Sources, Migration, Transformation, and Environmental Effects of Organic Carbon in Eutrophic Lakes: A Critical Review
Abstract
:1. Introduction
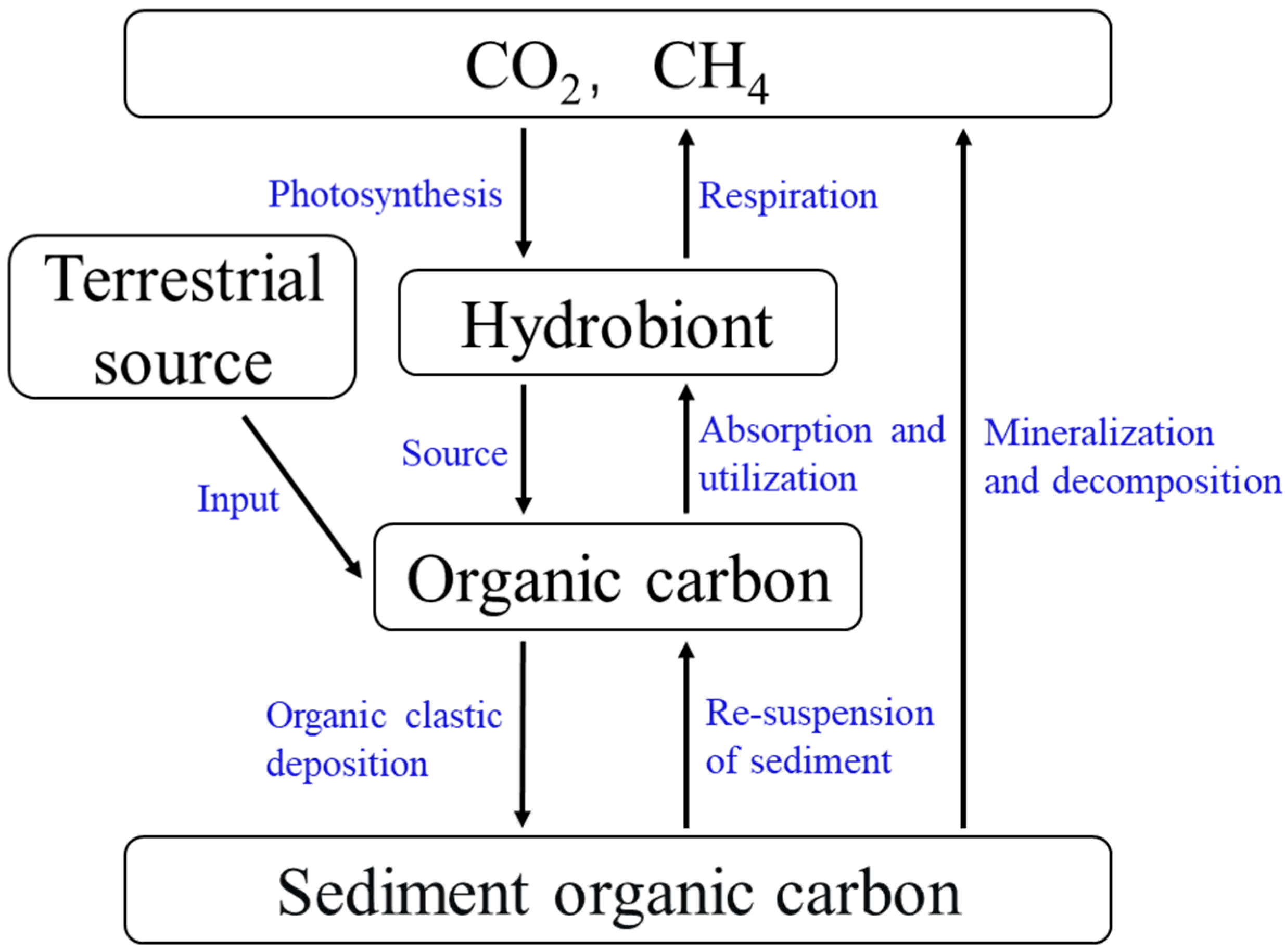
2. OC Source Analysis
2.1. Oc Sources
2.2. OC Tracing Technology
2.2.1. Carbon Stable Isotope
2.2.2. Biomarker Method
N-alkanes Tracing
Fatty Acid Tracing
2.2.3. Three-Dimensional Fluorescence Spectroscopy (3D-EEMs)
2.2.4. Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS)
3. OC Migration and Transformation
3.1. Migration and Transformation Processes of Oc at Different Phase Interfaces
3.1.1. Aqueous Phase–Gas Phase Interface
3.1.2. Aqueous Phase–Sediment Interface
3.2. Migration and Transformation Characteristics of Algae-Derived OC
3.3. Driving Mechanisms
3.3.1. Microbial Processes
3.3.2. Photodegradation Mechanisms
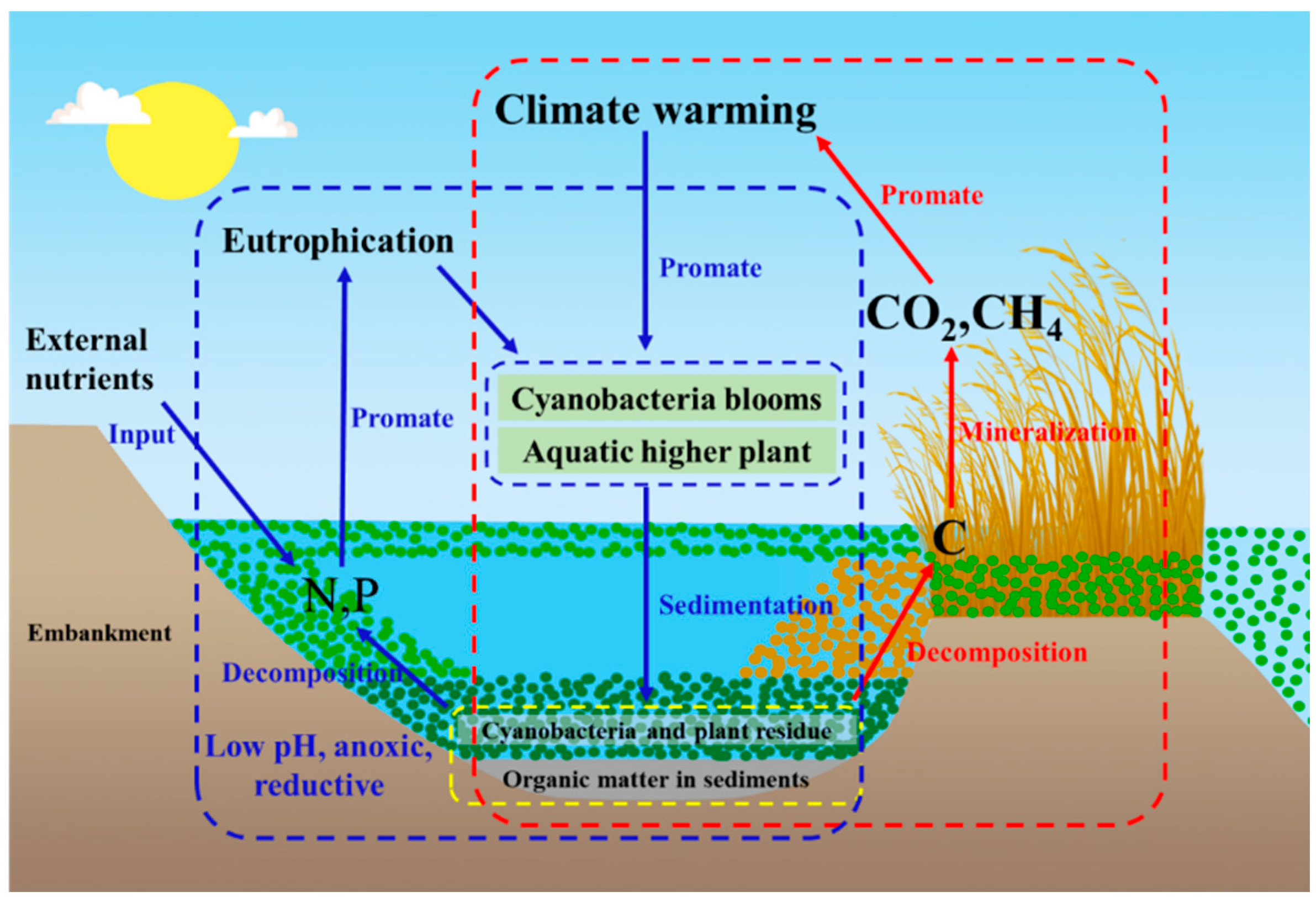
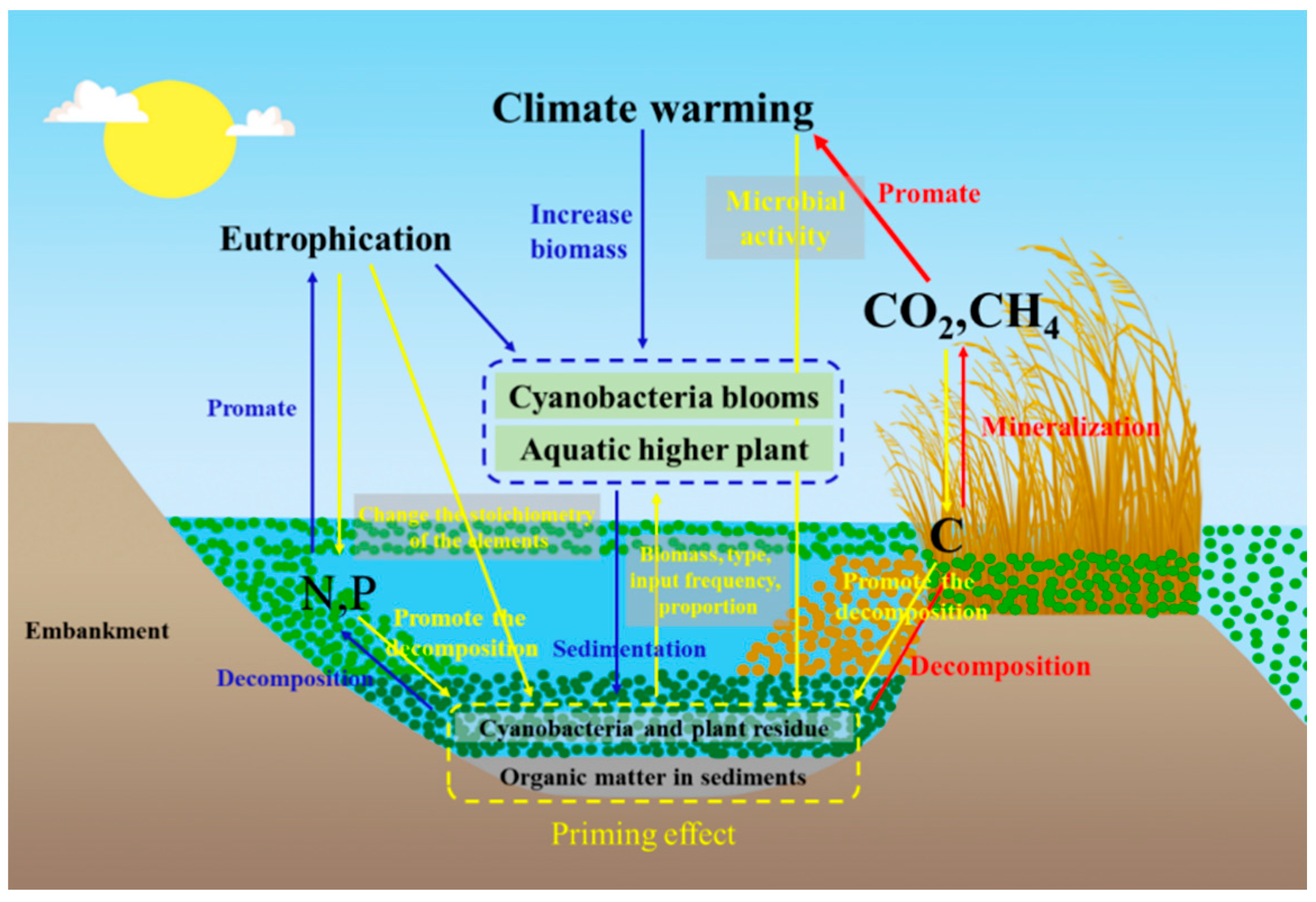
4. Environmental Effects of OC
4.1. Eutrophication Aggravates the Formation of Anaerobic Environments in Water Columns
4.2. Eutrophication Aggravates the Release of Nitrogen and Phosphorus Elements
4.3. Eutrophication Promotes the Emission of Odoriferous Substances
4.4. Eutrophication Enhances Greenhouse Gas Emissions
4.5. Eutrophication Enhances “Lake Flooding” and “Black Water Mass”
5. Outlook on Eutrophication and Carbon Cycling Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.L.; Xing, P.; Li, H.B.; Zeng, J. Impacts of regime shift between phytoplankton and macrophyte on the microbial community structure and its carbon cycling in lakes. Microbiol. China 2013, 40, 87–97. [Google Scholar]
- Pilcher, D.J.; McKinley, G.A.; Bootsma, H.A.; Bennington, V. Physical and biogeochemical mechanisms of internal carbon cycling in Lake Michigan. J. Geophys. Res. Oceans 2015, 120, 2112–2128. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.J.; Caraco, N.F.; Kling, G.W.; Kratz, T.K. Carbon dioxide supersaturation in the surface waters of lakes. Science 1994, 265, 1568–1570. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.G.; Yang, H.Q.; Zeng, Y.; Guo, J.Y.; Song, Y.L.; Ding, W. Combined use of radiocarbon and stable carbon isotope to constrain the sources and cycling of particulate organic carbon in a large freshwater lake, China. Sci. Total Environ. 2018, 625, 27–38. [Google Scholar] [CrossRef]
- West, W.E.; McCarthy, S.M.; Jones, S.E. Phytoplankton lipid content influences freshwater lake methanogenesis. Fresh-Water Biol. 2015, 60, 2261–2269. [Google Scholar] [CrossRef]
- Sepulveda-Jauregui, A.; Hoyos-Santillan, J.; Martinez-Cruz, K.; Anthony, K.M.W.; Casper, P.; Belmonte-Izquierdo, Y.; Thalasso, F. Eutrophication exacerbates the impact of climate warming on lake methane emission. Sci. Total. Environ. 2018, 636, 411–419. [Google Scholar] [CrossRef]
- Chen, X.; McGowan, S.; Zeng, L.H.; Xu, L.; Yang, X.D. Changes in carbon and nitrogen cycling in a floodplain lake over recent decades linked to littoral expansion, declining riverine influx, and eutrophication. Hydrol. Process. 2017, 31, 3110–3121. [Google Scholar] [CrossRef]
- Klaus, M.; Bergstrom, A.K.; Jonsson, A.; Deininger, A.; Geibrink, E.; Karlsson, J. Weak response of greenhouse gas emissions to whole lake N enrichment. Limnol. Oceanogr. 2018, 63, S340–S353. [Google Scholar] [CrossRef]
- Velthuis, M.; Kosten, S.; Aben, R.; Kazanjian, G.; Hilt, S.; Peeters, E.T.H.M.; van Donk, E.; Bakker, E.S. Warming enhances sedimentation and decomposition of organic carbon in shallow macrophyte-dominated systems with zero net effect on carbon burial. Glob. Chang. Biol. 2018, 24, 5231–5242. [Google Scholar] [CrossRef] [Green Version]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Ma, J.; He, F.; Yan, X.C.; Shi, R.J.; Ji, M.; Xu, B.; Wu, X.D.; Li, Z.C.; Xu, X.G.; Wang, G.X. Stoichiometric flexibility regulates the co-metabolism effect during organic carbon mineralization in eutrophic lacustrine sediments. J. Oceanol. Limnol. 2022, 40, 1974–1984. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Jeppesen, E.; Zhang, Y.L.; Niu, C.; Shi, K.; Liu, X.H.; Zhu, G.W.; Qin, B.Q. Chromophoric dissolved organic matter of black waters in a highly eutrophic Chinese lake: Freshly produced from algal scums? J. Hazard. Mater. 2015, 299, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.C.; Xu, X.G.; Wang, M.Y.; Wang, G.X.; Wu, S.J.; Li, Z.C.; Sun, H.; Shi, A.; Yang, Y.H. Climate warming and cy-anobacteria blooms: Looks at their relationships from a new perspective. Water Res. 2017, 125, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Zhou, L.; Zhang, Y.L.; de Souza, J.G.; Podgorski, D.C.; Spencer, R.G.M.; Jeppesen, E.; Davidson, T.A. Au-tochthonous dissolved organic matter potentially fuels methane ebullition from experimental lakes. Water Res. 2019, 166, 115048. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Song, K.; Han, R.M.; Riya, S.; Xu, X.G.; Yeerken, S.; Geng, S.X.; Ma, Y.; Terada, A. Nonlinear response of methane release to increased trophic state levels coupled with microbial processes in shallow lakes. Environ. Pollut. 2020, 265, 114919. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wu, F.C.; Zhang, R.Y.; Li, W.; Liao, H.Q. Characterization of dissolved organic matter fractions from Lake Hongfeng, Southwestern China Plateau. J. Environ. Sci. 2009, 21, 581–588. [Google Scholar] [CrossRef]
- Heathcote, A.J.; Anderson, N.J.; Prairie, Y.T.; Engstrom, D.R.; del Giorgio, P.A. Large increases in carbon burial in northern lakes during the Anthropocene. Nat. Commun. 2015, 6, 10016. [Google Scholar] [CrossRef] [Green Version]
- Kothawala, D.N.; Stedmon, C.A.; Muller, R.A.; Weyhenmeyer, G.A.; Kohler, S.J.; Tranvik, L.J. Controls of dissolved organic matter quality: Evidence from a large-scale boreal lake survey. Glob. Chang. Biol. 2014, 20, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.E.; Chen, W.; Cheng, Q.; Yang, L.Y. Assessing the priming effect of dissolved organic matter from typical sources using fluorescence EEMs-PARAFAC. Chemosphere 2021, 264, 128600. [Google Scholar] [CrossRef]
- Shen, Y.; Chapelle, F.H.; Strom, E.W.; Benner, R. Origins and bioavailability of dissolved organic matter in groundwater. Biogeochemistry 2015, 122, 61–78. [Google Scholar] [CrossRef]
- Lavorivska, L.; Boyer, E.W.; DeWalle, D.R. Atmospheric deposition of organic carbon via precipitation. Atmos. Environ. 2016, 146, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.Y.; Chen, W.; Zhuang, W.E.; Cheng, Q.; Li, W.X.; Wang, H.; Guo, W.D.; Chen, C.T.A.; Liu, M.H. Characteriza-tion and bioavailability of rainwater dissolved organic matter at the southeast coast of China using absorption spec-troscopy and fluorescence EEM-PARAFAC. Estuar. Coast. Shelf Sci. 2019, 217, 45–55. [Google Scholar] [CrossRef]
- Li, J.C.; Wang, L.Y.; Geng, J.J.; Li, S.N.; Yu, Q.M.; Xu, K.; Ren, H.Q. Distribution and removal of fluorescent dissolved organic matter in 15 municipal wastewater treatment plants in China. Chemosphere 2020, 251, 126375. [Google Scholar] [CrossRef]
- Wu, F.C.; Wang, L.Y.; Li, W.; Zhang, R.Y.; Fu, P.Q.; Liao, H.Q.; Bai, Y.C.; Guo, J.Y.; Wang, J. Natural organic matter and its significance in terrestrial surface environment. J. Lake Sci. 2008, 20, 1–12. [Google Scholar]
- Olefeldt, D.; Devito, K.J.; Turetsky, M.R. Sources and fate of terrestrial dissolved organic carbon in lakes of a Boreal Plains region recently affected by wildfire. Biogeosciences 2013, 10, 6247–6265. [Google Scholar] [CrossRef] [Green Version]
- Li, C.L.; Li, Q.; Zhao, L.; Ge, S.D.; Chen, D.D.; Dong, Q.M.; Zhao, X.Q. Land-use effects on organic and inorganic carbon patterns in the topsoil around Qinghai Lake basin, Qinghai-Tibetan Plateau. Catena 2016, 147, 345–355. [Google Scholar] [CrossRef]
- Ye, L.L.; Wu, X.D.; Liu, B.; Yan, D.Z.; Kong, F.X. Dynamics and sources of dissolved organic carbon during phyto-plankton bloom in hypereutrophic Lake Taihu (China). Limnologica 2015, 54, 5–13. [Google Scholar] [CrossRef]
- Zhu, G.W.; Chen, Y.X. A Review of Geochemical Behaviors and Environmental Effects of Organic Matter in Sediments. J. Lake Sci. 2001, 13, 272–279. [Google Scholar]
- Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Van de Bogert, M.; Bade, D.L.; Bastviken, D.; Gille, C.M.; Hodgson, J.R.; Kitchell, J.F.; Kritzberg, E.S. Ecosystem subsidies: Terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology 2005, 86, 2737–2750. [Google Scholar] [CrossRef]
- Farjalla, V.F.; Azevedo, D.A.; Esteves, F.A.; Bozelli, R.L.; Roland, F.; Enrich-Prast, A. Influence of hydrological pulse on bacterial growth and DOC uptake in a clear-water Amazonian lake. Microb. Ecol. 2006, 52, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Nara, F.; Imai, A.; Yoneda, M.; Matsushige, K.; Komatsu, K.; Nagai, T.; Shibata, Y.; Watanabe, T. Seasonal variation in sources of dissolved organic carbon in a lacustrine environment revealed by paired isotopic measurements (Δ14C and delta δ13C). Radiocarbon 2007, 49, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.C. Dynamic Distribution of Organic Carbon in Water-Sediment during Cyanobacteria Bloom Decline in Taihu Lake. Ph.D. Thesis, Nanjing Normal University, Nanjing, China, 15 March 2019. [Google Scholar]
- Ye, L.L.; Wu, X.D.; Yan, D.Z.; Liu, B. Seasonal dynamics of particulate organic carbon concentration in surface water and its source in the northwest of Lake Taihu. Acta Sci. Circumst. 2017, 37, 1323–1329. [Google Scholar]
- Xu, J. Remote Sensing Estimation of the Concentration and Source of Particulate Organic Carbon in Lakes. Ph.D. Thesis, Nanjing Normal University, Nanjing, China, 1 March 2021. [Google Scholar]
- Karlsson, J.; Jonsson, A.; Meili, M.; Jansson, M. Control of zooplankton dependence on allochthonous organic carbon in humic and clear-water lakes in northern Sweden. Limnol. Oceanogr. 2003, 48, 269–276. [Google Scholar] [CrossRef]
- Cole, J.J.; Carpenter, S.R.; Pace, M.L.; Van de Bogert, M.C.; Kitchell, J.L.; Hodgson, J.R. Differential support of lake food webs by three types of terrestrial organic carbon. Ecol. Lett. 2006, 9, 558–568. [Google Scholar] [CrossRef]
- Huang, Y.D. Relationship between Organic Matter from Different Sources and Bioaccumulation and Sedimentation of Typical Persistent Organic Pollutants in Lakes and Reservoirs. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 14 December 2017. [Google Scholar]
- Bianchi, T.S. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proc. Natl. Acad. Sci. USA 2011, 108, 19473–19481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Blodau, C.; Agethen, S.; Broder, T.; Knorr, K.H. Gradients of organic matter quality, mineralization and sequestration in Cook’s Bay of Lake Simcoe, Canada. Limnologica 2018, 68, 92–104. [Google Scholar] [CrossRef]
- Chen, X.C.; Feng, M.H.; Ke, F.; Pan, J.Z.; Fan, F.; Wang, Y.R.; Li, W.C. Source and Biogeochemical Distribution of Organic Matter in Surface Sediment in the Deep Oligotrophic Lake Fuxian, China. Aquat. Geochem. 2018, 24, 55–77. [Google Scholar] [CrossRef]
- Meng, L.Z.; Zhao, Z.L.; Lu, L.F.; Zhou, J.; Luo, D.; Fan, R.; Li, S.D.; Jiang, Q.L.; Huang, T.; Yang, H.; et al. Source identification of particulate organic carbon using stable isotopes and n-alkanes: Modeling and application. Water Res. 2021, 197, 117083. [Google Scholar] [CrossRef]
- Piatka, D.R.; Frank, A.H.; Kohler, I.; Castiglione, K.; van Geldern, R.; Barth, J.A.C. Balance of carbon species combined with stable isotope ratios show critical switch towards bicarbonate uptake during cyanobacteria blooms. Sci. Total Environ. 2022, 807, 151067. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Gao, Y.; Jia, J.J.; Lu, Y.; Sun, K.; Ha, X.R.; Li, Z.X.; Deng, W.Q. Vertically stratified water source characteristics and associated driving mechanisms of particulate organic carbon in a large floodplain lake system. Water Res. 2022, 209, 117963. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Wang, X.J.; Zhang, E.L.; Zhao, C.Y.; Liu, X.Q. Spatial distribution and sources of organic carbon in the surface sediment of Bosten Lake, China. Biogeosciences 2015, 12, 6605–6615. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.L.; Li, Y.M.; Liu, X.L.; Li, K.Y.; Chen, F.Z.; Gulati, R.D.; Liu, Z.W. The fate of cyanobacterial detritus in the food web of Lake Taihu: A mesocosm study using 13C and 15N labeling. Hydrobiologia 2013, 710, 39–46. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, B.; Hur, J.; Min, J.O.; Ha, S.Y.; Ra, K.; Kim, K.T.; Shin, K.H. Biodegradability of algal-derived organic matter in a large artificial lake by using stable isotope tracers. Environ. Sci. Pollut. Res. Int. 2016, 23, 8358–8366. [Google Scholar] [CrossRef] [PubMed]
- Brett, M.T.; Kainz, M.J.; Taipale, S.J.; Seshan, H. Phytoplankton, not allochthonous carbon, sustains herbivorous zoo-plankton production. Proc. Natl. Acad. Sci. USA 2009, 106, 21197–21201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluijver, A.D.; Yu, J.; Houtekamer, M.; Middelburg, J.J.; Liu, Z.W. Cyanobacteria as a carbon source for zooplankton in eutrophic Lake Taihu, China, measured by 13C labeling and fatty acid biomarkers. Limnol. Oceanogr. 2012, 57, 1245–1254. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, J. Application of molecular markers in the study of marine organic geochemistry: The occurrence and use of lipids. Marin. Environ. Sci. 2001, 20, 71–77. [Google Scholar]
- Schefuss, E.; Ratmeyer, V.; Stuut, J.B.W.; Jansen, J.H.F.; Damste, J.S.S. Carbon isotope analyses of n-alkanes in dust from the lower atmosphere over the central eastern Atlantic. Geochim. Cosmochim. Acta 2003, 67, 1757–1767. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, H. Spatial characters of n-alkane δ13C and δD and their paleoenvironmental significance. J. Nanjing Nor. Univ. 2011, 11, 83–88. [Google Scholar]
- Ren, W.X.; Wu, X.D.; Chen, B.F.; Chao, J.Y.; Ge, X.G.; Yang, J.Y.; Yang, H. Optical characteristics of chromophoric dissolved organic matter (CDOM) in upstream and downstream lakes of Taihu Lake basin: New insights for water environmental management. Chin. Geogr. Sci. 2022, 32, 606–619. [Google Scholar] [CrossRef]
- Chen, X.F.; Chuai, X.M.; Liu, T.; Yang, L.Y. Characteristics and source identification of the dissolved organic matter in the lakes of west Jiangsu by spectroscopy. J. Lake Sci. 2012, 24, 259–266. [Google Scholar]
- Chen, S.Y.; Li, Y.; Li, A.M. Application of Three-dimensional Fluorescence Spectroscopy in the Study of Dissolved Organic Matter. Environ. Sci. Tech. 2015, 38, 64–68. [Google Scholar]
- Chen, S.; Xie, Q.R.; Su, S.H.; Wu, L.B.; Zhong, S.J.; Zhang, Z.M.; Ma, C.; Qi, Y.L.; Hu, W.; Deng, J.J.; et al. Source and formation process impact the chemodiversity of rainwater dissolved organic matter along the Yangtze River Basin in summer. Water Res. 2022, 211, 118024. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.L.; Fujii, M.; Kwon, E. Development of an Internal Calibration Algorithm for Ultrahigh-Resolution Mass Spectra of Dissolved Organic Matter. Anal. Chem. 2022, 94, 10589–10594. [Google Scholar] [CrossRef]
- Qi, Y.L.; Xie, Q.R.; Wang, J.J.; He, D.; Bao, H.Y.; Fu, Q.L.; Su, S.H.; Sheng, M.; Li, S.L.; Volmer, D.A.; et al. Deciphering dissolved organic matter by Fourier transform ion cyclotron resonance mass spectrometry(FT-ICR MS): From bulk to fractions and individuals. Carbon Res. 2022, 1, 1–22. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, H.; Hong, Z.C.; Fu, G.Q.; Zheng, Y.; Li, Z.; Cui, F.Y. Photo-Reactivity and Photo-Transformation of Algal Dissolved Organic Matter Unraveled by Optical Spectroscopy and High-Resolution Mass Spectrometry Analysis. Environ. Sci. Technol. 2022, 56, 13439–13448. [Google Scholar] [CrossRef]
- He, C.; He, D.; Chen, C.M.; Shi, Q. Application of Fourier transform ion cyclotron resonance mass spectrometry in molecular characterization of dissolved organic matter. Sci. China Earth Sci. 2022, 65, 1–19. [Google Scholar] [CrossRef]
- Chen, Q.; Zhuang, W.E.; Yang, L.Y. Priming effect of dissolved organic matter in aquatic ecosystems: A review. Environ. Chem. 2018, 37, 10–18. [Google Scholar]
- Austin, A.T.; Ballare, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.R. Impact and effects of paralytic shellfish poisoning toxins derived from harmful algal blooms to marine fish. Fish Fish. 2016, 17, 226–248. [Google Scholar] [CrossRef]
- Rolton, A.; Rhodes, L.; Hutson, K.S.; Biessy, L.; Bui, T.; MacKenzie, L.; Symonds, J.E.; Smith, K.F. Effects of harmful algal blooms on fish and shellfish species: A case study of New Zealand in a changing environment. Toxins 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, F.S.; Roland, F.; Downing, J.A. Eutrophication reverses whole-lake carbon budgets. Inland Waters 2014, 4, 41–48. [Google Scholar] [CrossRef]
- Froelich, P.N.; Klinkhammer, G.P.; Bender, M.L.; Luedtke, N.A.; Heath, G.R.; Cullen, D.; Dauphin, P.; Hammond, D.; Hartman, B.; Maynard, V. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochim. Cosmochim. Acta 1979, 43, 1075–1090. [Google Scholar] [CrossRef]
- Gruca-Rokosz, R.; Tomaszek, J.A. Methane and Carbon Dioxide in the Sediment of a Eutrophic Reservoir: Production Pathways and Diffusion Fluxes at the Sediment-Water Interface. Water Air Soil Pollut. 2015, 226, 16. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, M.; Shi, X.L.; Kong, F.X.; Ma, R.H.; Yu, Y. Nutrient reduction magnifies the impact of extreme weather on cyanobacterial bloom formation in large shallow Lake Taihu (China). Water Res. 2016, 103, 302–310. [Google Scholar] [CrossRef]
- Hanamachi, Y.; Hama, T.; Yanai, T. Decomposition process of organic matter derived from freshwater phytoplankton. Limnology 2008, 9, 57–69. [Google Scholar] [CrossRef]
- Ye, L.L.; Shi, X.; Wu, X.D.; Zhang, M.; Yu, Y.; Li, D.M.; Kong, F.X. Dynamics of dissolved organic carbon after a cyanobacterial bloom in hypereutrophic Lake Taihu (China). Limnologica 2011, 41, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.F.; Huang, W.; Ma, S.Z.; Feng, M.H.; Liu, C.; Gu, X.Z.; Chen, K.N. Characterization of Chromophoric Dissolved Organic Matter in the Littoral Zones of Eutrophic Lakes Taihu and Hongze during the Algal Bloom Season. Water 2018, 10, 861. [Google Scholar] [CrossRef]
- Yang, X.F.; Zheng, X.; Wu, L.J.; Cao, X.; Li, Y.; Niu, J.F.; Meng, F.G. Interactions between algal (AOM) and natural organic matter (NOM): Impacts on their photodegradation in surface waters. Environ. Pollut. 2018, 242, 1185–1197. [Google Scholar] [CrossRef]
- Li, K.; Guan, B.H.; Liu, Z.W. Experiments on decomposition rate and release forms of nitrogen and phosphorus from the decomposing cyanobacterial detritus. J. Lake Sci. 2011, 23, 919–925. [Google Scholar]
- Li, W.C.; Chen, K.N.; Wu, Q.L.; Pan, J.Z. Experimental Studies on Decomposition Process of Aquatic Plant Material from East Taihu Lake. J. Lake Sci. 2001, 13, 331–336. [Google Scholar]
- Jensen, H.L. Carbon Nutrition of Some Microorganisms Decomposing Halogen-substituted Aliphatic Acids. Acta Agric. Scand. 1963, 13, 404–412. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Hu, H.Y.; Xi, J.Y. Aerobic Biodegradation Performance of Six Volatile Organic Compounds by Activated Sludge Acclimated with Toluene. Chin. J. Environ. Sci. 2003, 24, 83–89. [Google Scholar]
- Dai, J.H.; Sun, M.Y.; Culp, R.A.; Noakes, J.E. A laboratory study on biochemical degradation and microbial utilization of organic matter comprising a marine diatom, land grass, and salt marsh plant in estuarine ecosystems. Aquat. Ecol. 2009, 43, 825–841. [Google Scholar] [CrossRef]
- Canfield, D.E. Factors influencing organic carbon preservation in marine sediments. Chem. Geol. 1994, 114, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Benoy, G.; Cash, K.; McCauley, E.; Wrona, F. Carbon dynamics in lakes of the boreal forest under a changing climate. Environ. Rev. 2007, 15, 175–189. [Google Scholar] [CrossRef]
- Marotta, H.; Duarte, C.M.; Pinho, L.; Enrich-Prast, A. Rainfall leads to increased pCO2 in Brazilian coastal lakes. Biogeosciences 2010, 7, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.F.; He, Z.B.; Du, J.; Yang, J.J.; Zhu, X. Response of soil carbon cycling to climate warming: Challenges and perspectives. Acta Prataculturae Sin. 2015, 24, 183–194. [Google Scholar]
- O’Reilly, C.M.; Sharma, S.; Gray, D.K.; Hampton, S.E.; Read, J.S.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; McIntyre, P.B.; Kraemer, B.M.; et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 10773–10781. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Zhou, Y.Q.; Zhou, L.; Zhang, Y.L.; Xu, H.; Jang, K.S.; Kothawala, D.N.; Spencer, R.G.M.; Jeppesen, E.; Brookes, J.D.; et al. Changes in water chemistry associated with rainstorm events increase carbon emissions from the inflowing river mouth of a major drinking water reservoir. Environ. Sci. Technol. 2022, 56, 16494–16505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Yu, X.Q.; Zhou, L.; Zhang, Y.L.; Xu, H.; Zhu, M.Y.; Zhu, G.W.; Jang, K.S.; Spencer, R.G.M.; Jeppesen, E.; et al. Rainstorms drive export of aromatic and concurrent bio-labile organic matter to a large eutrophic lake and its major tributaries. Water Res. 2022, 229, 119448. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, M.; Middelboe, M. A cross-system analysis of labile dissolved organic carbon. Mar. Ecol. Prog. Ser. 1995, 118, 283–294. [Google Scholar] [CrossRef]
- Krause, S.; Lewandowski, J.; Grimm, N.B.; Hannah, D.M.; Pinay, G.; McDonald, K.; Marti, E.; Argerich, A.; Pfister, L.; Klaus, J.; et al. Ecohydrological interfaces as hot spots of ecosystem processes. Water Resour. Res. 2017, 53, 6359–6376. [Google Scholar] [CrossRef] [Green Version]
- Marin-Spiotta, E.; Gruley, K.E.; Crawford, J.; Atkinson, E.E.; Miesel, J.R.; Greene, S.; Cardona-Correa, C.; Spencer, R.G.M. Paradigm shifts in soil organic matter research affect interpretations of aquatic carbon cycling: Transcending disciplinary and ecosystem boundaries. Biogeochemistry 2014, 117, 279–297. [Google Scholar] [CrossRef]
- Farrer, E.C.; Suding, K.N. Teasing apart plant community responses to N enrichment: The roles of resource limitation, competition and soil microbes. Ecol. Lett. 2016, 19, 1287–1296. [Google Scholar] [CrossRef]
- Li, Y.B.; Bezemer, T.M.; Yang, J.J.; Lu, X.T.; Li, X.Y.; Liang, W.J.; Han, X.G. Changes in litter quality induced by N deposition alter soil microbial communities. Soil Biol. Biochem. 2019, 130, 33–42. [Google Scholar] [CrossRef]
- Song, N.; Yan, Z.S.; Cai, H.Y.; Jiang, H.L. Effect of temperature on submerged macrophyte litter decomposition within sediments from a large shallow and subtropical freshwater lake. Hydrobiologia 2013, 714, 131–144. [Google Scholar] [CrossRef]
- Song, N.; Bai, L.L.; Xu, H.C.; Jiang, H.L. The composition difference of macrophyte litter-derived dissolved organic matter by photodegradation and biodegradation: Role of reactive oxygen species on refractory component. Chemosphere 2020, 242, 125155. [Google Scholar] [CrossRef]
- Ren, D.; Chen, F.; Pu, H.Y.; Zhang, Y.; Li, Y.P. Photochemical Behaviors and Environmental Effects of Dissolved Organic Matter. J. Ecol. Rural Environ. 2019, 35, 563–572. [Google Scholar]
- Rutledge, S.; Campbell, D.I.; Baldocchi, D.; Schipper, L.A. Photodegradation leads to increased carbon dioxide losses from terrestrial organic matter. Glob. Chang. Biol. 2010, 16, 3065–3074. [Google Scholar] [CrossRef]
- Chin, Y.P.; Miller, P.L.; Zeng, L.K.; Cawley, K.; Weavers, L.K. Photosensitized degradation of bisphenol a by dissolved organic matter. Environ. Sci. Technol. 2004, 38, 5888–5894. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Buchwald, C.; Diaz, J.M.; Ossolinski, J.E.; Dyhrman, S.T.; Van Mooy, B.A.S.; Polyviou, D. Dynamics of extracellular superoxide production by Trichodesmium colonies from the Sargasso Sea. Limnol. Oceanogr. 2016, 61, 1188–1200. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Wen, Y.L.; Cheng, M. Effects of enhanced UV-B radiation on greenhouse gas emissions in terrestrial ecosystem: Research progress. Chin. Agric. Sci. Bull. 2022, 38, 80–88. [Google Scholar]
- Helms, J.R.; Mao, J.D.; Stubbins, A.; Schmidt-Rohr, K.; Spencer, R.G.M.; Hernes, P.J.; Mopper, K. Loss of optical and molecular indicators of terrigenous dissolved organic matter during long-term photobleaching. Aquat. Sci. 2014, 76, 353–373. [Google Scholar] [CrossRef]
- Cory, R.M.; Crump, B.C.; Dobkowski, J.A.; Kling, G.W. Surface exposure to sunlight stimulates CO2 release from permafrost soil carbon in the Arctic. Proc. Natl. Acad. Sci. USA 2013, 110, 3429–3434. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.M.; Pignatello, J.J.; Mitch, W.A. Influence of Ionic Strength on Triplet-State Natural Organic Matter Loss by Energy Transfer and Electron Transfer Pathways. Environ. Sci. Technol. 2013, 47, 10987–10994. [Google Scholar] [CrossRef]
- Yin, Y.G.; Liu, J.F.; Jiang, G.B. Sunlight-Induced Reduction of Ionic Ag and Au to Metallic Nanoparticles by Dissolved Organic Matter. ACS Nano 2012, 6, 7910–7919. [Google Scholar] [CrossRef]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, P.; Nie, Y. Concentration and Bioaccumulation of Cyanobacterial Bioactive and Odorous Metabolites Occurred in a Large, Shallow Chinese Lake. Bull. Environ. Contam. Toxicol. 2014, 93, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.Y.; Zhu, G.W.; Zhao, L.L.; Yao, X.; Zhang, Y.L.; Gao, G.; Qin, B.Q. Influence of algal bloom degradation on nutrient release at the sediment-water interface in Lake Taihu, China. Environ. Sci. Pollut. Res. 2013, 20, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Y.; Zhu, G.W.; Wang, Y.P. Influence of Scum of Algal Bloom on the Release of N and P from Sediments of Lake Taihu. Chin. J. Environ. Sci. 2011, 32, 409–415. [Google Scholar]
- Ma, J. Co-Metabolic Effect of Organic Carbon In typical Eutrophic Lake Sediments and Its Influencing Mechanism. Ph.D. Thesis, Nanjing Normal University, Nanjing, China, 1 May 2021. [Google Scholar]
- Shen, A.C.; Xu, Z.A.; Wu, D.H. Relationship between Accumulation and Dying of Cyanobacteria and Black Spot. J. Hydrol. 2012, 33, 68–72. [Google Scholar]
- Feng, Z.Y.; Fan, C.X.; Huang, W.Y.; Ding, S.M. Microorganisms and typical organic matter responsible for lacustrine “black bloom”. Sci. Total Environ. 2014, 470, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.X.; Ke, F.; Li, W.C.; Xu, X.G.; Song, Y.Y.; Feng, M.H. Laboratory research on the contaminants release during the anaerobic decomposition of high-density cyanobacteria. J. Lake Sci. 2013, 25, 47–54. [Google Scholar]
- Wu, Y.T.; Qi, C.; Xu, X.X.; Zhou, Y.; Wang, M.Y.; Wang, G.X. Simulation of cyanobacteia decay’s impacts on nutrients in Water. Acta Sci. Circumst. 2017, 37, 2846–2853. [Google Scholar]
- Li, H.B.; Xing, P.; Chen, M.J.; Bian, Y.Q.; Wu, Q.L.L. Short-term bacterial community composition dynamics in response to accumulation and breakdown of Microcystis blooms. Water Res. 2011, 45, 1702–1710. [Google Scholar] [CrossRef]
- Wu, T.T. Ecological Effects of Cyanobacteria Accumulation on Water Hyacinth and Decomposition of Plant Residues. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2015. [Google Scholar]
- Lu, X.; Fan, C.X.; He, W.; Deng, J.C.; Yin, H.B. Sulfur-containing amino acid methionine as the precursor of volatile organic sulfur compounds in algea-induced black bloom. J. Environ. Sci. 2013, 25, 33–43. [Google Scholar] [CrossRef]
- Shen, Q.S.; Liu, C.; Zhou, Q.L.; Shang, J.G.; Zhang, L.; Fan, C.X. Effects of physical and chemical characteristics of surface sediments in the formation of shallow lake algae-induced black bloom. J. Environ. Sci. 2013, 25, 2353–2360. [Google Scholar] [CrossRef]
- Yu, D.Z.; Xie, P.; Zeng, C.; Xie, L.J.; Chen, J. In situ enclosure experiments on the occurrence, development and decline of black bloom and the dynamics of its associated taste and odor compounds. Ecol. Eng. 2016, 87, 246–253. [Google Scholar] [CrossRef]
- Zhang, X.J.; Chen, C.; Ding, J.Q.; Hou, A.X.; Li, Y.; Niu, Z.B.; Su, X.Y.; Xu, Y.J.; Laws, E.A. The 2007 water crisis in Wuxi, China: Analysis of the origin. J. Hazard. Mater. 2010, 182, 130–135. [Google Scholar] [CrossRef] [PubMed]
- West, W.E.; Coloso, J.J.; Jones, S.E. Effects of algal and terrestrial carbon on methane production rates and methanogen community structure in a temperate lake sediment. Freshw. Biol. 2012, 57, 949–955. [Google Scholar] [CrossRef]
- Schwarz, J.I.K.; Eckert, W.; Conrad, R. Response of the methanogenic microbial community of a profundal lake sediment (Lake Kinneret, Israel) to algal deposition. Limnol. Oceanogr. 2008, 53, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.T.; Tang, Q.; Sun, W.; Zhu, L.F.; Xing, P. Dissolved methane dynamics during the degradation of organic matter derived from cyanobacterial bloom. China Environ. Sci. 2017, 37, 702–710. [Google Scholar]
- Grasset, C.; Mendonca, R.; Saucedo, G.V.; Bastviken, D.; Roland, F.; Sobek, S. Large but variable methane production in anoxic freshwater sediment upon addition of allochthonous and autochthonous organic matter. Limnol. Oceanogr. 2018, 63, 1488–1501. [Google Scholar] [CrossRef]
- Davidson, T.A.; Audet, J.; Svenning, J.C.; Lauridsen, T.L.; Sondergaard, M.; Landkildehus, F.; Larsen, S.E.; Jeppesen, E. Eutrophication effects on greenhouse gas fluxes from shallow-lake mesocosms override those of climate warming. Glob. Chang. Biol. 2015, 21, 4449–4463. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Ai, H.N.; Qiu, Y.X.; He, Q.; He, Y.X.; Yang, C.; Kang, L.; Luo, H.R.; Li, W.; Mao, Y.F.; Hu, M.J.; et al. Turn the potential greenhouse gases into biomass in harmful algal blooms waters: A microcosm study. Sci. Total Environ. 2019, 655, 520–528. [Google Scholar] [CrossRef]
- Xing, P.; Guo, L.; Tian, W.; Wu, Q.L.L. Novel Clostridium populations involved in the anaerobic degradation of Microcystis blooms. ISME J. 2011, 5, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.C.; Zhang, Z.Q.; Ji, M.; Wang, M.Y.; Ran, S.S.; Xu, X.X.; Wang, G.X. Concentration of dissolved greenhouse gas and its influence factors in the summer surface water of eutrophic lake. J. Lake Sci. 2018, 30, 1420–1428. [Google Scholar]
- Mitsch, W.J. Solving Lake Erie’s harmful algal blooms by restoring the Great Black Swamp in Ohio. Ecol. Eng. 2017, 108, 406–413. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Zhong, W.; Shang, S.T.; Ye, S.S.; Tang, X.W.; Xue, J.B.; Ouyang, J.; Smol, J.P. Lacustrine mineral magnetic record of postglacial environmental changes from Dahu Swamp, southern China. Glob. Planet. Chang. 2018, 170, 62–75. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wu, C.; Xie, D.; Ma, J. Sources, Migration, Transformation, and Environmental Effects of Organic Carbon in Eutrophic Lakes: A Critical Review. Int. J. Environ. Res. Public Health 2023, 20, 860. https://doi.org/10.3390/ijerph20010860
Xu X, Wu C, Xie D, Ma J. Sources, Migration, Transformation, and Environmental Effects of Organic Carbon in Eutrophic Lakes: A Critical Review. International Journal of Environmental Research and Public Health. 2023; 20(1):860. https://doi.org/10.3390/ijerph20010860
Chicago/Turabian StyleXu, Xiaoguang, Chao Wu, Dongyu Xie, and Jie Ma. 2023. "Sources, Migration, Transformation, and Environmental Effects of Organic Carbon in Eutrophic Lakes: A Critical Review" International Journal of Environmental Research and Public Health 20, no. 1: 860. https://doi.org/10.3390/ijerph20010860




