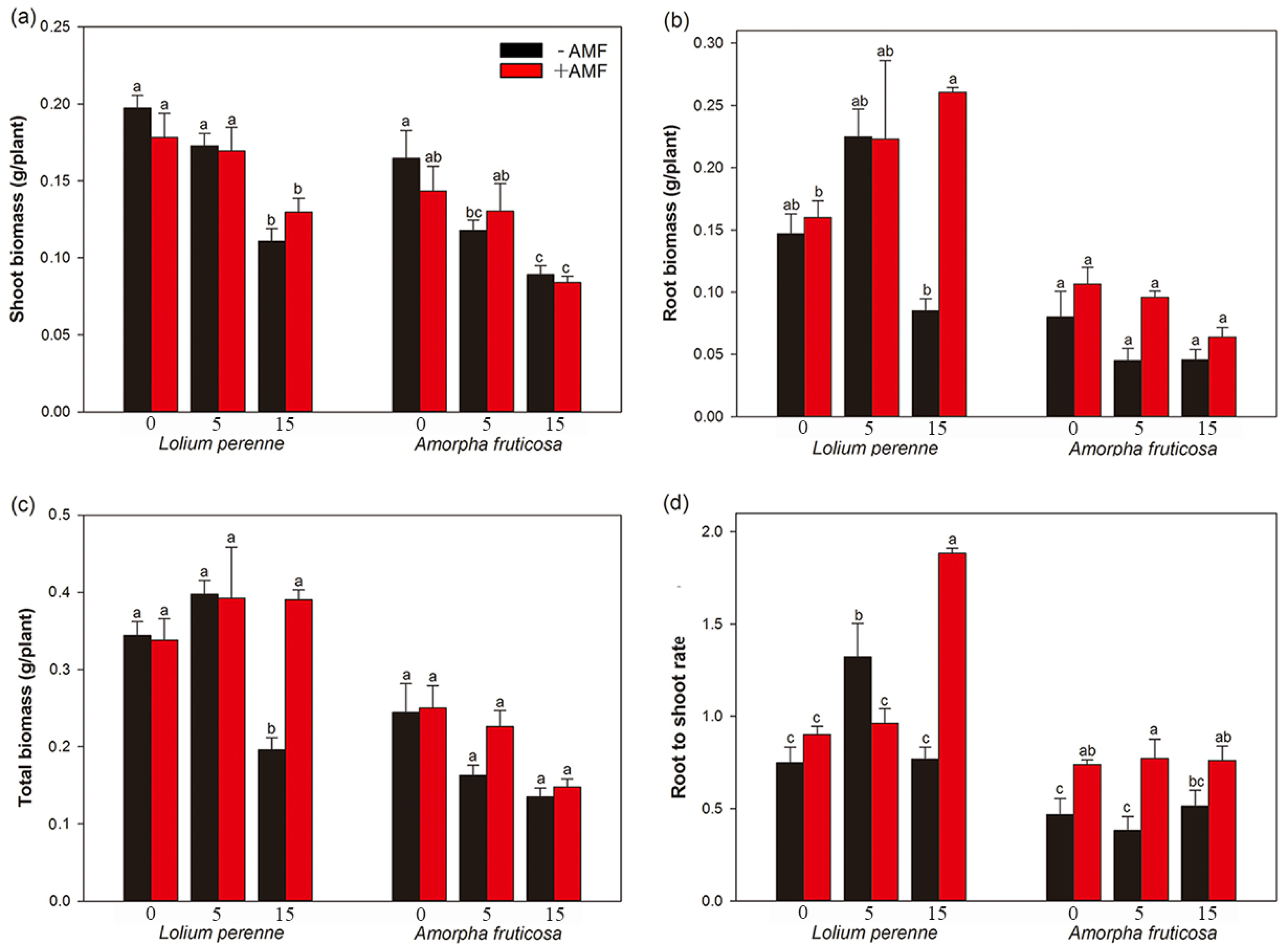
3.2. Plant Biomass
3.3. Soil Parameters
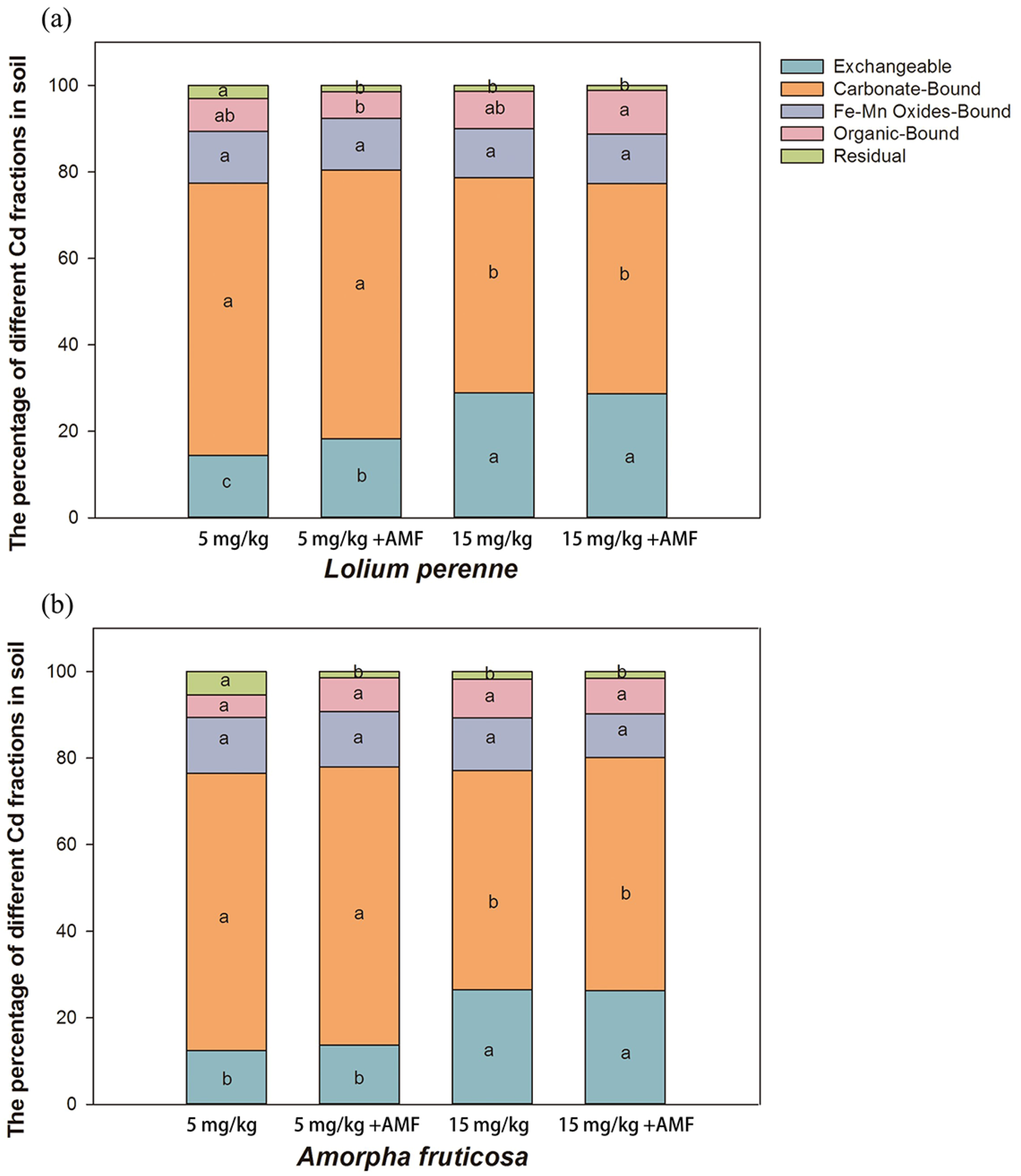
3.4. Soil Cd Fractions
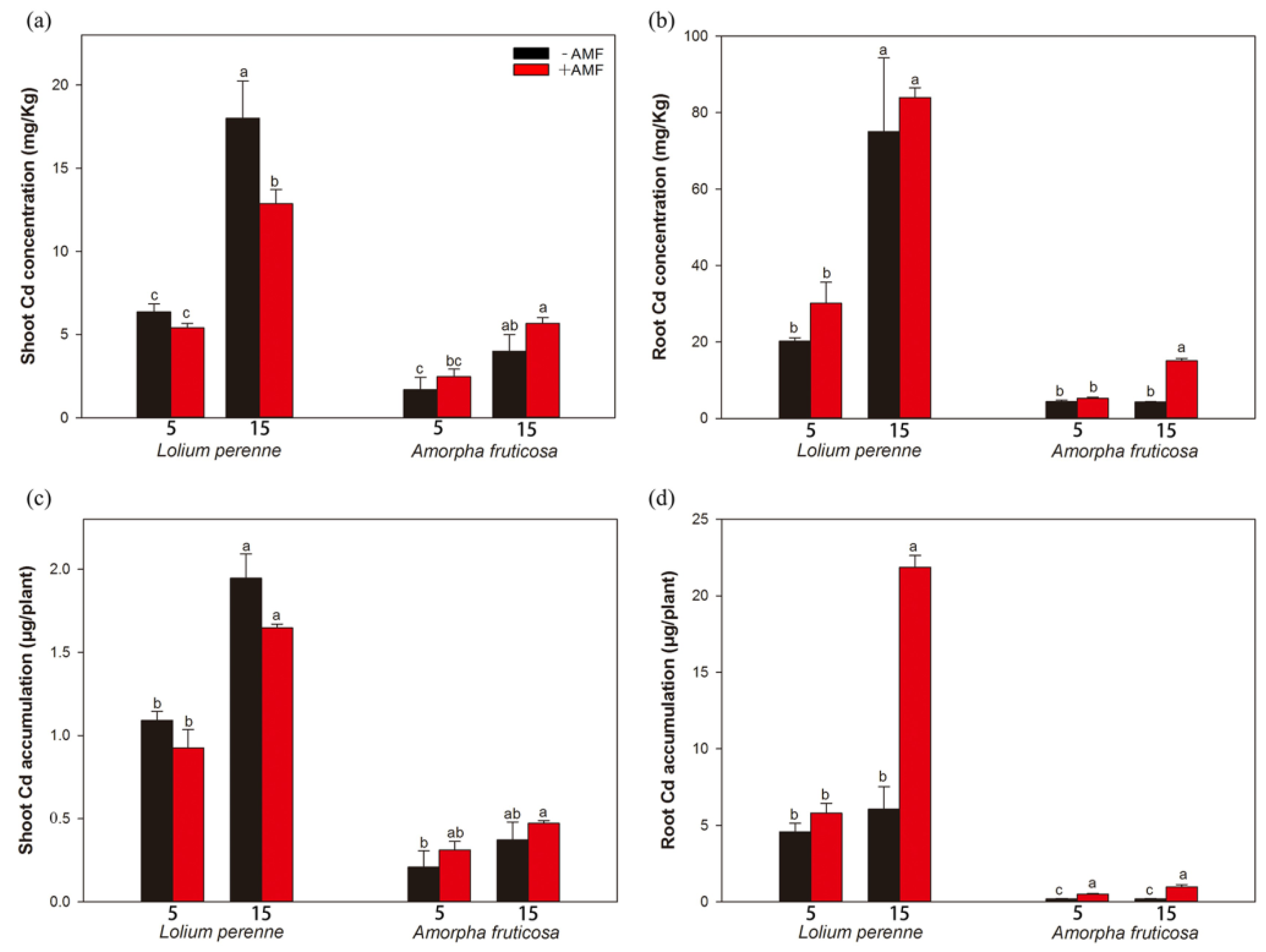
3.5. Plant Cd Concentration and Accumulation
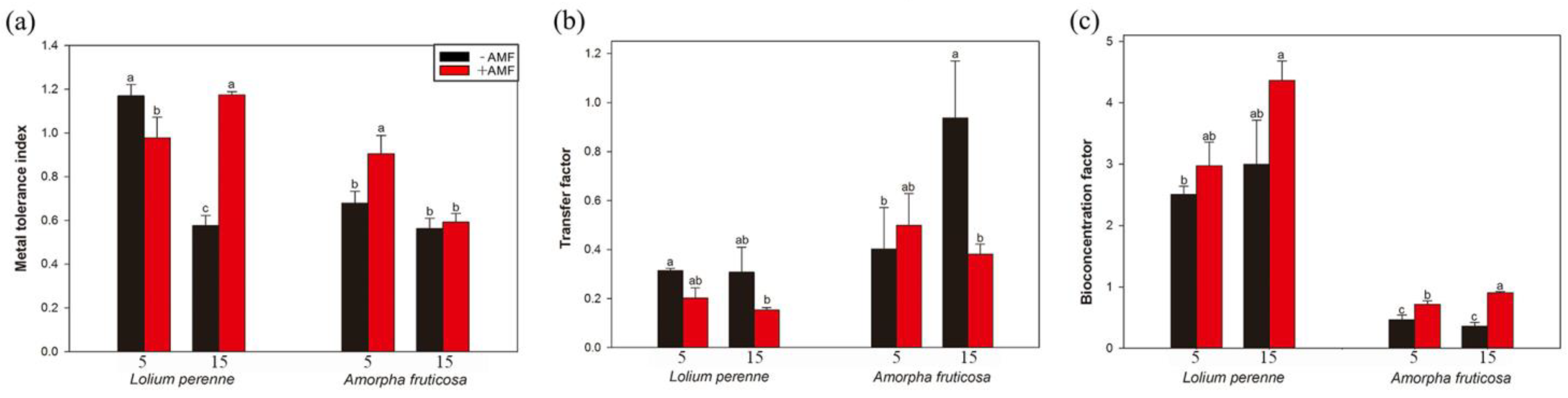
3.6. Metal Tolerance Index, Transfer Factor, and Bioconcentration Factor (BCF)
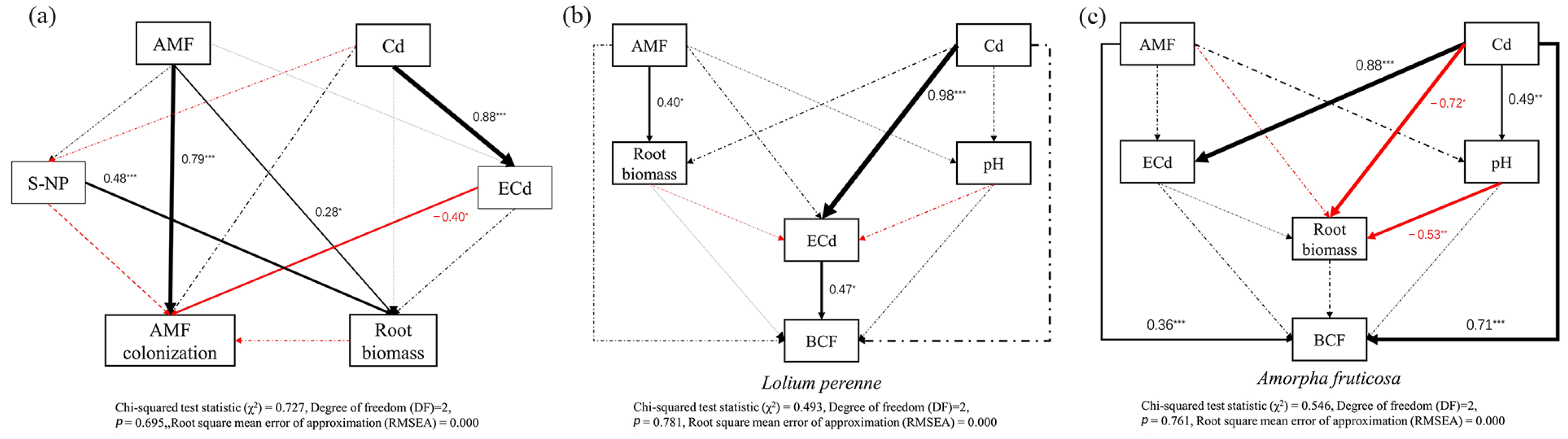
3.7. Pathways of AMF D. eburnea and Cd Treatment Effects on D. eburnea Colonization Rate and Bioconcentration Factor
4. Discussion
4.1. Cd in Soil Affected the Colonization Rate of AMF D. eburnea
4.2. AMF Inoculation and Cd Addition Affected Cd Fractions in Soil
4.3. AMF Impact on Plant Biomass and Cd Uptake
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haider, F.U.; Cai, L.Q.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.Z.; Ma, W.J.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Cameselle, C.; Gouveia, S.; Urrejola, S. Benefits of phytoremediation amended with DC electric field. Application to soils contaminated with heavy metals. Chemosphere 2019, 229, 481–488. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Boller, T. Different arbuscular mycorrhizal fungal species are potential determinants of plant community. Ecology 1998, 79, 2082. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Etemadi, N. Physiological response of Arizona cypress to Cd-contaminated soil inoculated with arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria. Rhizosphere 2021, 18, 100354. [Google Scholar] [CrossRef]
- Bisht, A.; Garg, N. AMF species improve yielding potential of Cd stressed pigeonpea plants by modulating sucrose-starch metabolism, nutrients acquisition and soil microbial enzymatic activities. Plant Growth Regul. 2022, 96, 409–430. [Google Scholar] [CrossRef]
- De Andrade, S.A.L.; da Silveira, A.P.D.; Jorge, R.A.; de Abreu, M.F. Cadmium accumulation in sunflower plants influenced by arbuscular mycorrhiza. Int. J. Phytoremediat. 2008, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Benti, G.; Wang, D.; Yang, Z.; Xiao, R. Effect of Alteration in Precipitation Amount on Soil Microbial Community in a Semi-Arid Grassland. Front. Microbiol. 2022, 13, 842446. [Google Scholar] [CrossRef]
- Li, J.; Charles, L.S.; Yang, Z.; Du, G.; Fu, S. Differential Mechanisms Drive Species Loss Under Artificial Shade and Fertilization in the Alpine Meadow of the Tibetan Plateau. Front. Plant Sci. 2022, 13, 832473. [Google Scholar] [CrossRef]
- Li, S.Q.; Li, G.D.; Peng, K.M.; Yang, L.H.; Huang, X.F.; Lu, L.J.; Liu, J. The combined effect of Diversispora versiformis and sodium bentonite contributes on the colonization of Phragmites in cadmium-contaminated soil. Chemosphere 2022, 293, 133613. [Google Scholar] [CrossRef]
- Mollavali, M.; Bolandnazar, S.A.; Schwarz, D.; Rohn, S.; Riehle, P.; Nahandi, F.Z. Flavonol glucoside and antioxidant enzyme biosynthesis affected by mycorrhizal fungi in various cultivars of onion (Allium cepa L.). J. Agric. Food Chem. 2015, 64, 71–77. [Google Scholar]
- Wang, G.; Wang, L.; Ma, F.; Yang, D.; You, Y. Earthworm and arbuscular mycorrhiza interactions: Strategies to motivate antioxidant responses and improve soil functionality. Environ. Pollut. 2020, 272, 115980. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Ma, F.; You, Y.; Wang, Y.; Yang, D. Integration of earthworms and arbuscular mycorrhizal fungi into phytoremediation of cadmium-contaminated soil by Solanum nigrum L. J. Hazard. Mater. 2020, 389, 121873. [Google Scholar] [CrossRef]
- Hassan, S.E.; Hijri, M.; St-Arnaud, M. Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. New Biotechnol. 2013, 30, 780–787. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, R.; Jiang, L.; Chen, K.; Zhu, W. Alleviation of Cadmium Toxicity to Medicago Truncatula by AMF Involves the Changes of Cd Speciation in Rhizosphere Soil and Subcellular Distribution. Phyton-Int. J. Exp. Bot. 2021, 90, 403–415. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Zhang, L.J.; Zhao, H.M.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; Mo, C.H. Do arbuscular mycorrhizal fungi affect cadmium uptake kinetics, subcellular distribution and chemical forms in rice? Sci. Total Environ. 2016, 571, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, L.; Zhu, S.; Ho, S.-H.; Wu, J.; Kalita, P.K.; Ma, F. Unraveling the effects of arbuscular mycorrhizal fungus on uptake, translocation, and distribution of cadmium in Phragmites australis (Cav.) Trin. ex Steud. Ecotoxicol. Environ. Saf. 2018, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.T.; Straker, C.J.; Mavri-Damelin, D.; Weiersbye, I.M. Diversity of arbuscular mycorrhizal (AM) fungi colonising roots of indigenous Vachellia and Senegalia trees on gold and uranium mine tailings in South Africa. S. Afr. J. Bot. 2019, 121, 34–44. [Google Scholar] [CrossRef]
- Alguacil, M.M.; Torrecillas, E.; Caravaca, F.; Fernández, D.; Azcón, R.; Roldán, A. The application of an organic amendment modifies the arbuscular mycorrhizal fungal communities colonizing native seedlings grown in a heavy-metal-polluted soil. Soil Biol. Biochem. 2011, 43, 1498–1508. [Google Scholar] [CrossRef]
- Sikdar, A.; Wang, J.; Hasanuzzaman, M.; Liu, X.; Feng, S.; Roy, R.; Sial, T.A.; Lahori, A.H.; Arockiam Jeyasundar, P.G.S.; Wang, X. Phytostabilization of Pb-Zn Mine Tailings with Amorpha fruticosa Aided by Organic Amendments and Triple Superphosphate. Molecules 2020, 25, 1617. [Google Scholar] [CrossRef]
- Bahmani-Babanari, L.; Mirzahosseini, Z.; Shabani, L.; Sabzalian, M.R. Effect of arbuscular mycorrhizal fungus, Funneliformis fasciculatum, on detoxification of Nickel and expression of TIP genes in Lolium perenne L. Biologia 2021, 76, 1675–1683. [Google Scholar] [CrossRef]
- Malicka, M.S.; Magurno, F.; Posta, K.; Chmura, D.; Piotrowska-Seget, Z. Differences in the effects of single and mixed AMF species on the growth and oxidative stress defense in Lolium perenne exposed to hydrocarbons. Ecotoxicol. Environ. Saf. 2021, 217, 112252. [Google Scholar] [CrossRef]
- Bi, Y.; Guo, Y.; Christie, P. Mining subsidence area reconstruction with N2-fixing plants promotes arbuscular mycorrhizal fungal biodiversity and microbial biomass C:N:P stoichiometry of cyanobacterial biocrusts. For. Ecol. Manag. 2022, 503, 119763. [Google Scholar] [CrossRef]
- Song, F.Q.; Qi, D.D.; Liu, X.; Kong, X.S.; Gao, Y.; Zhou, Z.X.; Wu, Q. Proteomic analysis of symbiotic proteins of Glomus mosseae and Amorpha fruticosa. Sci. Rep. 2015, 5, 18031. [Google Scholar] [CrossRef]
- Detheridge, A.P.; Brand, G.; Fychan, R.; Crotty, F.V.; Sanderson, R.; Griffith, G.W.; Marley, C.L. The legacy effect of cover crops on soil fungal populations in a cereal rotation. Agric. Ecosyst. Environ. 2016, 228, 49–61. [Google Scholar] [CrossRef]
- Redecker, D.; Berswordt-Wallrabe, P.V.; Beck, D.P.; Werner, D. Influence of inoculation with arbuscular mycorrhizal fungi on stable isotopes of nitrogen in Phaseolus vulgaris. Biol. Fert. Soils. 1997, 24, 344–346. [Google Scholar] [CrossRef]
- Zhao, B.; Trouvelot, A.; Gianinazzi, S.; Gianinazzi-Pearson, V. Influence of two legume species on hyphal production and activity of two arbuscular mycorrhizal fungi. Mycorrhiza 1997, 7, 179–185. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 32. [Google Scholar]
- Tessier, A.P.; Campbell, P.G.C.; Bisson, M.X. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Kormanik, P.P.; Bryan, W.C.; Schultz, R.C. Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay. Can. J. Microbiol. 1980, 26, 536–538. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Rizwan, M.; Sultan, T.; Chaudhary, H.J.; Isik, M.; Aydin, O. Effects of Rhizophagus clarus and biochar on growth, photosynthesis, nutrients, and cadmium (Cd) concentration of maize (Zea mays) grown in Cd-spiked soil. Environ. Sci. Pollut. Res. 2019, 26, 20689–20700. [Google Scholar] [CrossRef]
- Gai, J.P.; Fan, J.Q.; Zhang, S.B.; Mi, N.N.; Christie, P.; Li, X.L.; Feng, G. Direct effects of soil cadmium on the growth and activity of arbuscular mycorrhizal fungi. Rhizosphere 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Del Val, C.; Barea, J.M.; Azcon-Aguilar, C. Diversity of arbuscular mycorrhizal fungus populations in heavy-metal-contaminated soils. Appl. Environ. Microbiol. 1999, 65, 718–723. [Google Scholar] [CrossRef]
- Dandan, Z.; Tao, L.I.; Zhiwei, Z. Research advances in arbuscular mycorrhizal fungi-legumes-rhizobia symbiosis. Chin. J. Ecol. 2006, 25, 327–333. [Google Scholar]
- Chen, Z.; Ai, Y.; Fang, C.; Wang, K.; Li, W.; Liu, S.; Li, C.; Xiao, J.; Huang, Z. Distribution and phytoavailability of heavy metal chemical fractions in artificial soil on rock cut slopes alongside railways. J. Hazard. Mater. 2014, 273, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediat. 2019, 21, 849–856. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.B.; Martinez, C.E. Copper phytotoxicity in a contaminated soil: Remediation tests with adsorptive materials. Environ. Sci. Technol. 2000, 34, 4386–4391. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.B.; Shi, H.D.; Li, S.N.; Chen, S.B. Yielding hydroxyl radicals in the Fenton-like reaction induced by manganese (II) oxidation determines Cd mobilization upon soil aeration in paddy soil systems. Environ. Pollut. 2022, 292, 118311. [Google Scholar] [CrossRef]
- Stec, M.; Jagustyn, B.; Slowik, K.; Sciazko, M.; Iluk, T. Influence of High Chloride Concentration on pH Control in Hydroxide Precipitation of Heavy Metals. J. Sustain. Metall. 2020, 6, 239–249. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Wang, Z.X.; Du, C.Y.; Liu, W.L.; Gerson, A.R.; Pi, K.W. Properties and heavy metal leaching characteristics of leachate sludge-derived biochar. Water Environ. Res. 2021, 93, 3064–3074. [Google Scholar] [CrossRef]
- Mortimer, R.J.G.; Rae, J.E. Metal speciation (Cu, Zn, Pb, Cd) and organic matter in oxic to suboxic salt marsh sediments, Severn Estuary, southwest Britain. Mar. Pollut. Bull. 2000, 40, 377–386. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable Management with Mycorrhizae and Phosphate Solubilizing Bacteria for Enhanced Phosphorus Uptake in Calcareous Soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Treseder, K.K.; Allen, E.B.; Egerton-Warburton, L.M.; Hart, M.M.; Klironomos, J.N.; Maherali, H.; Tedersoo, L. Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait-based predictive framework. J. Ecol. 2018, 106, 480–489. [Google Scholar] [CrossRef]
- He, Y.M.; Fan, X.M.; Zhang, G.Q.; Li, B.; Li, T.G.; Zu, Y.Q.; Zhan, F.D. Effects of arbuscular mycorrhizal fungi and dark septate endophytes on maize performance and root traits under a high cadmium stress. S. Afr. J. Bot. 2020, 134, 415–423. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Yue, X.; He, Y.; Xia, Y.; Wang, Y. Arbuscular mycorrhizal fungi enhance antioxidant defense in the leaves and the retention of heavy metals in the roots of maize. Environ. Sci. Pollut. Res. 2018, 25, 24338–24347. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Kang, Y.; So, P.S.; Ng, C.W.W.; Wong, M.H. Arbuscular mycorrhizal fungi increase the proportion of cellulose and hemicellulose in the root stele of vetiver grass. Plant Soil 2018, 425, 309–319. [Google Scholar] [CrossRef]
- Rasouli-Sadaghiani, M.H.; Barin, M.; Khodaverdiloo, H.; Moghaddam, S.S.; Damalas, C.A.; Kazemalilou, S. Arbuscular Mycorrhizal Fungi and Rhizobacteria Promote Growth of Russian Knapweed (Acroptilon repens L.) in a Cd-Contaminated Soil. J. Plant Growth Regul. 2019, 38, 113–121. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghili, F.; Jafarinia, R. Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ. Sci. Pollut. Res. 2019, 26, 30794–30807. [Google Scholar] [CrossRef]
- He, S.; Wu, Q.; He, Z. Growth-Promoting Hormone DA-6 Assists Phytoextraction and Detoxification of Cd by Ryegrass. Int. J. Phytoremediat. 2015, 17, 597–603. [Google Scholar] [CrossRef]

| Parameters | Soil Properties | Sand Properties |
|---|---|---|
| pH | 8.43 ± 0.01 | 8.30 ± 0.01 |
| MBC (mg/kg) | 18.39 ± 0.09 | 19.26 ± 0.87 |
| MBN (mg/kg) | 3.61 ± 0.08 | 2.01 ± 0.07 |
| A-P (mg/kg) | 0.89 ± 0.04 | 1.51 ± 0.11 |
| NH4+-N (mg/kg) | 19.69 ± 0.57 | 12.15 ± 0.34 |
| NO3−-N (mg/kg) | 5.94 ± 0.14 | 3.67 ± 0.08 |
| SOC (g/kg) | 13.6 ± 0.05 | 4.01 ± 0.06 |
| TC (g/kg) | 15.10 ± 0.41 | 13.43 ± 0.24 |
| TN (g/kg) | 1.10 ± 0.20 | 3.98 ± 0.01 |
| Total Cd (mg/kg) | 0.87 ± 0.01 | 0.35 ± 0.01 |
| Treatment | CK | AMF | Cd5 | Cd5 + AMF | Cd15 | Cd15 + AMF | |
|---|---|---|---|---|---|---|---|
| L. perenne | pH | 9.44 ± 0.02 b | 9.54 ± 0.05 ab | 9.59 ± 0.02 a | 9.54 ± 0.07 ab | 9.51 ± 0.02 ab | 9.49 ± 0.02 ab |
| Available phosphorus (mg/kg) | 8.07 ± 0.9 a | 2.88 ± 0.5 c | 1.47 ± 0.18 c | 5.66 ± 1.4 ab | 5.64 ± 0.9 ab | 3.75 ± 0.77 bc | |
| NH4+-N (mg/kg) | 25.45 ± 2.62 ab | 23.82 ± 1.04 b | 24.45 ± 2.22 ab | 27.22 ± 1.63 ab | 25.95 ± 2.11 ab | 30.21 ± 1.46 a | |
| NO3−-N (mg/kg) | 0.44 ± 0.14 b | 0.79 ± 0.05 b | 0.72 ± 0.12 b | 0.99 ± 0.24 b | 0.91 ± 0.12 b | 1.52 ± 0.26 a | |
| Neutral phosphatase (U/g) | 0.1 ± 0.01 a | 0.11 ± 0.01 a | 0.1 ± 0.0042 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a | |
| Alkaline phosphatase (U/g) | 0.15 ± 0.01 a | 0.16 ± 0.01 a | 0.14 ± 0.01 a | 0.15 ± 0.01 a | 0.15 ± 0.01 a | 0.15 ± 0.01 a | |
| A. fruticosa | pH | 9.23 ± 0.08 a | 9.3 ± 0.04 a | 9.42 ± 0.02 a | 9.43 ± 0.04 a | 9.37 ± 0.02 a | 9.48 ± 0.04 a |
| Available phosphorus (mg/kg) | 1.5 ± 0.15 b | 2.11 ± 0.18 b | 1.98 ± 0.57 b | 2.16 ± 0.27 b | 6.46 ± 0.51 a | 5.96 ± 0.67 a | |
| NH4+-N (mg/kg) | 29.35 ± 2.85 a | 30.08 ± 0.65 a | 31.88 ± 1.32 a | 31.7 ± 2.09 a | 29.82 ± 1.06 a | 37.53 ± 4.58 a | |
| NO3−-N (mg/kg) | 4.3 ± 0.52 ab | 6.51 ± 0.61 a | 1.74 ± 0.47 b | 5.13 ± 0.8 ab | 1.73 ± 0.17 b | 2.87 ± 0.52 ab | |
| Neutral phosphatase (U/g) | 0.06 ± 0.001 a | 0.06 ± 0.0029 a | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.0018 a | |
| Alkaline phosphatase (U/g) | 0.14 ± 0.01 b | 0.18 ± 0.01 a | 0.16 ± 0.01 ab | 0.15 ± 0.01 ab | 0.17 ± 0.01 a | 0.14 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Jia, Q.; Li, Y.; Dong, K.; Xu, S.; Ren, Y.; Zhang, T.; Chen, J.; Shi, N.; Fu, S. Effect of Arbuscular Mycorrhiza Fungus Diversispora eburnea Inoculation on Lolium perenne and Amorpha fruticosa Growth, Cadmium Uptake, and Soil Cadmium Speciation in Cadmium-Contaminated Soil. Int. J. Environ. Res. Public Health 2023, 20, 795. https://doi.org/10.3390/ijerph20010795
Sun J, Jia Q, Li Y, Dong K, Xu S, Ren Y, Zhang T, Chen J, Shi N, Fu S. Effect of Arbuscular Mycorrhiza Fungus Diversispora eburnea Inoculation on Lolium perenne and Amorpha fruticosa Growth, Cadmium Uptake, and Soil Cadmium Speciation in Cadmium-Contaminated Soil. International Journal of Environmental Research and Public Health. 2023; 20(1):795. https://doi.org/10.3390/ijerph20010795
Chicago/Turabian StyleSun, Jiahua, Qiong Jia, Yi Li, Kanglong Dong, Shuai Xu, Yanan Ren, Ting Zhang, Jiayuan Chen, Nannan Shi, and Shenglei Fu. 2023. "Effect of Arbuscular Mycorrhiza Fungus Diversispora eburnea Inoculation on Lolium perenne and Amorpha fruticosa Growth, Cadmium Uptake, and Soil Cadmium Speciation in Cadmium-Contaminated Soil" International Journal of Environmental Research and Public Health 20, no. 1: 795. https://doi.org/10.3390/ijerph20010795
APA StyleSun, J., Jia, Q., Li, Y., Dong, K., Xu, S., Ren, Y., Zhang, T., Chen, J., Shi, N., & Fu, S. (2023). Effect of Arbuscular Mycorrhiza Fungus Diversispora eburnea Inoculation on Lolium perenne and Amorpha fruticosa Growth, Cadmium Uptake, and Soil Cadmium Speciation in Cadmium-Contaminated Soil. International Journal of Environmental Research and Public Health, 20(1), 795. https://doi.org/10.3390/ijerph20010795






