Potential Mechanism of Long-Term Immobilization of Pb/Cd by Layered Double Hydroxide Doped Chicken-Manure Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation and Characterization
2.2. Measurement of Material Stability
2.3. Batch Adsorption Experiments of Pb/Cd
2.3.1. Adsorption Isotherms
2.3.2. The Effect of pH on Adsorption
2.3.3. Post-Sorption Characterization
2.4. Immobilization of Pb/Cd in Soil
3. Results and Discussion
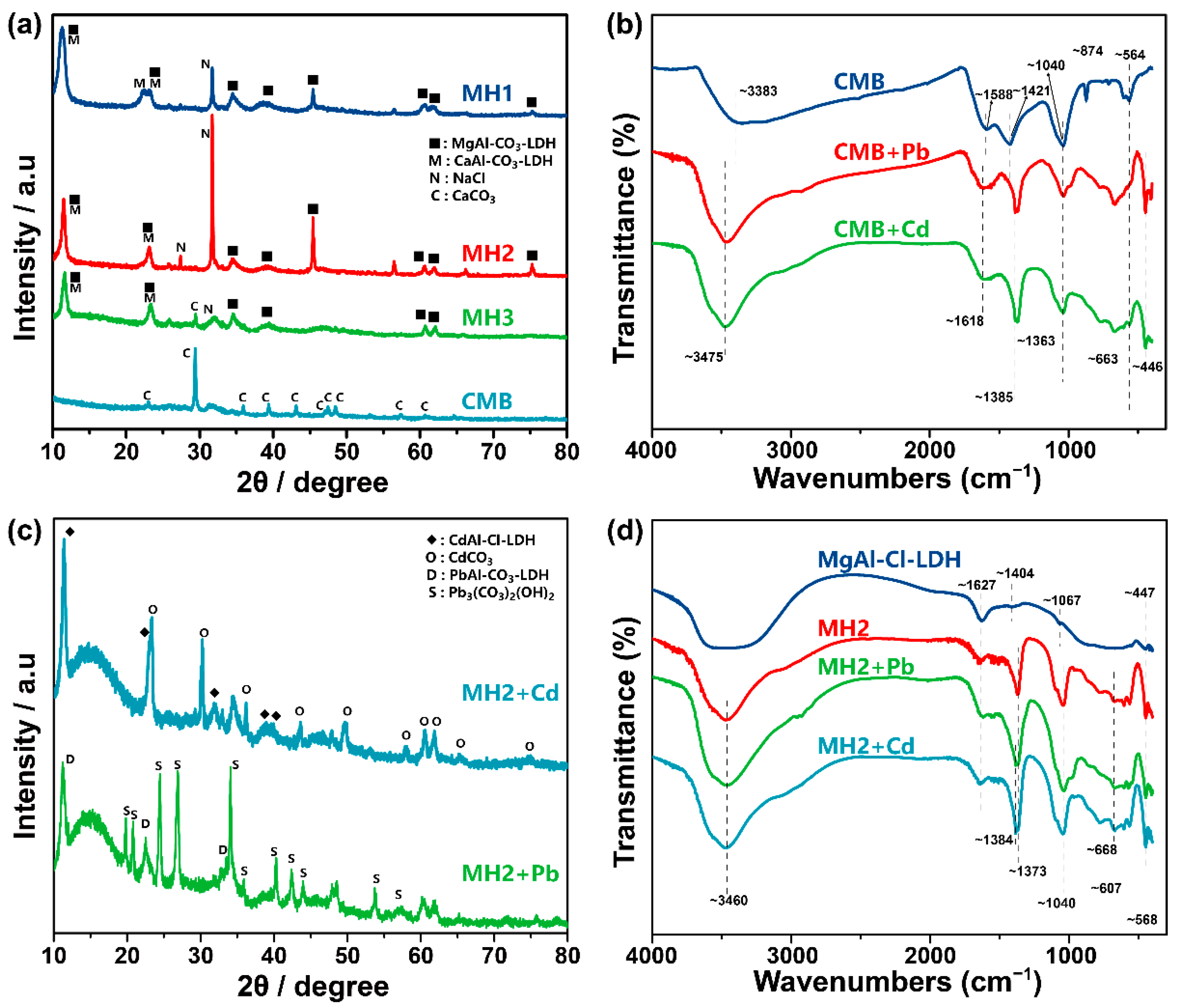
3.1. Material Characterization
3.1.1. Basic Physicochemical Properties of Synthetic Materials
3.1.2. Stability
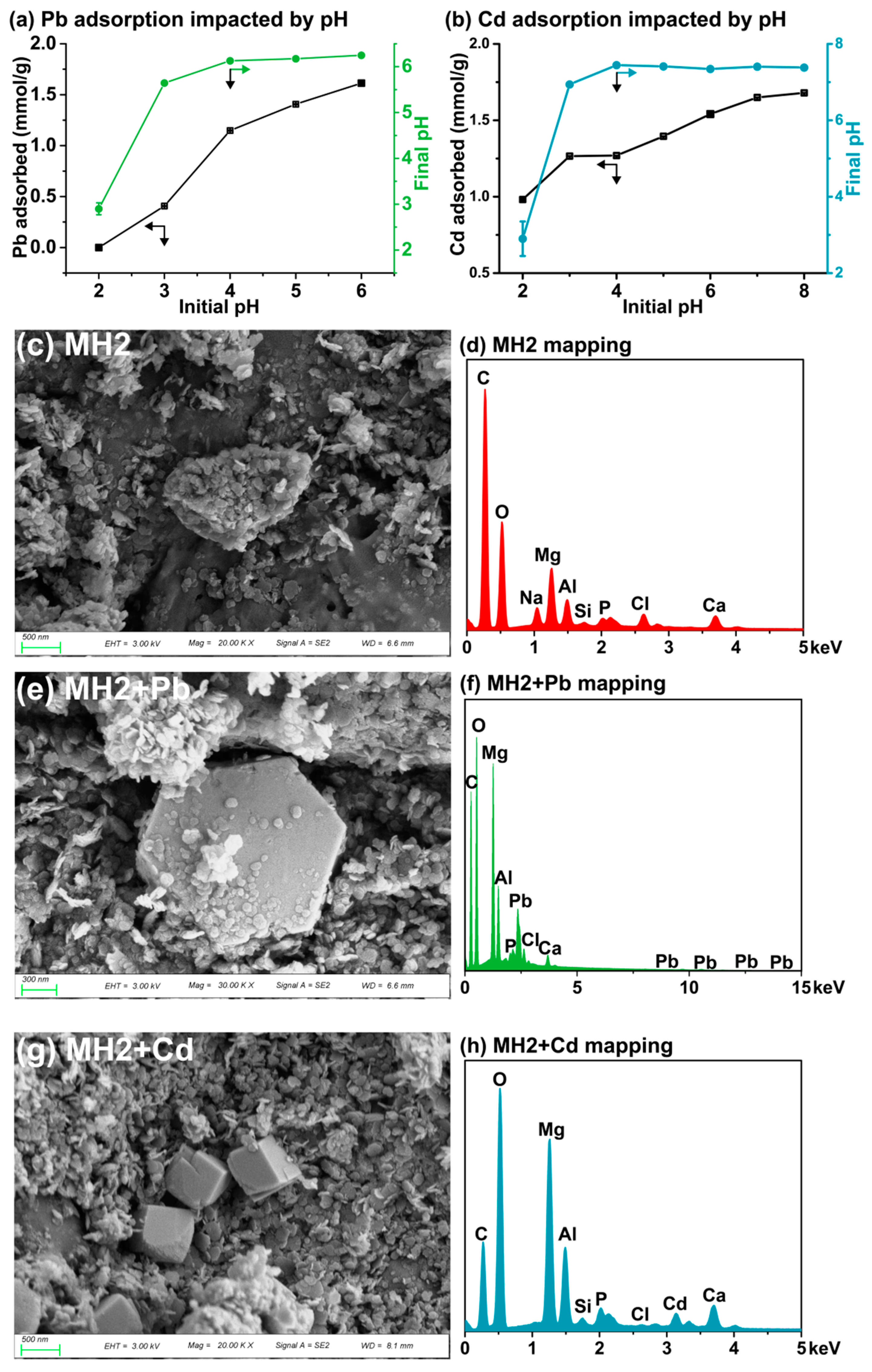
3.2. Aqueous Adsorption of Pb/Cd
3.2.1. Efficient Adsorption of Pb(II) and Cd(II) by LDH Doped CMB
3.2.2. Influence of Initial pH
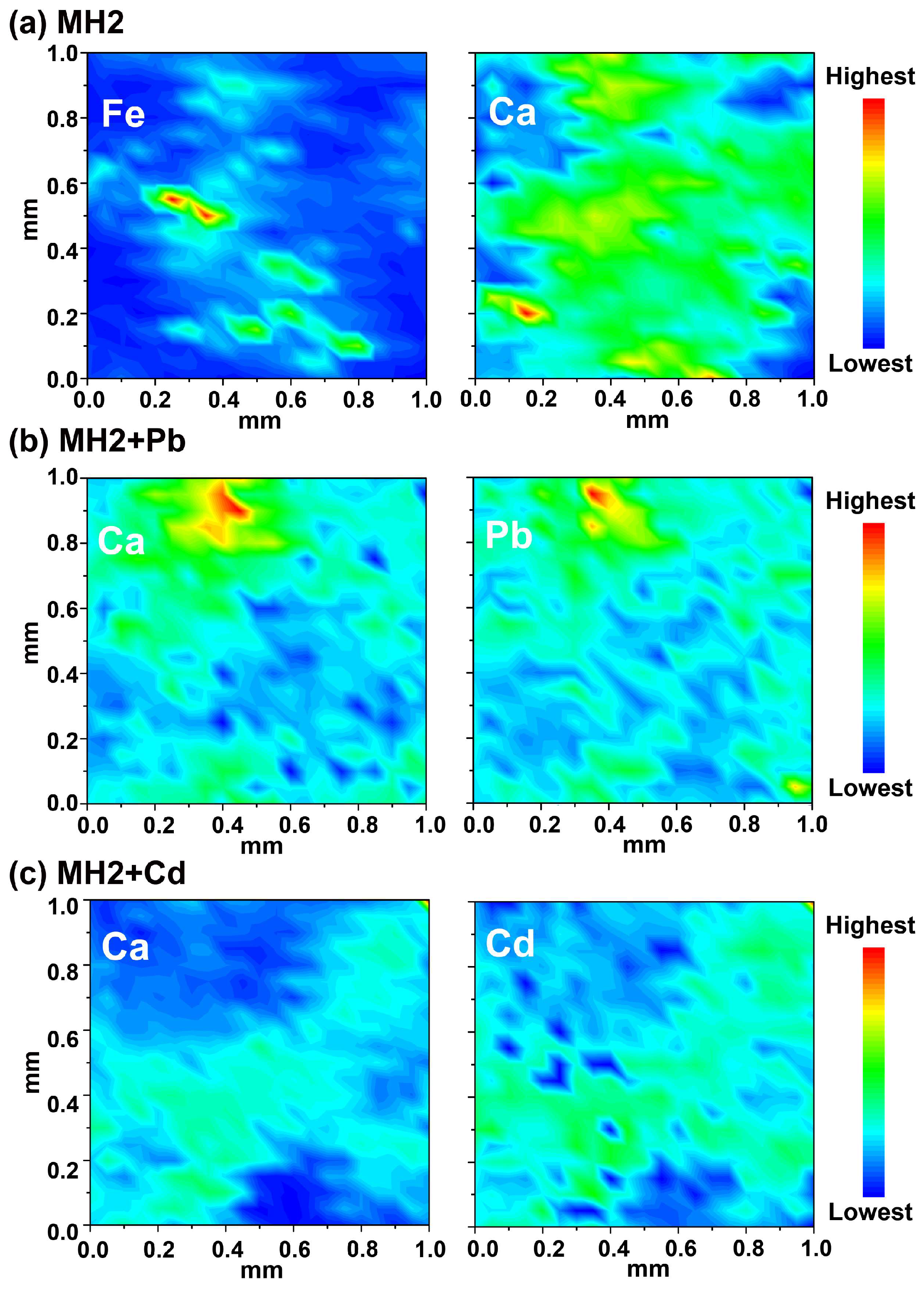
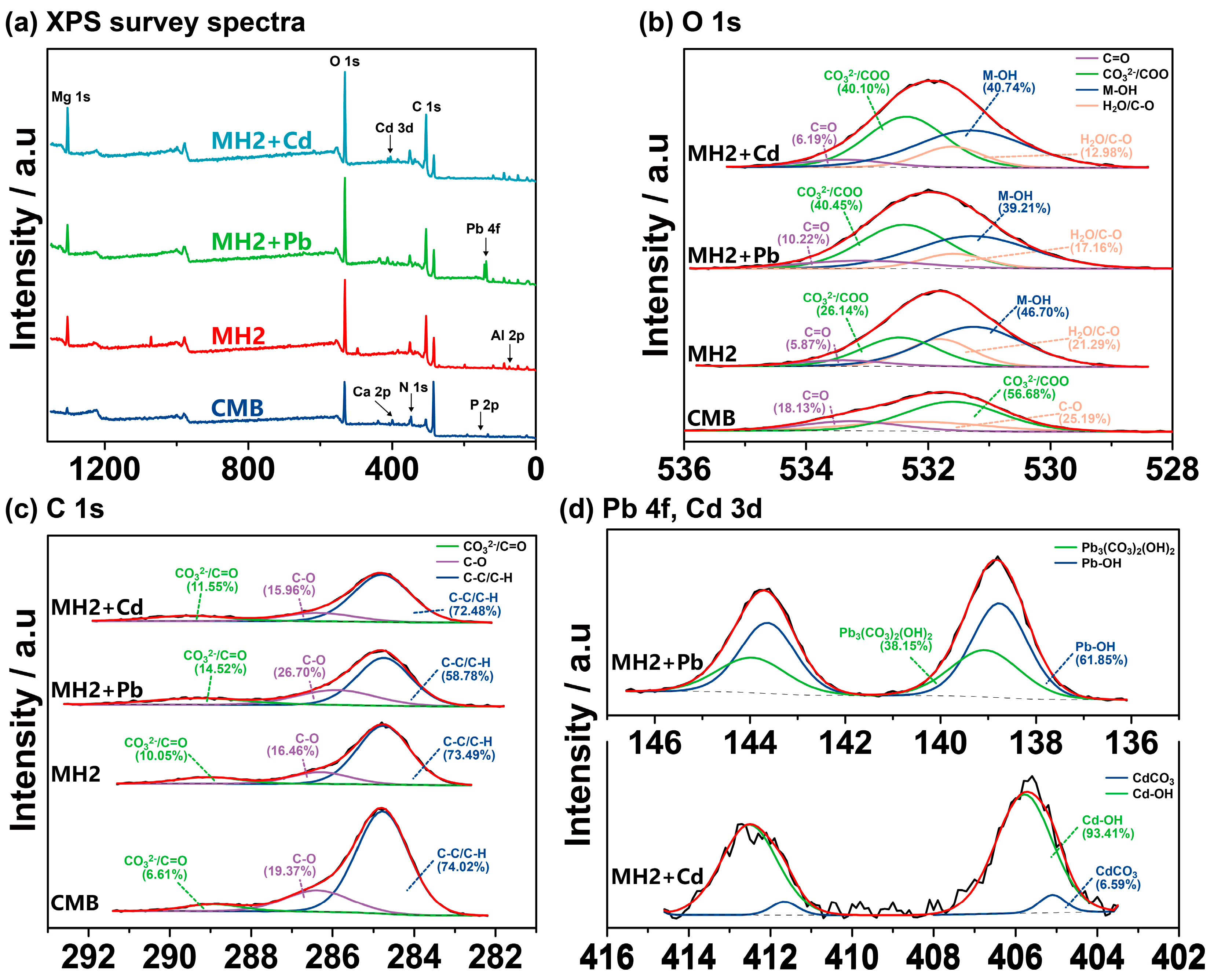
3.2.3. Pb/Cd Adsorption Mechanism of CMB
3.2.4. Isomorphous Substitution and Precipitation Facilitate Long-Term Immobilization of Pb/Cd by MHs
3.3. Immobilization of Pb/Cd in Soil
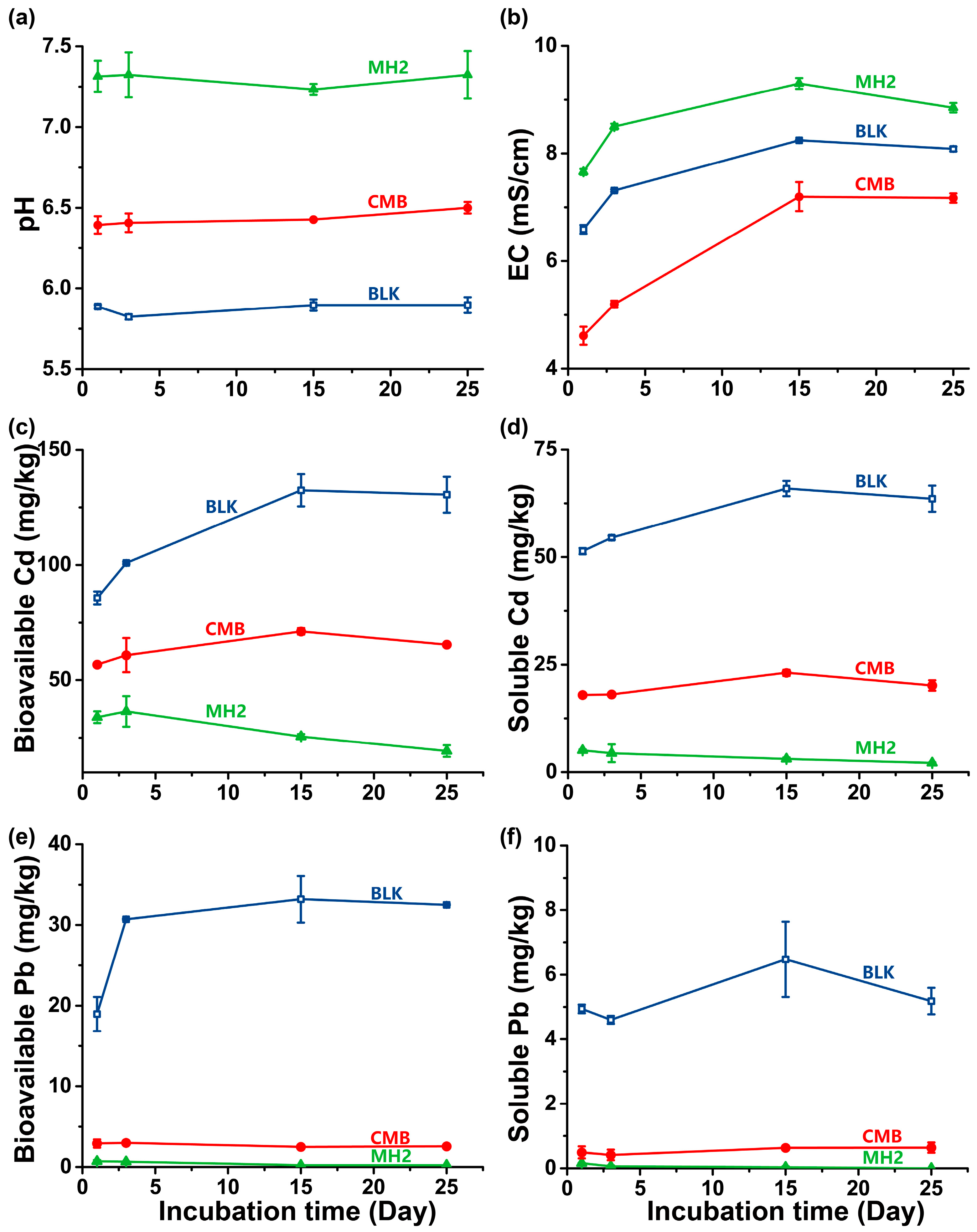
3.3.1. The Improvement on Physicochemical Properties of Soil
3.3.2. Immobilization Efficiency in Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Bureau of Statistics of China. China Statistical Yearbook-2021; China Statistics Press: Beijing, China, 2021. [Google Scholar]
- Xu, L.; Dai, H.; Skuza, L.; Xu, J.; Shi, J.; Wei, S. Co-high-efficiency washing agents for simultaneous removal of Cd, Pb and As from smelting soil with risk assessment. Chemosphere 2022, 300, 134581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Peng, C.; Liu, X.; Jiang, Z.; Guo, Z.; Xiao, X. Pollution and Risk Assessments of Heavy Metal(loid)s in the Soil around Lead-Zinc Smelteries via Data Integration Analysis. Int. J. Environ. Res. Public Health 2022, 19, 9698. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kwon, Y.K.; Yu, S.; Lee, P.K.; Park, H.S.; Song, N. Assessment of Zn pollution sources and apportionment in agricultural soils impacted by a Zn smelter in South Korea. J. Hazard. Mater. 2019, 364, 475–487. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Guo, Z.; Deng, Y.; Xu, M.; Zhang, S.; Yin, H.; Liang, Y.; Liu, H.; Miao, B.; et al. Spatial Distribution of Toxic Metal(loid)s and Microbial Community Analysis in Soil Vertical Profile at an Abandoned Nonferrous Metal Smelting Site. Int. J. Environ. Res. Public Health 2020, 17, 7101. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Xu, R.; Xie, H.; Xiao, X.; Peng, C. Contamination vertical distribution and key factors identification of metal(loid)s in site soil from an abandoned Pb/Zn smelter using machine learning. Sci. Total Environ. 2023, 856, 159264. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; M.M.S., C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Shikha, D.; Saw, S. Evaluation of potential toxic heavy metal contamination in soil, fly ash, vegetables and grain crops along with associated ecological and health risk assessment of nearby inhabitants of a thermal power station in Jharkhand (India). Environ. Sci. Pollut Res. 2022, 29, 1–18. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.P.; Xie, M.Y.; Yang, B.K. Ecological risk and health risk analysis of soil potentially toxic elements from oil production plants in central China. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Tyszka, R.; Pietranik, A.; Kierczak, J.; Zieliński, G.; Darling, J. Cadmium distribution in Pb-Zn slags from Upper Silesia, Poland: Implications for cadmium mobility from slag phases to the environment. J. Geochem. Explor. 2018, 186, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Kunene, S.C.; Lin, K.-S.; Mdlovu, N.V.; Lin, Y.-S.; Mdlovu, N.B. Speciation and fate of toxic cadmium in contaminated paddy soils and rice using XANES/EXAFS spectroscopy. J. Hazard. Mater. 2020, 383, 121167. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, J.y.; Ni, N.; Xu, R. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agr. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Lei, S.; Shi, Y.; Xue, C.; Wang, J.; Che, L.; Qiu, Y. Hybrid ash/biochar biocomposites as soil amendments for the alleviation of cadmium accumulation by Oryza sativa L. in a contaminated paddy field. Chemosphere 2020, 239, 124805. [Google Scholar] [CrossRef]
- Haider, F.U.; Wang, X.; Farooq, M.; Hussain, S.; Cheema, S.A.; Ain, N.u.; Virk, A.L.; Ejaz, M.; Janyshova, U.; Liqun, C. Biochar application for the remediation of trace metals in contaminated soils: Implications for stress tolerance and crop production. Ecotox. Environ. Safe 2022, 230, 113165. [Google Scholar] [CrossRef]
- Huang, F.; Gao, L.Y.; Deng, J.H.; Chen, S.H.; Cai, K.Z. Quantitative contribution of Cd2+ adsorption mechanisms by chicken-manure-derived biochars. Environ. Sci. Pollut. Res. Int. 2018, 25, 28322–28334. [Google Scholar] [CrossRef]
- Piash, M.I.; Iwabuchi, K.; Itoh, T.; Uemura, K. Release of essential plant nutrients from manure- and wood-based biochars. Geoderma 2021, 397, 115100. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Park, J.H.; Li, R.; Dodla, S.K.; Zhang, Z. Biochar produced from mineral salt-impregnated chicken manure: Fertility properties and potential for carbon sequestration. Waste Manag. 2018, 78, 802–810. [Google Scholar] [CrossRef]
- Lei, S.C.; Zhu, L.; Xue, C.; Hong, C.Y.; Wang, J.L.; Che, L.; Hu, Y.F.; Qiu, Y.P. Mechanistic insights and multiple characterizations of cadmium binding to animal-derived biochar. Environ. Pollut. 2020, 258, 113675. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, L.; Rui, X.; Hu, J.; Zhu, N. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Siatecka, A.; Oleszczuk, P. Mechanism of aging of biochars obtained at different temperatures from sewage sludges with different composition and character. Chemosphere 2022, 287, 132258. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Enhanced Arsenic Removal by Hydrothermally Treated Nanocrystalline Mg/Al Layered Double Hydroxide with Nitrate Intercalation. Environ. Sci. Technol. 2009, 43, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, C.L.; Seo, Y.J.; Yeo, S.K.; Choi, J.; Komarneni, S.; Lee, J.H. Reactions of Cu2+ and Pb2+ with Mg/Al layered double hydroxide. Appl. Clay Sci. 2007, 37, 143–148. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Qiu, Y.; Yin, D. Mg-Fe layered double hydroxide assembled on biochar derived from rice husk ash: Facile synthesis and application in efficient removal of heavy metals. Environ. Sci. Pollut. Res. Int. 2018, 25, 24293–24304. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, Z.; Wei, S. A new hydrotalcite-like absorbent FeMnMg-LDH and its adsorption capacity for Pb2+ ions in water. Appl. Clay Sci. 2018, 153, 29–37. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, X.; Wang, C.; Sun, H.; Ma, A.; Zhang, G.; Wang, N. Sorption of cadmium onto Mg-Fe Layered Double Hydroxide (LDH)-Kiwi branch biochar. Environ. Pollut. Bioavailab. 2019, 31, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Ge, R.; Liu, T.; Xu, S.; Hao, P.; Zhao, X.; Li, Z.; Lei, X.; Duan, H. Super-stable mineralization of cadmium by calcium-aluminum layered double hydroxide and its large-scale application in agriculture soil remediation. Chem. Eng. J. 2021, 407, 127178. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Li, Y. Phosphate adsorption from wastewater using ZnAl-LDO-loaded modified banana straw biochar. Environ. Sci. Pollut. Res. Int. 2019, 26, 18343–18353. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Y.; Guo, L.; Wang, Q.; Guan, C.Y.; Yang, F.; Chen, Q. Arsenic adsorption on layered double hydroxides biochars and their amended red and calcareous soils. J. Environ. Manage 2020, 271, 111045. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Y.; Sun, R.; Zhou, Y.; Tsang, D.C.W.; Chen, Q. Optimizing the synthesis of Fe/Al (Hydr)oxides-Biochars to maximize phosphate removal via response surface model. J. Clean. Prod. 2019, 237, 117770. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Han, X.; Wu, Z.; Lai, C.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Tsang, D.C.W.; Cheng, K.; Ok, Y.S. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery. J. Hazard. Mater. 2019, 365, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Harvey, O.R.; Kuo, L.-J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars). Environ. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The Interfacial Behavior between Biochar and Soil Minerals and Its Effect on Biochar Stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Kim, S.W.; Chae, Y.; Moon, J.; Kim, D.; Cui, R.; An, G.; Jeong, S.W.; An, Y.J. In Situ Evaluation of Crop Productivity and Bioaccumulation of Heavy Metals in Paddy Soils after Remediation of Metal-Contaminated Soils. J. Agric. Food Chem. 2017, 65, 1239–1246. [Google Scholar] [CrossRef]
- Ji, J.; Zhao, Y.; Wang, H.; Jiang, L.; Yuan, X.; Wang, H. Resource utilization of chicken manure to produce biochar for effective removal of levofloxacin hydrochloride through peroxymonosulfate activation: The synergetic function of graphitization and nitrogen functionality. Chemosphere 2022, 309, 136419. [Google Scholar] [CrossRef]
- Grover, K.; Komarneni, S.; Katsuki, H. Uptake of arsenite by synthetic layered double hydroxides. Water Res. 2009, 43, 3884–3890. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Hu, J.; Chen, C.; Wang, X. The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem. Eng. J. 2011, 171, 167–174. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef]
- Liang, X.F.; Hou, W.G.; Xu, Y.M.; Sun, G.H.; Wang, L.; Sun, Y.; Qin, X. Sorption of lead ion by layered double hydroxide intercalated with diethylenetriaminepentaacetic acid. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 50–57. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Bi, Y.; Rehman, S.; Dang, Z.; Wu, P. Multifunctional magnetic MgMn-oxide composite for efficient purification of Cd2+ and paracetamol pollution: Synergetic effect and stability. J. Hazard. Mater. 2020, 388, 122078. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, H.; Li, Q.; Zhou, G.; Ye, Q.; Wang, Q.; Zhang, J. Panda manure biochar-based green catalyst to remove organic pollutants by activating peroxymonosulfate: Important role of non-free radical pathways. J. Environ. Chem. Eng. 2021, 9, 106485. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.; Huang, X.; Wang, G.; Zhu, C. Pre-magnetic bamboo biochar cross-linked CaMgAl layered double-hydroxide composite: High-efficiency removal of As(III) and Cd(II) from aqueous solutions and insight into the mechanism of simultaneous purification. Sci. Total Environ. 2022, 823, 153743. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Zhao, G.; Zhang, Z.; Xu, C.; Liu, Y.; Chen, J.; Zhuang, L.; Haya, T.; Wang, X. Enhanced adsorption of U(VI) and 241Am(III) from wastewater using Ca/Al layered double hydroxide@carbon nanotube composites. J. Hazard. Mater. 2018, 347, 67–77. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; Jiang, C.; Jiang, X.; Guan, Y. Simultaneous and Efficient Capture of Inorganic Nitrogen and Heavy Metals by Polyporous Layered Double Hydroxide and Biochar Composite for Agricultural Nonpoint Pollution Control. ACS Appl. Mater. Interfaces 2018, 10, 43013–43030. [Google Scholar] [CrossRef]
- Li, S.S.; Jiang, M.; Jiang, T.J.; Liu, J.H.; Guo, Z.; Huang, X.J. Competitive adsorption behavior toward metal ions on nano-Fe/Mg/Ni ternary layered double hydroxide proved by XPS: Evidence of selective and sensitive detection of Pb(II). J. Hazard. Mater. 2017, 338, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; He, J.; Guo, C. Second staging of tartrate and carbonate anions in Mg–Al layered double hydroxide. Appl. Clay Sci. 2008, 39, 166–171. [Google Scholar] [CrossRef]
- Ay, A.N.; Zumreoglu-Karan, B.; Temel, A.; Rives, V. Bioinorganic magnetic core-shell nanocomposites carrying antiarthritic agents: Intercalation of ibuprofen and glucuronic acid into Mg-Al-layered double hydroxides supported on magnesium ferrite. Inorg. Chem. 2009, 48, 8871–8877. [Google Scholar] [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energ. Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Chen, M.; Zhuo, G.; Ji, R.; Wang, S.; Zhang, L.; Zhang, M.; Li, H. Comparison of Biochar Materials Derived from Coconut Husks and Various Types of Livestock Manure, and Their Potential for Use in Removal of H2S from Biogas. Sustainability 2021, 13, 6262. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Qian, G.; Xu, Z.P.; Chen, C.; Liu, Q. Reinvestigation of Dehydration and Dehydroxylation of Hydrotalcite-like Compounds through Combined TG-DTA-MS Analyses. J. Phys. Chem. C 2010, 114, 10768–10774. [Google Scholar] [CrossRef]
- Pang, X.; Liu, Y.; Chen, L.; Zhong, Y.; Li, Z.; Liu, M.; Li, S. Preparation and formation mechanism of pure phase Ca2Al-layered double hydroxides nanosheets synthesized by a T-type microchannel reactor: Application as hardening accelerator for mortar. Appl. Clay Sci. 2018, 166, 174–180. [Google Scholar] [CrossRef]
- Wang, Q.W.; Chai, L.Y.; Wang, Y.Y.; Li, Q.Z.; Shu, Y.D. Novel Technology for Treatment of Acidic Wastewater Containing Mercury in Zinc Smelter by Biologics. In Proceedings of the Symposium on Extraction and Processing Division Held at the TMS 2009 Annual Meeting and Exhibition, San Francisco, CA, USA, 15–19 February 2009; pp. 1013–1017. [Google Scholar]
- Xu, Z.P.; Jin, Y.; Liu, S.; Hao, Z.P.; Lu, G.Q. Surface charging of layered double hydroxides during dynamic interactions of anions at the interfaces. J. Colloid Interface Sci. 2008, 326, 522–529. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). Acs. Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Wang, F.; Jin, L.T.; Guo, C.N.; Min, L.J.; Zhang, P.; Sun, H.W.; Zhu, H.K.; Zhang, C.P. Enhanced heavy metals sorption by modified biochars derived from pig manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Zhang, C.; Liu, Y.G.; Zeng, G.M.; Lai, C.; Gong, X.M.; Cheng, M.; Wan, J.; Zhang, Q. Insights into the effect of chemical treatment on the physicochemical characteristics and adsorption behavior of pig manure-derived biochars. Environ. Sci. Pollut. R 2019, 26, 1962–1972. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.X.; Fan, G.J.; Wang, X.; Li, H.B.; Li, H.; Xu, X.Y. Enhanced adsorption of Pb(II) by phosphorus-modified chicken manure and Chinese medicine residue co-pyrolysis biochar. Microsc. Res. Tech. 2022, 85, 3589–3599. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Zhang, J.Q.; Huang, L.; Yuan, Z.H.; Li, Z.J.; Liu, M.C. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Integr. Agr. 2019, 18, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, H.; Zhang, C.; Huang, D.; Zhu, Y.; Chai, X. The mechanism of Cd sorption by silkworm excrement organic fertilizer and its effect on Cd accumulation in rice. J. Soils Sediments 2022, 22, 2184–2195. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Yuan, L.; Zhou, D. Comparison of adsorption behavior studies of Cd2+ by vermicompost biochar and KMnO4-modified vermicompost biochar. J. Environ. Manag. 2020, 256, 109959. [Google Scholar] [CrossRef]
- Hong, C.; Dong, Z.; Zhang, J.; Zhu, L.; Che, L.; Mao, F.; Qiu, Y. Effectiveness and mechanism for the simultaneous adsorption of Pb(II), Cd(II) and As(III) by animal-derived biochar/ferrihydrite composite. Chemosphere 2022, 293, 133583. [Google Scholar] [CrossRef]
- González, M.A.; Pavlovic, I.; Rojas-Delgado, R.; Barriga, C. Removal of Cu2+, Pb2+ and Cd2+ by layered double hydroxide–humate hybrid. Sorbate and sorbent comparative studies. Chem. Eng. J. 2014, 254, 605–611. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Ma, J.; Xia, P.; Zhao, J. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste–struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Chen, Z.; Zhou, X.; Wang, J.; Chen, Z. Engineered biochar with anisotropic layered double hydroxide nanosheets to simultaneously and efficiently capture Pb2+ and CrO42− from electroplating wastewater. Bioresour. Technol. 2020, 306, 123118. [Google Scholar] [CrossRef]
- Lee, S.; Dyer, J.A.; Sparks, D.L.; Scrivner, N.C.; Elzinga, E.J. A multi-scale assessment of Pb(II) sorption on dolomite. J. Colloid Interface Sci. 2006, 298, 20–30. [Google Scholar] [CrossRef]
- Kim, E.J.; Herrera, J.E. Characteristics of Lead Corrosion Scales Formed during Drinking Water Distribution and Their Potential Influence on the Release of Lead and Other Contaminants. Environ. Sci. Technol. 2010, 44, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Morales, G.E.M.; Garcia, M.E.A.; Cruz, S.C.; Plata, B.R.; Moreno, O.P.; Perez, R.G. CdCO3 nanocrystalline thin film grown by chemical bath and its transition to porous CdO by thermal annealing treatment. Optik 2018, 171, 347–355. [Google Scholar] [CrossRef]
- Li, B.; Zhou, J.; Lu, Y.; Xiong, Z.Q. Field-aged biochar reduces the greenhouse gas balance in a degraded vegetable field treated by reductive soil disinfestation. Environ. Sci. Pollut. Res. 2019, 26, 10609–10620. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, T.; Zhang, J.; Zhu, L. Potential Mechanism of Long-Term Immobilization of Pb/Cd by Layered Double Hydroxide Doped Chicken-Manure Biochar. Int. J. Environ. Res. Public Health 2023, 20, 867. https://doi.org/10.3390/ijerph20010867
Zhang X, Liu T, Zhang J, Zhu L. Potential Mechanism of Long-Term Immobilization of Pb/Cd by Layered Double Hydroxide Doped Chicken-Manure Biochar. International Journal of Environmental Research and Public Health. 2023; 20(1):867. https://doi.org/10.3390/ijerph20010867
Chicago/Turabian StyleZhang, Xiaoxian, Tingran Liu, Jichen Zhang, and Ling Zhu. 2023. "Potential Mechanism of Long-Term Immobilization of Pb/Cd by Layered Double Hydroxide Doped Chicken-Manure Biochar" International Journal of Environmental Research and Public Health 20, no. 1: 867. https://doi.org/10.3390/ijerph20010867
APA StyleZhang, X., Liu, T., Zhang, J., & Zhu, L. (2023). Potential Mechanism of Long-Term Immobilization of Pb/Cd by Layered Double Hydroxide Doped Chicken-Manure Biochar. International Journal of Environmental Research and Public Health, 20(1), 867. https://doi.org/10.3390/ijerph20010867





