Efficient Remediation of Cadmium- and Lead-Contaminated Water by Using Fe-Modified Date Palm Waste Biochar-Based Adsorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Production
2.2. Biochar Modification
2.3. Characterization
2.4. Adsorption Experiments
3. Results and Discussion
3.1. This Adsorbent Characterization
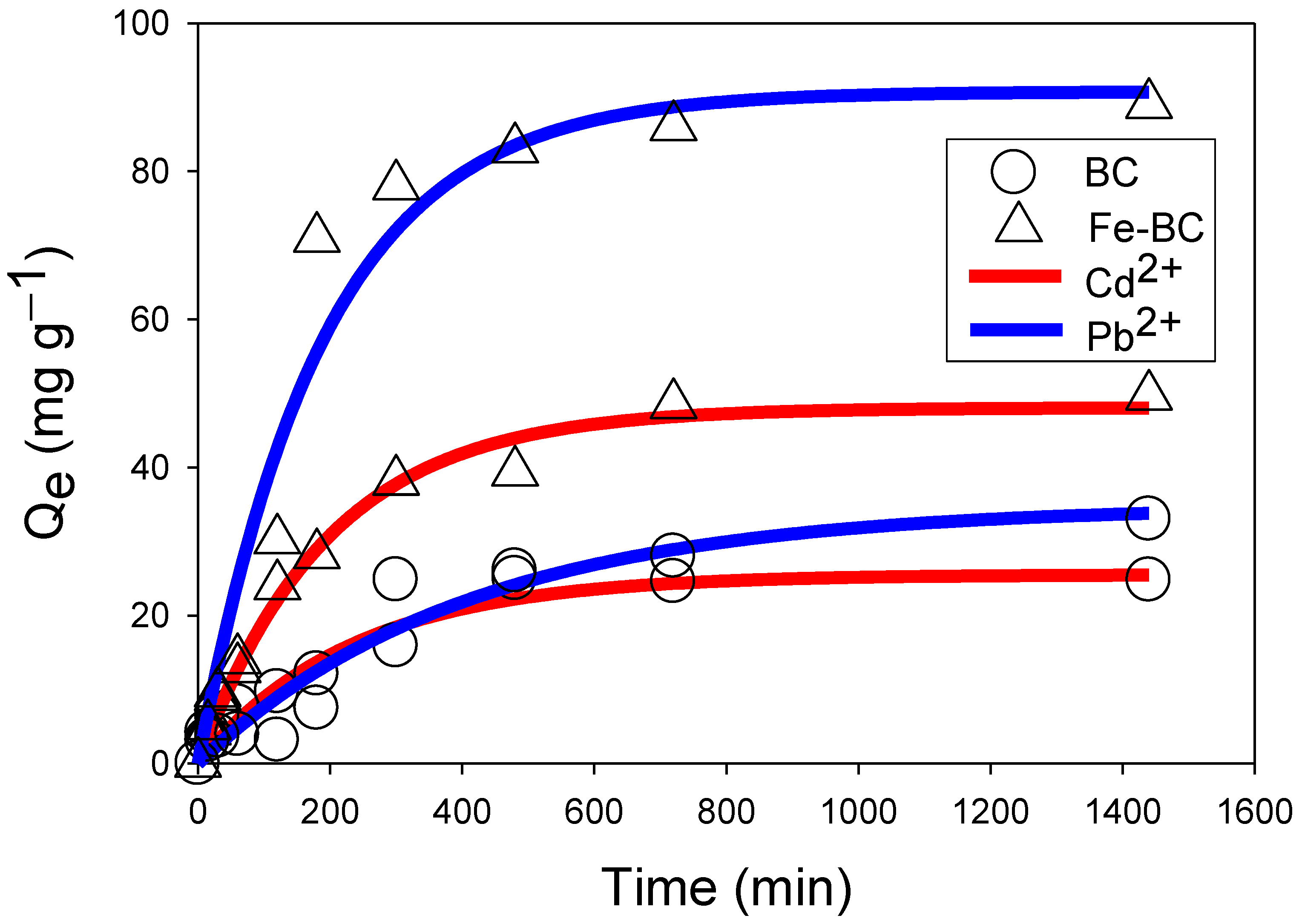
3.2. Kinetic Adsorption of Cd2+ or Pb2+
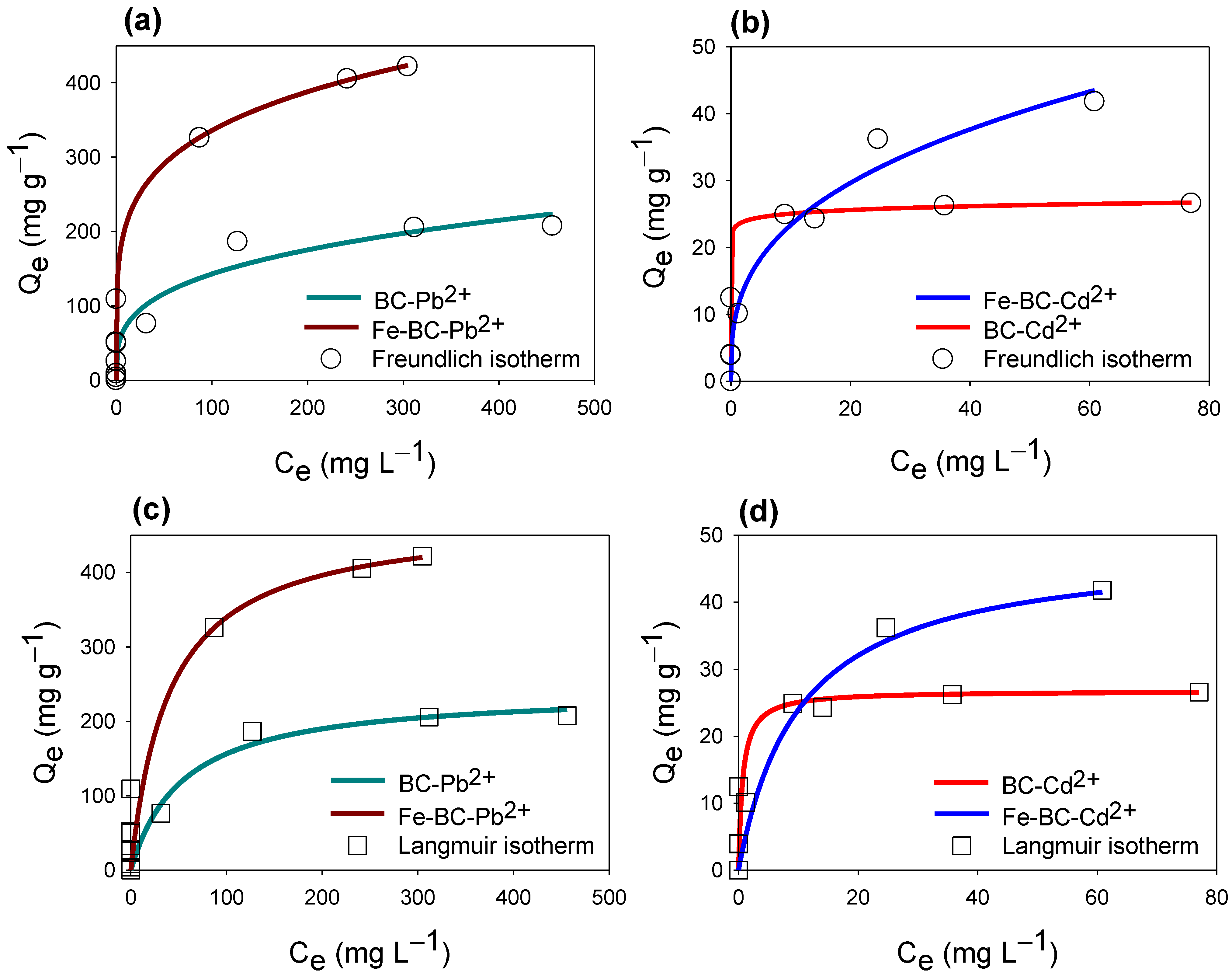
3.3. Equilibrium Adsorption of Cd2+ or Pb2+
3.4. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- UN-Water. 2018 UN World Water Development Report, Nature-Based Solutions for Water; UNESCO: Paris, France, 2018. [Google Scholar]
- UNICEF; WHO. Progress on Drinking Water, Sanitation and Hygiene; UNICEF: New York, NY, USA; WHO: Geneva, Switzerland, 2017.
- Pandit, A.B.; Kumar, J.K. Clean Water for Developing Countries. Annal. Rev. Chem. Biomol. Eng. 2015, 6, 217–246. [Google Scholar] [CrossRef]
- Ali, I. The Quest for Active Carbon Adsorbent Substitutes: Inexpensive Adsorbents for Toxic Metal Ions Removal from Wastewater. Separ. Purif. Rev. 2010, 39, 95–171. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Brit. Medic. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, H.N.M.E.; Obidul Huq, A.K.; binti Yahya, R. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Biosorption of copper (II) and lead (II) from aqueous solutions by nonliving green algae Cladophora fascicularis: Equilibrium, kinetics and environmental effects. Adsorption 2006, 12, 267–277. [Google Scholar] [CrossRef]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.A.; Al-Wabel, M.I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manag. 2019, 246, 214–228. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrol. 2021, 155, 105081. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef]
- Farrell, M.; Macdonald, L.M.; Butler, G.; Chirino-Valle, I.; Condron, L.M. Biochar and fertiliser applications influence phosphorus fractionation and wheat yield. Biol. Fertil. Soils 2014, 50, 169–178. [Google Scholar] [CrossRef]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol. Fertil. Soils 2014, 50, 695–702. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Reddy, D.H.; Lee, S.M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Ahmad, M.; Usman, A.R.; Rafique, M.I.; Al-Wabel, M.I. Engineered biochar composites with zeolite, silica, and nano-zerovalent iron for the efficient scavenging of chlortetracycline from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 15136–15152. [Google Scholar] [CrossRef]
- Ok, Y.S.; Chang, S.X.; Gao, B.; Chung, H.-J. SMART biochar technology—A shifting paradigm towards advanced materials and healthcare research. Environ. Technol. Innov. 2015, 4, 206–209. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Conver. Biorefin. 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Wu, Z.; Zhang, L.; Zeng, G.; Zhou, C. Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 85–90. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Li, R.; Ali, A.; Chen, A.; Zhang, Z. Enhanced aqueous Cr (VI) removal using chitosan-modified magnetic biochars derived from bamboo residues. Chemosphere 2020, 261, 127694. [Google Scholar] [CrossRef] [PubMed]
- ASTM D1762-84; Standard Methods for Chemical Analysis of Wood Charcoal. American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 1989.
- Richard, L.A. Diagnoses and Improvement of Saline and Alkali Soils; Agriculture Handbook, 60: Washington, DC, USA, 1954. [Google Scholar]
- Ahmad, M.; Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Abduljabbar, A.S.; Al-Wabel, M.I. Biochar composites with nano zerovalent iron and eggshell powder for nitrate removal from aqueous solution with coexisting chloride ions. Environ. Sci. Pollut. Res. 2018, 26, 25757–25771. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Singh, R.; Misra, V.; Singh, R.P. Synthesis, characterization and role of zero-valent iron nanoparticle in removal of hexavalent chromium from chromium-spiked soil. J. Nanopart. Res. 2011, 13, 4063–4073. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Mayakaduwa, S.S.; Kumarathilaka, P.; Herath, I.; Ahmad, M.; Al-Wabel, M.; Ok, Y.S.; Usman, A.; Abduljabbar, A.; Vithanage, M. Equilibrium and kinetic mechanisms of woody biochar on aqueous glyphosate removal. Chemosphere 2016, 144, 2516–2521. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, T.; Liu, Y.; Yan, L.; Wei, Q.; Du, B.; Xu, W. EDTA functionalized magnetic biochar for Pb (II) removal: Adsorption performance, mechanism and SVM model prediction. Sep. Purif. Technol. 2019, 227, 115696. [Google Scholar] [CrossRef]
- Ho, S.H.; Wang, D.; Wei, Z.S.; Chang, J.S.; Ren, N.Q. Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis. Bioresour. Technol. 2018, 247, 463–470. [Google Scholar]
- Oladipo, A.A.; Ahaka, E.O.; Gazi, M. High adsorptive potential of calcined magnetic biochar derived from banana peels for Cu2+, Hg2+, and Zn2+ ions removal in single and ternary systems. Environ. Sci. Pollut. Res. 2019, 31, 31887–31899. [Google Scholar] [CrossRef] [PubMed]
- Zahedifar, M.; Seyedi, N.; Shafiei, S.; Basij, M. Surface-modified magnetic biochar: Highly efficient adsorbents for removal of Pb(ΙΙ) and Cd(ΙΙ). Mater. Chem. Phys. 2021, 271, 124860. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman Jr, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Trakal, L.; Bingöl, D.; Pohořelý, M.; Hruška, M.; Komárek, M. Geochemical and spectroscopic investigations of Cd and Pb sorption mechanisms on contrasting biochars: Engineering implications. Bioresour. Technol. 2014, 171, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kong, L.; Qu, Z.; Li, L.; Shen, G. Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain. Chem. Eng. 2015, 3, 125–132. [Google Scholar] [CrossRef]
- Yap, M.W.; Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Qiu, W.; Islam, M.S.; Song, Z. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere 2020, 246, 125701. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 7, 5223–5232. [Google Scholar] [CrossRef]
- Ni, B.J.; Huang, Q.S.; Wang, C.; Ni, T.Y.; Sun, J.; Wei, W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, J.Q.; Huang, L.; Yuan, Z.H.; Li, Z.J.; Liu, M.C. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Integr. Agric. 2019, 1, 201–210. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Inter. Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef] [PubMed]
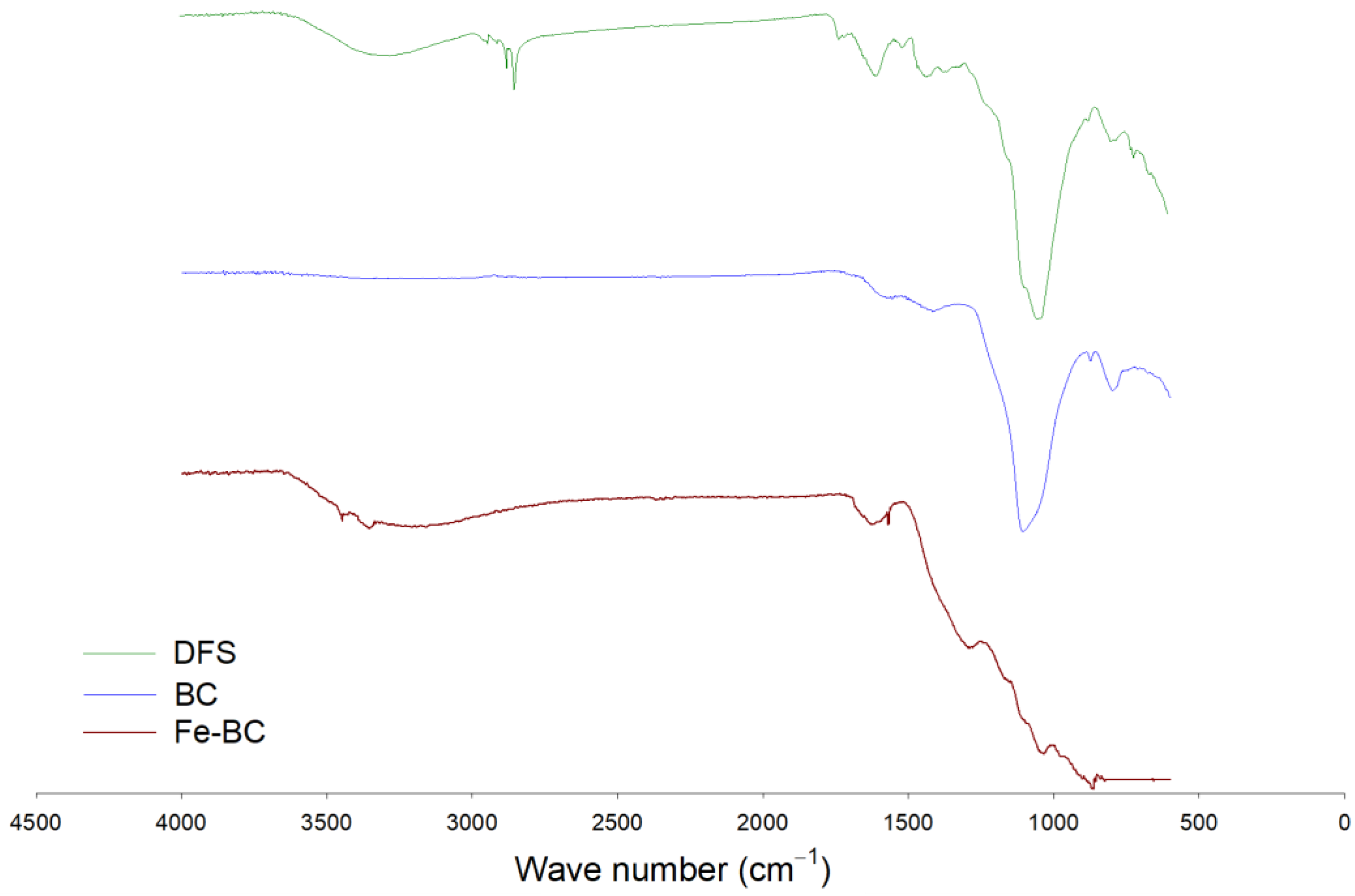
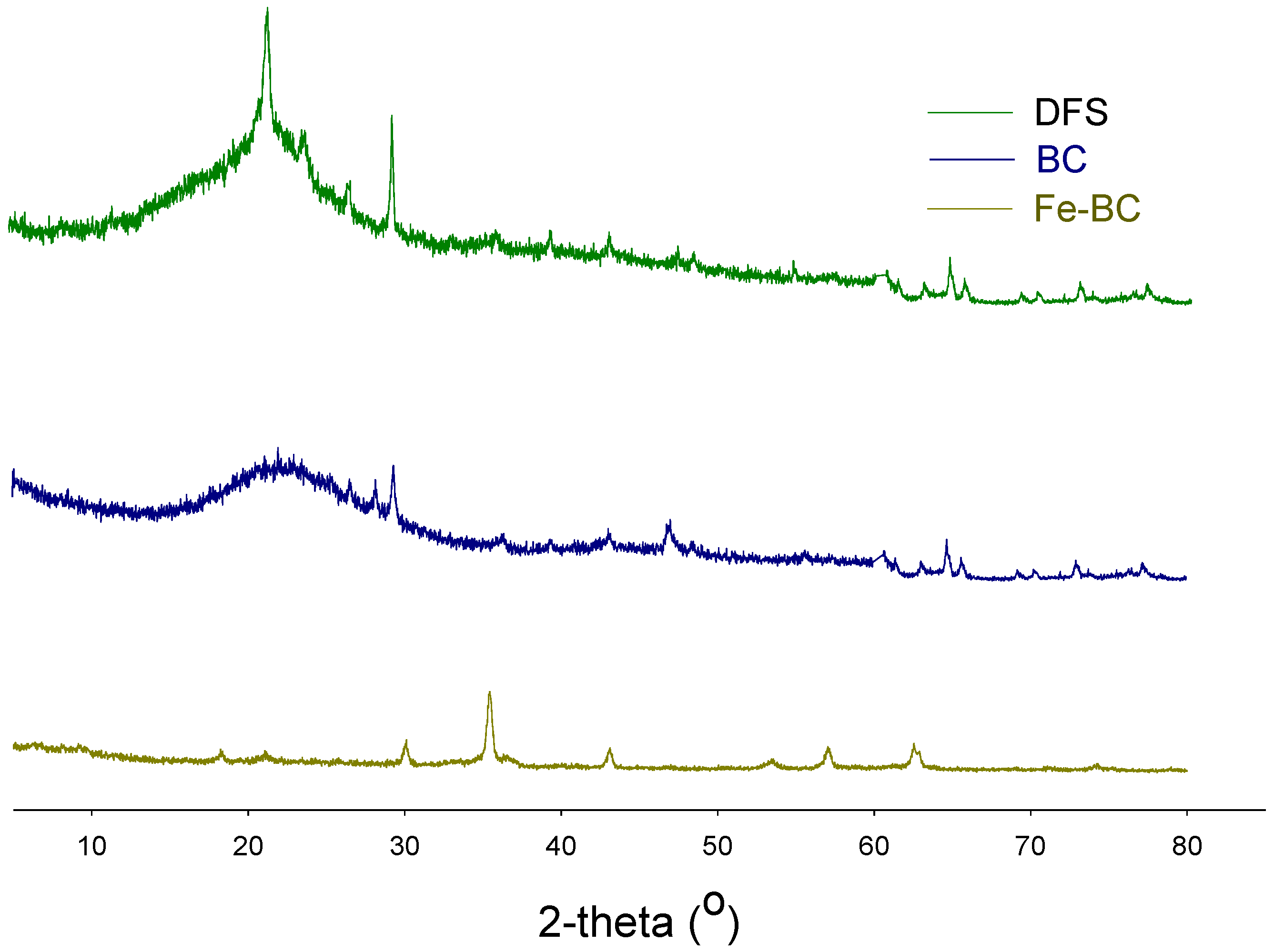
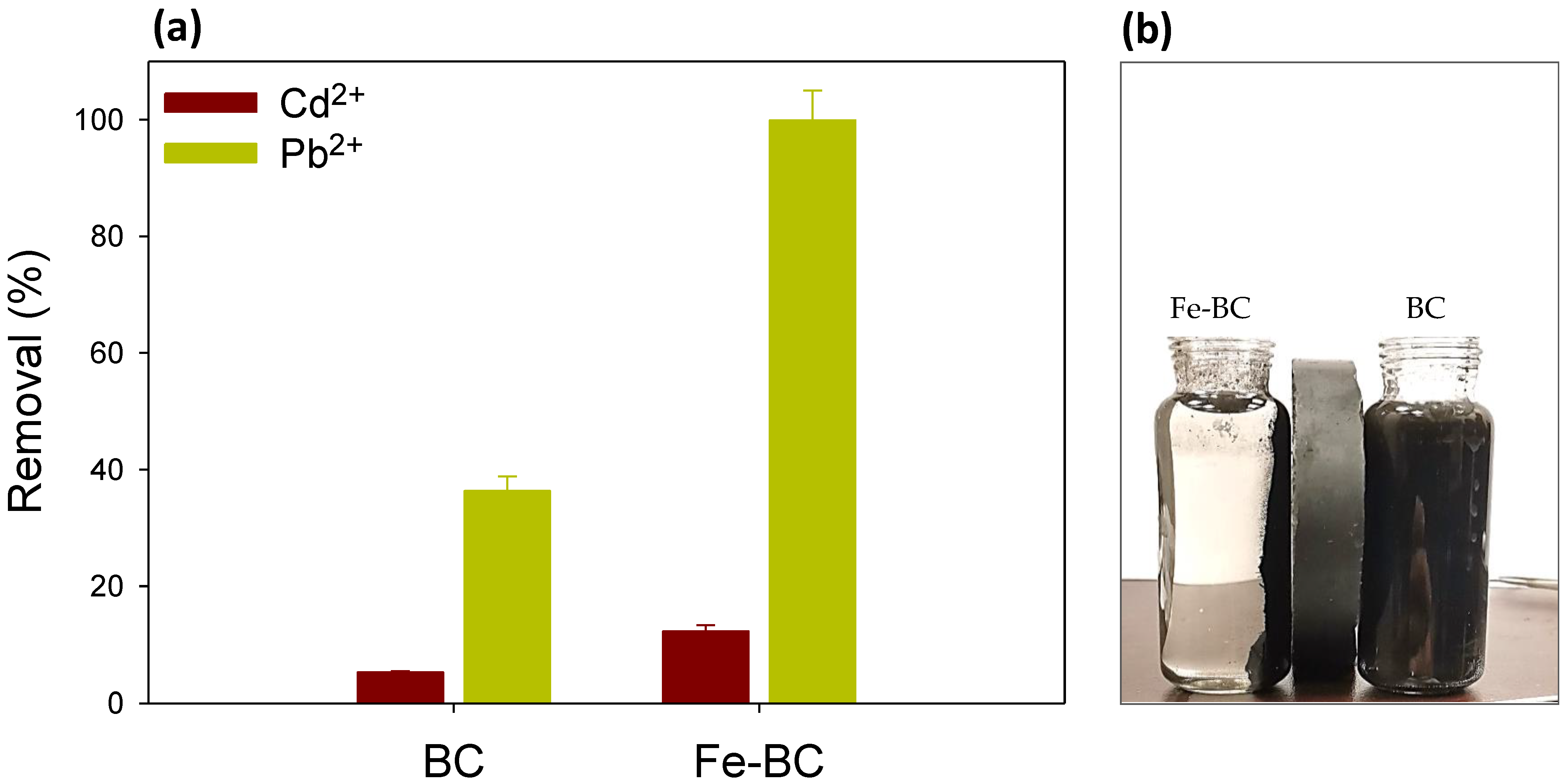
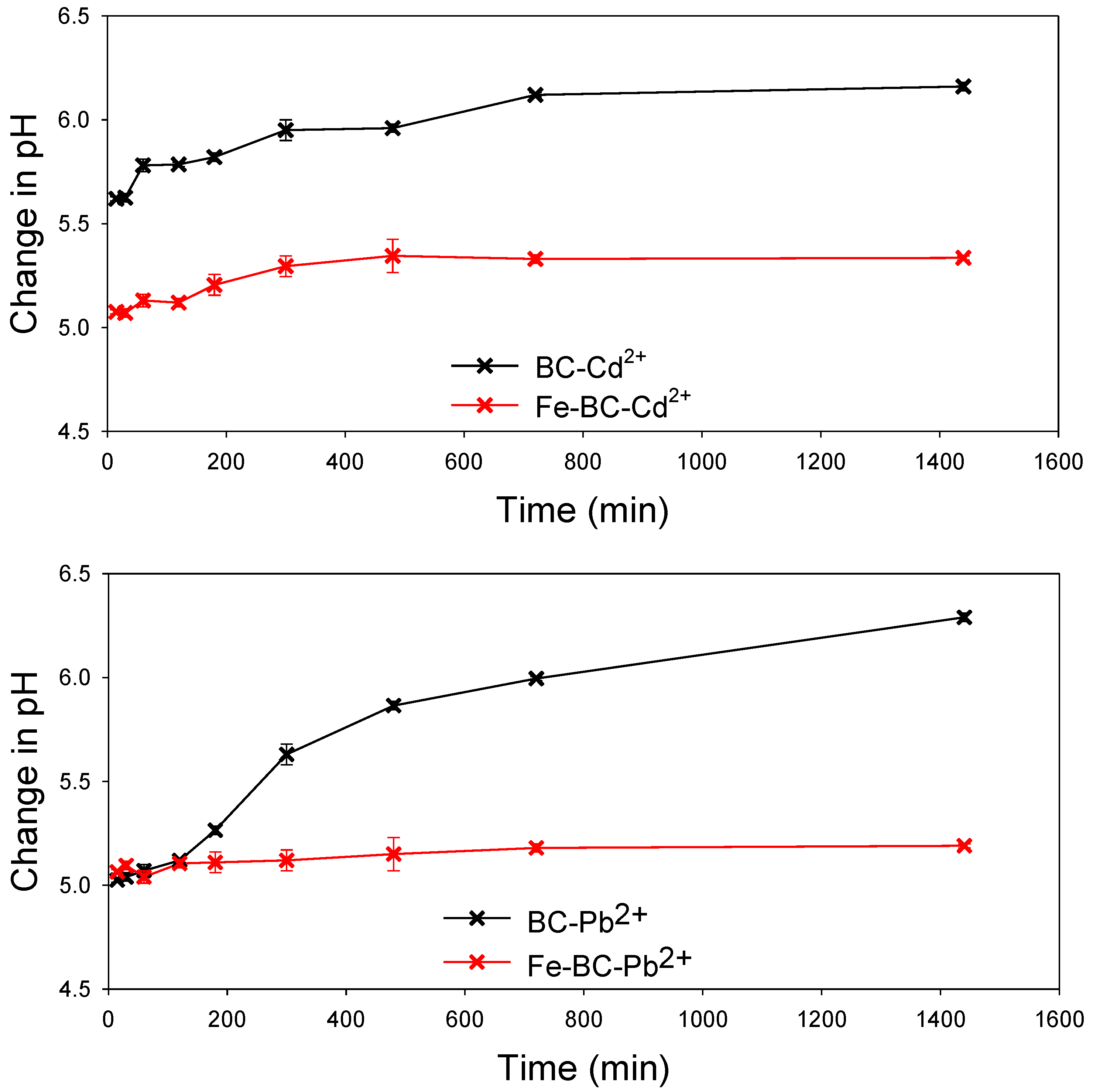
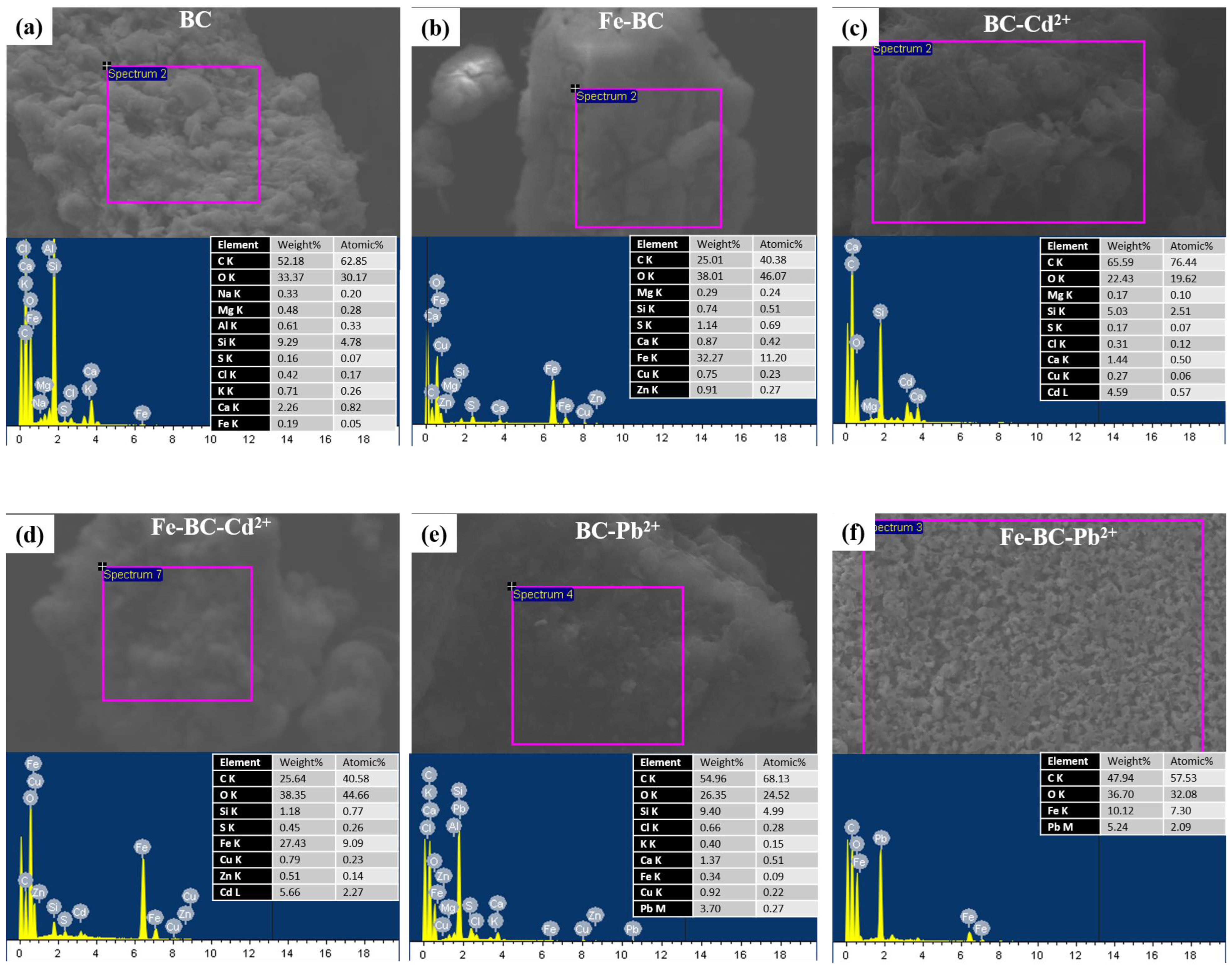
| Yield (%) | Moisture (%) | Volatile Matter (%) | Resident Matter (%) | Ash (%) | pH | Cation Exchange Capacity (cmol kg−1) | Fe Contents (%) | |
|---|---|---|---|---|---|---|---|---|
| DFS | - | 3.21 ± 0.12 | 62.88 ± 2.14 | 18.51 ± 1.26 | 15.45 ± 1.08 | 6.78 ± 0.24 | 43.78 ± 2.74 | - |
| BC | 35.47 ± 3.25 | 1.02 ± 0.06 | 16.13 ± 1.87 | 56.79 ± 2.54 | 25.98 ± 2.81 | 9.26 ± 0.39 | 63.19 ± 3.52 | 0.12 ± 0.00 |
| Fe-BC | - | 2.08 ± 0.14 | 13.47 ± 1.58 | 33.49 ± 2.11 | 51.03 ± 1.25 | 7.12 ± 0.10 | 81.74 ± 3.78 | 19.05 ± 2.11 |
| C (%) | H (%) | N (%) | O (%) | S (%) | Surface Area (m2 g−1) | Pore Size (nm) | Total Volume in Pores (cm3 g−1) | |
|---|---|---|---|---|---|---|---|---|
| DFS | 41.54 | 11.87 | 3.08 | 43.45 | 0 | 3.28 | 31.71 | 0.00019 |
| BC | 68.82 | 6.46 | 1.12 | 24.13 | 0 | 68.49 | 9.18 | 0.0983 |
| Fe-BC | 72.98 | 9.36 | 1.23 | 16.77 | 0 | 119.82 | 4.72 | 0.1590 |
| Adsorption of Cd2+ | Adsorption of Pb2+ | ||||
|---|---|---|---|---|---|
| BC | Fe-BC | BC | Fe-BC | ||
| Pseudo-first order | k1′ | −2.6 × 10−3 | −3.0 × 10−3 | −2.5 × 10−3 | −3.3 × 10−3 |
| qe | 2.79 | 3.62 | 3.43 | 4.10 | |
| R2 | 0.73 | 0.86 | 0.95 | 0.87 | |
| Pseudo-second order | k2′ | 2.9 × 104 | 1.3 × 104 | 4.0 × 10−5 | 4.6 × 10−5 |
| qe | 27.12 | 54.49 | 45.89 | 105.49 | |
| h | 0.23 | 0.39 | 0.08 | 0.52 | |
| R2 | 0.97 | 0.98 | 0.93 | 0.95 | |
| Elovich | a | 3.90 | 7.95 | 4.95 | 15.60 |
| β | −4.92 | −11.10 | −9.42 | −25.06 | |
| R2 | 0.84 | 0.88 | 0.68 | 0.79 | |
| Intraparticle diffusion | kid | 0.74 | 1.48 | 1.03 | 2.88 |
| c | 2.27 | 3.85 | 1.66 | 4.61 | |
| R2 | 0.74 | 0.90 | 0.87 | 0.79 | |
| Power function | kf | 0.46 | 0.58 | 0.52 | 0.70 |
| b | 0.13 | 0.13 | 0.10 | 0.02 | |
| R2 | 0.98 | 0.97 | 0.85 | 0.95 | |
| Sorbents | Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|---|
| QL (mg g−1) | KL (L g−1) | R2 | KF (L g−1) | 1/n | R2 | ||
| Cd2+ | BC | 26.78 | 1.453 | 0.86 | 10.54 | 0.345 | 0.76 |
| Fe-BC | 48.44 | 0.097 | 0.96 | 23.30 | 0.031 | 0.78 | |
| Pb2+ | BC | 160.07 | 0.013 | 0.96 | 36.66 | 0.295 | 0.92 |
| Fe-BC | 475.14 | 0.025 | 0.94 | 129.06 | 0.207 | 0.94 | |
| Adsorbent | Modification Type | Heavy Metal | Maximum Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|---|
| BC | - | Cd2+ | 26.78 | This study |
| Pb2+ | 160.07 | |||
| Fe-BC | Fe3+/Fe2+ | Cd2+ | 48.44 | |
| Pb2+ | 475.14 | |||
| Oak bark | Fe2+/Fe3+ SO4 solution | Cd2+ | 7.40 | [37] |
| Pb2+ | 30. | |||
| Oak wood | Fe2+/Fe3+ SO4 solution | Cd2+ | 2.87 | |
| Pb2+ | 10.10 | |||
| Wheat straw | FeSO47H2O | Cd2+ | 75.30 | [38] |
| Pb2+ | 311.0 | |||
| Grape husk | FeSO47H2O | Cd2+ | 38.30 | |
| Pb2+ | 204 | |||
| Rice hull | Fe(acac)3 + calcination | Pb2+ | 23.90 | [39] |
| Rice hull | Fe(acac)3 + calcination + ZnS | Pb2+ | 368.0 | |
| Coconut shell | FeCl3·6H2O | Cd2+ | 3.846 | [40] |
| Pb2+ | 4.097 | |||
| Corn straw | 600 °C BC + Ferric nitrate + calcination | Cd2+ | 28.71 | [41] |
| 800 °C BC + Ferric nitrate + calcination | 46.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.G.; Alasmary, Z. Efficient Remediation of Cadmium- and Lead-Contaminated Water by Using Fe-Modified Date Palm Waste Biochar-Based Adsorbents. Int. J. Environ. Res. Public Health 2023, 20, 802. https://doi.org/10.3390/ijerph20010802
Alghamdi AG, Alasmary Z. Efficient Remediation of Cadmium- and Lead-Contaminated Water by Using Fe-Modified Date Palm Waste Biochar-Based Adsorbents. International Journal of Environmental Research and Public Health. 2023; 20(1):802. https://doi.org/10.3390/ijerph20010802
Chicago/Turabian StyleAlghamdi, Abdulaziz G., and Zafer Alasmary. 2023. "Efficient Remediation of Cadmium- and Lead-Contaminated Water by Using Fe-Modified Date Palm Waste Biochar-Based Adsorbents" International Journal of Environmental Research and Public Health 20, no. 1: 802. https://doi.org/10.3390/ijerph20010802
APA StyleAlghamdi, A. G., & Alasmary, Z. (2023). Efficient Remediation of Cadmium- and Lead-Contaminated Water by Using Fe-Modified Date Palm Waste Biochar-Based Adsorbents. International Journal of Environmental Research and Public Health, 20(1), 802. https://doi.org/10.3390/ijerph20010802






