Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case–Control Studies
Abstract
:1. Introduction
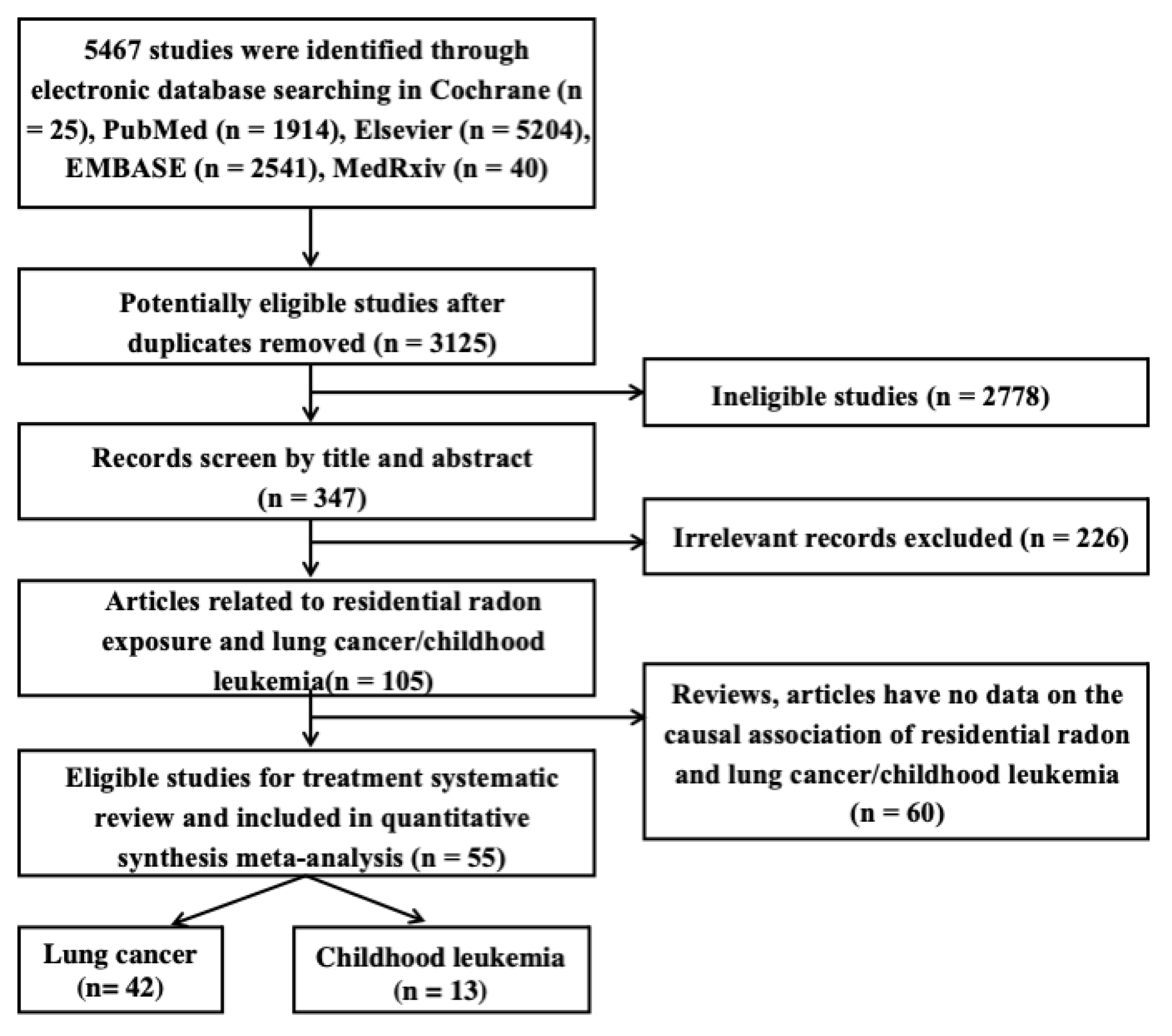
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection and Data Extraction
2.2.1. Study Selection
2.2.2. Data Extraction
2.3. Meta-Analysis
2.4. Quality Assessment of Included Studies
3. Results and Discussion
3.1. Characteristics of Included Studies
Description of Included Studies
3.2. Association between Residential Radon Exposure and Human Cancers
3.2.1. Increased Incidence of Lung Cancer
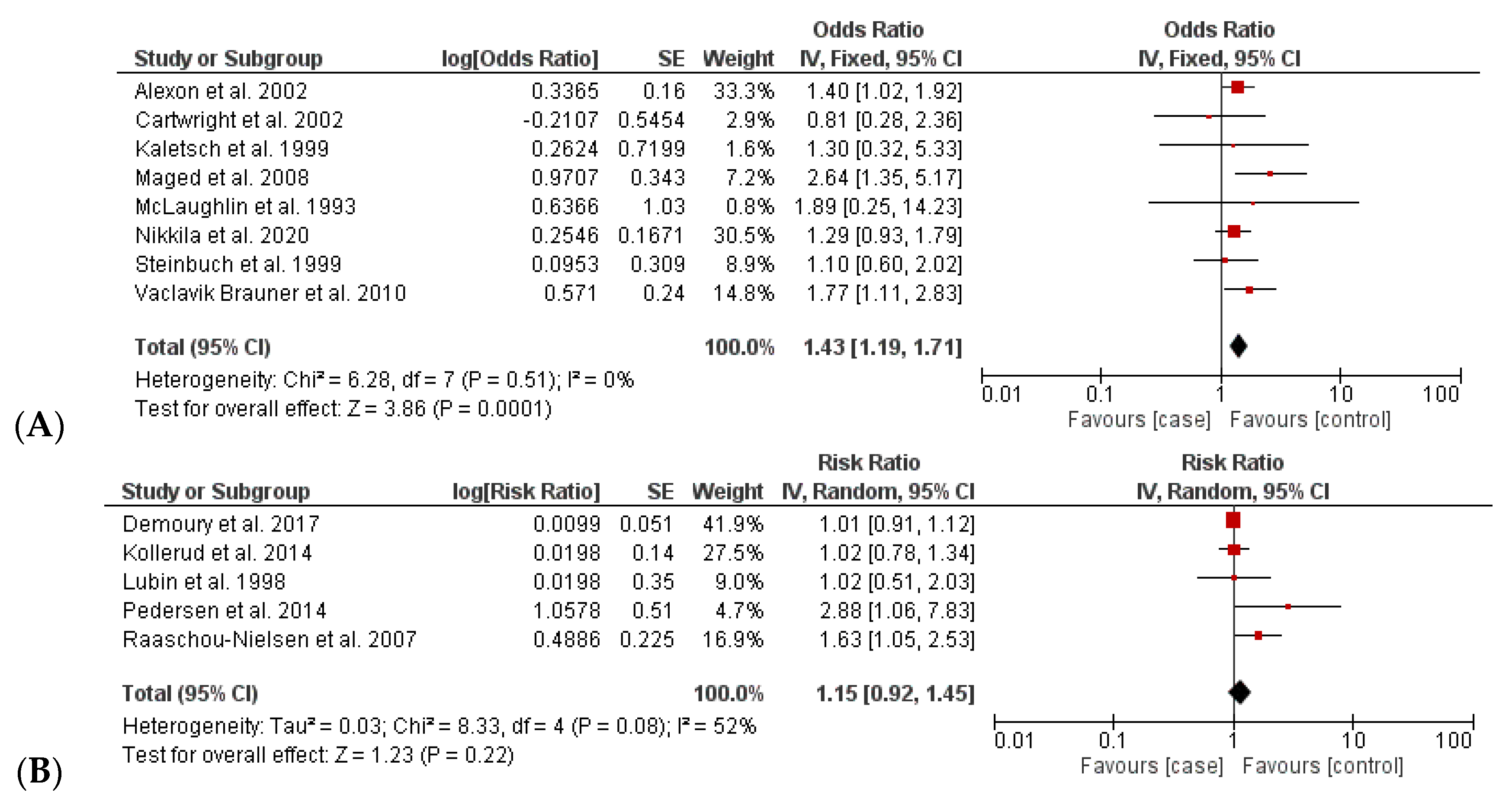
3.2.2. Increased Incidence of Childhood Leukemia
3.3. Subgroup Analysis
3.4. Dose–Response Analyses
3.5. Analysis Bias
4. Discussion
5. Limitations of Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Radon and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/radon-and-health (accessed on 3 November 2022).
- Li, C.; Wang, C.; Yu, J.; Fan, Y.; Liu, D.; Zhou, W.; Shi, T. Residential Radon and Histological Types of Lung Cancer: A Meta-Analysis of Case—Control Studies. Int. J. Environ. Res. Public Health 2020, 17, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Observatory, G. Cancer Estimated Number of New Cases and Number of Deaths in 2020, World, Both Sexes, All Ages. Available online: https://gco.iarc.fr/ (accessed on 12 December 2022).
- Darby, S.; Whitley, E.; Silcocks, P.; Thakrar, B.; Green, M.; Lomas, P.; Miles, J.; Reeves, G.; Fearn, T.; Doll, R. Risk of Lung Cancer Associated with Residential Radon Exposure in South-West England: A Case-Control Study. Br. J. Cancer 1998, 78, 394–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo-González, M.; Ruano-Ravina, A.; Torres-Durán, M.; Kelsey, K.T.; Provencio, M.; Parente-Lamelas, I.; Leiro-Fernández, V.; Vidal-García, I.; Castro-Añón, O.; Martínez, C.; et al. Lung Cancer and Residential Radon in Never-Smokers: A Pooling Study in the Northwest of Spain. Environ. Res. 2019, 172, 713–718. [Google Scholar] [CrossRef]
- Riudavets, M.; Garcia de Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef] [PubMed]
- Binger, C.M.; Ablin, A.R.; Feuerstein, R.C.; Kushner, J.H.; Zoger, S.; Mikkelsen, C. Childhood Leukemia. N. Engl. J. Med. 1969, 280, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Buffler, P.A.; Kwan, M.L.; Reynolds, P.; Urayama, K.Y. Environmental and Genetic Risk Factors for Childhood Leukemia: Appraising the Evidence. Cancer Investig. 2005, 23, 60–75. [Google Scholar] [CrossRef]
- Little, M.P.; Wakeford, R.; Borrego, D.; French, B.; Zablotska, L.B.; Adams, M.J.; Allodji, R.; de Vathaire, F.; Lee, C.; Brenner, A. V Leukaemia and Myeloid Malignancy among People Exposed to Low Doses (<100 MSv) of Ionising Radiation during Childhood: A Pooled Analysis of Nine Historical Cohort Studies. Lancet Haematol. 2018, 5, e346–e358. [Google Scholar]
- Laurent, O.; Ancelet, S.; Richardson, D.B.; Hémon, D.; Ielsch, G.; Demoury, C.; Clavel, J.; Laurier, D. Potential Impacts of Radon, Terrestrial Gamma and Cosmic Rays on Childhood Leukemia in France: A Quantitative Risk Assessment. Radiat. Environ. Biophys. 2013, 52, 195–209. [Google Scholar] [CrossRef]
- Mclaughlin, J.R.; King, W.D.; Anderson, T.W.; Clarke, E.A.; Ashmore, J.P.; King, W.D.; Clarke, E.A.; Anderson, T.W. Paternal Radiation Exposure and Leukemia in Offspring: The Ontario Case-Control Study. BMJ 1993, 307, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Bräuner, E.V.; Andersen, C.E.; Andersen, H.P.; Gravesen, P.; Lind, M.; Ulbak, K.; Hertel, O.; Schüz, J.; Raaschou-Nielsen, O. Is There Any Interaction between Domestic Radon Exposure and Air Pollution from Traffic in Relation to Childhood Leukemia Risk? Cancer Causes Control 2010, 21, 1961–1964. [Google Scholar] [CrossRef]
- Bochicchio, F.; Forastiere, F.; Farchi, S.; Quarto, M.; Axelson, O. Residential Radon Exposure, Diet and Lung Cancer: A Case-Control Study in a Mediterranean Region. Int. J. Cancer 2005, 114, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.L.; Wang, X.R.; Qiu, H.; Yu, I.T.S. Risk Factors for Lung Cancer: A Case-Control Study in Hong Kong Women. Cancer Causes Control 2010, 21, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Gerken, M.; Kreienbrock, L.; Wellmann, J.; Wichmann, H.E. Lung Cancer in Lifetime Nonsmoking Men—Results of a Case-Control Study in Germany. Br. J. Cancer 2001, 84, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbuch, M.; Weinberg, C.R.; Buckley, J.D.; Robison, L.L.; Sandler, D.P. Indoor Residential Radon Exposure and Risk of Childhood Acute Myeloid Leukaemia. Br. J. Cancer 1999, 81, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Yoo, H.K. Residential Radon Exposure and Leukemia: A Meta-Analysis and Dose-Response Meta-Analyses for Ecological, Case-Control, and Cohort Studies. Environ. Res. 2021, 202, 111714. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021; ISBN 1119558387. [Google Scholar]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; pp. 1–12. [Google Scholar]
- Alavanja, M.C.R.; Lubin, J.H.; Mahaffey, J.A.; Brownson, R.C. Residential Radon Exposure and Risk of Lung Cancer in Missouri. Am. J. Public Health 1999, 89, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- Barros-Dios, J.M.; Barreiro, M.A.; Ruano-Ravina, A.; Figueiras, A. Exposure to Residential Radon and Lung Cancer in Spain: A Population-Based: Case-Control Study. Am. J. Epidemiol 2002, 156, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Alavanja, M.C.R.; Brownson, R.C.; Benichou, J.; Swanson, C.; Boice, J.D. Attributable Risk of Lung Cancer in Lifetime Nonsmokers and Long-Term Ex-Smokers (Missouri, United States). Cancer Causes Control 1995, 6, 209–216. [Google Scholar] [CrossRef]
- Field, R.W.; Steck, D.J.; Smith, B.J.; Brus, C.P.; Fisher, E.L.; Neuberger, J.S.; Platz, C.E.; Robinson, R.A.; Woolson, R.F.; Lynch, C.F. Residential Radon Gas Exposure and Lung Cancer: The Iowa Radon Lung Cancer Study. Am. J. Epidemiol. 2000, 151, 1091–1102. [Google Scholar] [CrossRef] [Green Version]
- Field, R.W.; Steck, D.J.; Smith, B.J.; Brus, C.P.; Fisher, E.L.; Neuberger, J.S.; Lynch, C.F. The Iowa Radon Lung Cancer Study—Phase I: Residential Radon Gas Exposure and Lung Cancer. Sci. Total Environ. 2001, 272, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Axelsson, G.; Damber, L.; Mellander, H.; Nyberg, F.; Pershagen, G. Residential Radon and Lung Cancer among Never-Smokers in Sweden. Epidemiology 2001, 12, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Falk, R.; Almren, K.; Nyberg, F.; Svensson, H.; Pershagen, G. Glass-Based Radon-Exposure Assessment and Lung Cancer Risk. J. Expo. Sci. Environ. Epidemiol. 2002, 12, 344–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demoury, C.; Marquant, F.; Ielsch, G.; Goujon, S.; Debayle, C.; Faure, L.; Coste, A.; Laurent, O.; Guillevic, J.; Laurier, D.; et al. Residential Exposure to Natural Background Radiation and Risk of Childhood. Environ. Health Perspect. 2017, 125, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Nyberg, F.; Bellander, T.; Pershagen, G.; Gustavsson, P. Urban Air Pollution and Lung Cancer in Stockholm. Epidemiology 2001, 12, 591–592. [Google Scholar] [CrossRef]
- Del Risco Kollerud, R.; Blaasaas, K.G.; Claussen, B. Risk of Leukaemia or Cancer in the Central Nervous System among Children Living in an Area with High Indoor Radon Concentrations: Results from a Cohort Study in Norway. Br. J. Cancer 2014, 111, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, C.; Bräuner, E.V.; Rod, N.H.; Albieri, V.; Andersen, C.E.; Ulbak, K.; Hertel, O.; Johansen, C.; Schüz, J.; Raaschou-Nielsen, O. Distance to High-Voltage Power Lines and Risk of Childhood Leukemia—An Analysis of Confounding by and Interaction with Other Potential Risk Factors. PLoS ONE 2014, 9, e107096. [Google Scholar] [CrossRef] [Green Version]
- Nikkilä, A.; Arvela, H.; Mehtonen, J.; Raitanen, J.; Heinäniemi, M.; Lohi, O.; Auvinen, A. Predicting Residential Radon Concentrations in Finland: Model Development, Validation, and Application to Childhood Leukemia. Scand. J. Work Environ. Health 2020, 46, 278–292. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, C.E.; Andersen, H.P.; Gravesen, P.; Lind, M.; Schüz, J.; Ulbak, K. Domestic Radon and Childhood Cancer in Denmark. Epidemiology 2007, 18, S107. [Google Scholar] [CrossRef]
- Alavanja, M.C.R.; Brownson, R.C.; Lubin, J.H.; Berger, E.; Chang, J.; Boice, J.D., Jr. Residential Radon Exposure and Lung Cancer among Nonsmoking Women. JNCI J. Natl. Cancer Inst. 1994, 86, 1829–1837. [Google Scholar] [CrossRef]
- Auvinen, A.; Mäkeläinen, I.; Hakama, M.; Castrén, O.; Pukkala, E.; Reisbacka, H.; Rytömaa, T. Indoor Radon Exposure and Risk of Lung Cancer: A Nested Case—Control Study in Finland. JNCI J. Natl. Cancer Inst. 1996, 88, 966–972. [Google Scholar] [CrossRef]
- Barros-Dios, J.M.; Ruano-Ravina, A.; Pérez-Ríos, M.; Castro-Bernárdez, M.; Abal-Arca, J.; Tojo-Castro, M. Residential Radon Exposure, Histologic Types, and Lung Cancer Risk. A Case—Control Study in Galicia, Spain. Cancer Epidemiol. Biomark. Prev. 2012, 21, 951–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baysson, H.; Tirmarche, M.; Tymen, G.; Gouva, S.; Caillaud, D.; Artus, J.C.; Vergnenegre, A.; Ducloy, F.; Laurier, D. Indoor Radon and Lung Cancer in France. Epidemiology 2004, 15, 709–716. [Google Scholar] [CrossRef]
- Hystad, P.; Brauer, M.; Demers, P.A.; Johnson, K.C.; Setton, E.; Cervantes-Larios, A.; Poplawski, K.; McFarlane, A.; Whitehead, A.; Nicol, A.M. Geographic Variation in Radon and Associated Lung Cancer Risk in Canada. Can. J. Public Health 2014, 105, e4–e10. [Google Scholar] [CrossRef] [PubMed]
- Kreienbrock, L.; Kreuzer, M.; Gerken, M.; Dingerkus, G.; Wellmann, J.; Keller, G.; Erich Wichmann, H. Case-Control Study on Lung Cancer and Residential Radon in Western Germany. Am. J. Epidemiol. 2001, 153, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuzer, M.; Heinrich, J.; Wölke, G.; Rosario, A.S.; Gerken, M.; Wellmann, J.; Keller, G.; Kreienbrock, L.; Wichmann, H.-E. Residential Radon and Risk of Lung Cancer in Eastern Germany. Epidemiology 2003, 14, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Yoshinaga, S.; Li, X.; Lei, S.; Zhang, S.; Sun, Q.; Koriyama, C.; Akiba, S.; Tokonami, S. The First Attempt to Reevaluate Radon and Thoron Exposure in Gansu Province Study Using Radon-Thoron Discriminating Measurement Technique. Front Public Health 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Letourneau, E.G.; Krewski, D.; Choi, N.W.; Goddard, M.J.; McGregor, R.G.; Zielinski, J.M.; Du, J. Case-Control Study of Residential Radon and Lung Cancer in Winnipeg, Manitoba, Canada. Am. J. Epidemiol. 1994, 140, 310–322. [Google Scholar] [CrossRef]
- Lorenzo-Gonzalez, M.; Ruano-Ravina, A.; Torres-Duran, M.; Kelsey, K.T.; Provencio, M.; Parente-Lamelas, I.; Piñeiro-Lamas, M.; Varela-Lema, L.; Perez-Rios, M.; Fernandez-Villar, A.; et al. Lung Cancer Risk and Residential Radon Exposure: A Pooling of Case-Control Studies in Northwestern Spain. Environ. Res. 2020, 189, 109968. [Google Scholar] [CrossRef]
- Lubin, J.H. Studies of Radon and Lung Cancer in North America and China. Radiat. Prot. Dosimetry. 2003, 104, 315–319. [Google Scholar] [CrossRef]
- Lubin, J.H.; Wang, Z.Y.; Boice, J.D.; Xu, Z.Y.; Blot, W.J.; De Wang, L.; Kleinerman, R.A. Risk of Lung Cancer and Residential Radon in China: Pooled Results of Two Studies. Int. J. Cancer 2004, 109, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, H.; Kim, H.C.; Sheen, S.S.; Koh, S.B.; Park, K.S.; Cho, N.H.; Lee, C.M.; Kang, D.R. Residential Radon Exposure and Cigarette Smoking in Association with Lung Cancer: A Matched Case-Control Study in Korea. Int. J. Environ. Res. Public Health 2020, 17, 2946. [Google Scholar] [CrossRef] [PubMed]
- Pershagen, G.; Liang, Z.-H.; Hrubec, Z.; Svensson, C.; Boice, J.D., Jr. Residential Radon Exposure and Lung Cancer in Swedish Women. Health Phys. 1992, 63, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pershagen, G.; Akerblom, G.; Axelson, O.; Clavensjo, B.; Damber, L.; Desai, G.; Enflo, A.; Lagarde, F.; Mellander, H.; Svartengren, M. Residential Radon Exposure and Lung Cancer in Sweden. N. Engl. J. Med. 1994, 330, 159–164. [Google Scholar] [CrossRef]
- Pisa, F.E.; Barbone, F.; Betta, A.; Bonomi, M.; Alessandrini, B.; Bovenzi, M. Residential Radon and Risk of Lung Cancer in an Italian Alpine Area. Arch. Environ. Health Int. J. 2001, 56, 208–215. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Cameselle-Lago, C.; Torres-Durán, M.; Pando-Sandoval, A.; Dacal-Quintas, R.; Valdés-Cuadrado, L.; Hernández-Hernández, J.; Consuegra-Vanegas, A.; Tenes-Mayén, J.A.; Varela-Lema, L.; et al. Indoor Radon Exposure and COPD, Synergic Association? A Multicentric, Hospital-Based Case–Control Study in a Radon-Prone Area. Arch. Bronconeumol. 2021, 57, 630–636. [Google Scholar] [CrossRef]
- Sandler, D.P.; Weinberg, C.R.; Shore, D.L.; Archer, V.E.; Bishop Stone, M.; Lyon, J.L.; Rothney-Kozlak, L.; Shepherd, M.; Stolwijk, J.A.J. Indoor Radon and Lung Cancer Risk in Connecticut and Utah. J. Toxicol. Environ. Health A 2006, 69, 633–654. [Google Scholar] [CrossRef]
- Schoenberg, J.B.; Klotz, J.B.; Wilcox, H.B.; Nicholls, G.P.; Gil-del-Real, M.T.; Stemhagen, A.; Mason, T.J. Case-Control Study of Residential Radon and Lung Cancer among New Jersey Women. Cancer Res. 1990, 50, 6520–6524. [Google Scholar]
- Sobue, T.; Lee, V.S.; Ye, W.; Tanooka, H.; Mifune, M.; Suyama, A.; Koga, T.; Morishima, H.; Kondo, S. Residential Radon Exposure and Lung Cancer Risk in Misasa, Japan: A Case-Control Study. J. Radiat. Res. 2000, 41, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Svensson, C.; Pershagen, G.; Klominek, J. Lung Cancer in Women and Type of Dwelling in Relation to Radon Exposure. Cancer Res. 1989, 49, 1861–1865. [Google Scholar]
- Thompson, R.E. Epidemiological Evidence for Possible Radiation Hormesis from Radon Exposure: A Case-Control Study Conducted in Worcester, MA. Dose-Response 2011, 9, 59–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Durán, M.; Ruano-Ravina, A.; Parente-Lamelas, I.; Leiro-Fernández, V.; Abal-Arca, J.; Montero-Martínez, C.; Pena-Álvarez, C.; González-Barcala, F.J.; Castro-Añón, O.; Golpe-Gómez, A.; et al. Lung Cancer in Never-Smokers: A Case-Control Study in a Radon-Prone Area (Galicia, Spain). Eur. Respir. J. 2014, 44, 994–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Duran, M.; Ruano-Ravina, A.; Parente-Lamelas, I.; Leiro-Fernandez, V.; Abal-Arca, J.; Montero-Martinez, C.; Pena-Alvarez, C.; Castro-Anon, O.; Golpe-Gomez, A.; Martinez, C. Residential Radon and Lung Cancer Characteristics in Never Smokers. Int. J. Radiat. Biol. 2015, 91, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Tse, L.A.; Yu, I.T.S.; Qiu, H.; Au, J.S.K.; Wang, X.R. A Case-Referent Study of Lung Cancer and Incense Smoke, Smoking, and Residential Radon in Chinese Men. Environ. Health Perspect. 2011, 119, 1641–1646. [Google Scholar] [CrossRef]
- Tse, L.A.; Wang, F.; Wong, M.C.-S.; Au, J.S.-K.; Yu, I.T.-S. Risk Assessment and Prediction for Lung Cancer among Hong Kong Chinese Men. BMC Cancer 2022, 22, 585. [Google Scholar] [CrossRef]
- Wang, Z.; Lubin, J.H.; Wang, L.; Zhang, S.; Boice, J.D.; Cui, H.; Zhang, S.; Conrath, S.; Xia, Y.; Shang, B.; et al. Residential Radon and Lung Cancer Risk in a High-Exposure Area of Gansu Province, China. Am. J. Epidemiol. 2002, 155, 554–564. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, H.E.; Rosario, A.S.; Heid, I.M.; Kreuzer, M.; Heinrich, J.; Kreienbrock, L. Increased Lung Cancer Risk Due to Residential Radon in a Pooled and Extended Analysis of Studies in Germany. Health Phys. 2005, 88, 71–79. [Google Scholar] [CrossRef]
- Wilcox, H.B.; Al-Zoughool, M.; Garner, M.J.; Jiang, H.; Klotz, J.B.; Krewski, D.; Nicholson, W.J.; Schoenberg, J.B.; Villeneuve, P.J.; Zielinski, J.M. Case-Control Study of Radon and Lung Cancer in New Jersey. Radiat. Prot. Dosim. 2008, 128, 169–179. [Google Scholar] [CrossRef]
- Axelson, O.; Fredrikson, M.; Åkerblom, G.; Hardell, L. Leukemia in Childhood and Adolescence and Exposure to Ionizing Radiation in Homes Built from Uranium-Containing Alum Shale Concrete. Epidemiology 2002, 13, 146–150. [Google Scholar] [CrossRef]
- Kaletsch, U.; Kaatsch, P.; Meinert, R.; Schüz, J.; Czarwinski, R.; Michaelis, J. Childhood Cancer and Residential Radon Exposure—Results of a Population-Based Case-Control Study in Lower Saxony (Germany). Radiat. Environ. Biophys. 1999, 38, 211–215. [Google Scholar] [CrossRef]
- Lubin, J.H.; Linet, M.S.; Boice, J.D.; Buckley, J.; Conrath, S.M.; Hatch, E.E.; Kleinerman, R.A.; Tarone, R.E.; Wacholder, S.; Robison, L.L. Case-Control Study of Childhood Acute Lymphoblastic Leukemia and Residential Radon Exposure. J. Natl. Cancer Inst 1998, 90, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maged, A.F.; Mokhtar, G.M.; El-Tobgui, M.M.; Gabbr, A.A.; Attia, N.I.; Shady, M.M.A. Domestic Radon Concentration and Childhood Cancer Study in Cairo, Egypt. J. Environ. Sci. Health Part C 2000, 18, 153–170. [Google Scholar] [CrossRef]
- Cartwright, R.A.; Law, G.; Roman, E.; Gilman, E.; Eden, O.B.; Mott, M.; Muir, K.; Goodhead, D.; Kendall, G.; Doll, R.; et al. The United Kingdom Childhood Cancer Study of Exposure to Domestic Sources of Ionising Radiation: I: Radon Gas. Br. J. Cancer 2002, 86, 1721–1726. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Kropat, G.; Bochud, F.; Jaboyedoff, M.; Laedermann, J.-P.; Murith, C.; Palacios, M.; Baechler, S. Major Influencing Factors of Indoor Radon Concentrations in Switzerland. J. Environ. Radioact. 2014, 129, 7–22. [Google Scholar] [CrossRef]
- Symonds, P.; Rees, D.; Daraktchieva, Z.; McColl, N.; Bradley, J.; Hamilton, I.; Davies, M. Home Energy Efficiency and Radon: An Observational Study. Indoor Air 2019, 29, 854–864. [Google Scholar] [CrossRef] [Green Version]
- Peckham, E.C.; Scheurer, M.E.; Danysh, H.E.; Lubega, J.; Langlois, P.H.; Lupo, P.J. Residential Radon Exposure and Incidence of Childhood Lymphoma in Texas, 1995–2011. Int. J. Environ. Res. Public Health 2015, 12, 12110–12126. [Google Scholar] [CrossRef] [Green Version]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in Homes and Risk of Lung Cancer: Collaborative Analysis of Individual Data from 13 European Case-Control Studies. Br. Med. J. 2005, 330, 223–226. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Chen, Q.; Wei, J.; Cao, G.; Zhang, J. Domestic Radon Exposure and Risk of Childhood Leukemia: A Meta-Analysis. J. Buon 2020, 25, 1035–1041. [Google Scholar]


| References | Study Location | Period of Investigation | Ages | Human Cancers | Case/Control | Exposure Comparison (Bq/m3) | Types of Size Effects | Adjustment Criteria |
|---|---|---|---|---|---|---|---|---|
| Alavanja et al., 1994 [34] | USA | 1986–1992 | 55–80 | Lung cancer | 538/1183 | >100 vs. <29 | OR | Age, sex, and smoking status |
| Alavanja et al., 1995 [23] | USA | 1986–1992 | 55–75 | Lung cancer | 618/1402 | >200 vs. <50 | OR | Age and sex |
| Alavanja et al., 1999 [21] | USA | 1993–1994 | 65–84 | Lung cancer | 512/3886 | >148 vs. <37 Increase of 100 Bq/m3 | OR | Age, sex, education, and smoking status |
| Auvinen et al., 1996 [35] | Finland | 1986–1992 | 54–75 | Lung cancer | 1055/1544 | >200 vs. <50 Increase of 100 Bq/m3 | OR | Cigarette smoking, intensity, duration, and age |
| Barros-Dios et al., 2002 [22] | Spain | 1992–1994 | 35–70 | Lung cancer | 163/241 | 148 vs. <37 | OR | Sex, age, lifetime tobacco use, family history, and habitat |
| Barros-Dios et al., 2012 [36] | Spain | 2004–2008 | 50–70 | Lung cancer | 349/513 | >148 vs. <50 Increase of 100 Bq/m3 | OR | Age, sex, and tobacco consumption |
| Baysson et al., 2004 [37] | France | 1992–1998 | 40–75 | Lung cancer | 486/984 | >200 vs. <50 | OR | Age, sex, region, smoking status, and occupational exposure |
| Bochicchio et al., 2005 [13] | Sweden | 1993–1996 | 35–90 | Lung cancer | 384/404 | >200 vs. <50 Increase of 100 Bq/m3 | OR | Age, smoking, and diet |
| Chiu et al., 2010 [14] | Hong Kong | 2002–2004 | 50–70 | Lung cancer | 279/322 | >150 vs. <25 | OR | Age, employment, and years of education |
| Darby et al., 1998 [4] | UK | 1988–1993 | 45–73 | Lung cancer | 982/3185 | 200 vs. <25 | OR | Age, sex, smoking status, country of residence, and social class |
| Field et al., 2000 [24] | USA | 1993–1997 | 40–84 | Lung cancer | 413/614 | 148 vs. <50 | OR | Age, smoking, and education |
| Hystad et al., 2014 [38] | Canada | 1994–1997 | 59–63 | Lung cancer | 2390/3507 | >50 | OR | Age, sex, smoke consumption |
| Kreienbrock et al., 2001 [39] | Germany | 1990–1996 | 40–80 | Lung cancer | 1449/2297 | >140 vs. <50 Increase of 100 Bq/m3 | OR | Age and sex |
| Kreuzer et al., 2001 [15] | Germany | 1990–1996 | 50–74 | Lung cancer | 58/803 | Increase of 100 Bq/m3 | OR | Age, region, occupational carcinogens, and indoor radon |
| Kreuzer et al., 2003 [40] | Germany | 1990–1997 | 50–75 | Lung cancer | 1192/1640 | >140 vs. <50 | OR | Smoking and asbestos exposure |
| Kudo et al., 2021 [41] | China | 2005–2007 | 39–77 | Lung cancer | 30/39 | >100 vs. < 50 Increase of 100 Bq/m3 | OR | Age, smoking, and income |
| Lagarde et al., 2000 [26] | Sweden | 1980–1995 | 35–74 | Lung cancer | 258/487 | 140 vs. <50 | OR | Age, sex, and smoking status |
| Lagarde et al., 2002 [27] | Sweden | 1985–1995 | 40–70 | Lung cancer | 110/231 | 140 vs. <50 | RR | Age, sex, and smoking status |
| Letourneau et al., 1994 [42] | Canada | 1983–1990 | 25–76 | Lung cancer | 738/738 | 375 per month | OR | Age and sex |
| Lorenzo-Gonzalez et al., 2019 [5] | Spain | 2002–2017 | 57–78 | Lung cancer | 523/892 | ≥200 vs. ≤100 | OR | Age, sex, and environmental tobacco-smoke exposure |
| Lorenzo-Gonzalez et al., 2020 [43] | Spain | 2004–2019 | 54–71 | Lung cancer | 1842/1862 | >200 vs. <50 | OR | Age, sex, and never smoker |
| Lubin et al., 2003 [44] | USA | 1992–2000 | 35–70 | Lung cancer | 4081/5281 | Increase of 100 Bq/m3 | OR | Age and sex |
| Lubin et al., 2004 [45] | China | 1985–1987 | 40–75 | Lung cancer | 1053/1997 | 200 vs. <100 Increase of 100 Bq/m3 | OR | Age, sex, and smoking status |
| Nyberg et al., 2000 [29] | Sweden | 1950–1990 | 40–75 | Lung cancer | 1042/2364 | >116 vs. <78 Increase of 100 Bq/m3 | OR | Tobacco smoking, socioeconomic status, residential radon, and occupational exposures |
| Park et al., 2020 [46] | Korea | 2015–2018 | 57–72 | Lung cancer | 519/519 | ≥100 vs. 25 | OR | Age, sex, and indoor hours, and smoking status |
| Pershagen et al., 1992 [47] | Swedish | 1983–1986 | 35–70 | Lung cancer | 210/209 | >150 vs. <24 | OR | Age, sex, and smoking status |
| Pershagen et al., 1994 [48] | Sweden | 1980–1984 | 35–74 | Lung cancer | 1360/2847 | >400 vs. <50 Increase of 100 Bq/m3 | OR | Age and sex |
| Pisa et al., 2000 [49] | Italy | 1991–1997 | 30–70 | Lung cancer | 138/291 | >200 vs. <40 | OR | Age and sex |
| Ruano-Ravina et al., 2021 [50] | Spain | 2018–2019 | 51–68 | Lung cancer | 189/747 | >200 vs. <50 | OR | Sex, age, and education |
| Sandler et al., 2007 [51] | USA | 1996–2006 | 40–79 | Lung cancer | 1474/1811 | <100 | RR | Age and sex |
| Schoenberg et al., 1990 [52] | USA | 1980–1989 | 35–60 | Lung cancer | 433/402 | >148 vs. <37 | OR | Age, sex, and smoking status |
| Sobue et al., 2000 [53] | Japan | 1976–1996 | 40–80 | Lung cancer | 28/36 | 100 vs. <25 | OR | Age, sex, occupational exposure, and smoking status |
| Svensson et al., 1989 [54] | Sweden | 1980–1986 | 40–70 | Lung cancer | 210/209 | >200 vs. <50 | RR | Smoking, age, and degree of urbanization |
| Thompson et al., 2011 [55] | USA | 1990–2010 | 40–70 | Lung cancer | 200/397 | ≥250 vs. <25 | OR | Smoking, residency, job exposure, income, and education |
| Torres-Duran et al., 2014 [56] | Spain | 2011–2013 | 61–79 | Lung cancer | 192/329 | 200 vs. <100 | OR | Age and sex |
| Torres-Duran et al., 2015 [57] | Spain | 2000–2012 | 50–70 | Lung cancer | 198/275 | >200 vs. <50 | OR | Age and sex |
| Tse et al., 2011 [58] | China | 2004–2006 | 35–79 | Lung cancer | 1208/1069 | >200 vs. <50 | OR | Age and sex |
| Tse et al., 2022 [59] | Hong Kong | 2004–2006 | 35–79 | Lung cancer | 1069/1208 | Increase of 100 Bq/m3 | OR | Age and sex |
| Wang et al., 2002 [60] | China | 1994–1998 | 30–75 | Lung cancer | 768/1659 | >300 vs. <50 Increase of 100 Bq/m3 | OR | Age, sex, prefecture, and tobacco use, |
| Wichmanm et al., 2005 [61] | Germany | 1990–1997 | 35–75 | Lung cancer | 2963/4232 | >140 vs. <50 Increase of 100 Bq/m3 | OR | Sex, age, and smoking status |
| Wilcox et al., 2008 [62] | Canada | 1989–1992 | 50–75 | Lung cancer | 561/740 | 150 vs. <25 Increase of 100 Bq/m3 | OR | Sex and age |
| William Field et al., 2001 [25] | USA | 1981–2001 | 40–84 | Lung cancer | 413/614 | 55–150 | OR | Age and sex |
| Axelson et al., 2002 [63] | Sweden | 1980–1989 | <20 | Childhood leukemia | 312/1418 | 100 | OR | Age, sex, and county |
| Demoury et al., 2017 [28] | France | 1990–2009 | <15 | Childhood leukemia | 9056/30000 | >89 | OR | Family income and age |
| Kaletsch et al., 1999 [64] | Germany | 1988–1993 | <15 | Childhood leukemia | 164/209 | 70 | RR | Age and sex |
| Kollerud et al., 2014 [30] | Norway | 1967–2009 | <15 | Childhood leukemia | 712/674 | >100 vs. 50 | RR | Socioeconomic |
| Lubin et al., 1998 [65] | USA | 1989–1993 | <5 | Childhood leukemia | 505/443 | ≥148 vs. <37 | RR | Age, income, sex, type of residence, parental smoking habits, and parental occupation |
| Maged et al., 2008 [66] | Egypt | 1996–1998 | 2–14 | Childhood leukemia | 50/110 | >90 vs. <40 | OR | Age and sex |
| McLaughlin et al., 1993 [11] | USA | 1950–1988 | <14 | Childhood leukemia | 112/890 | >50 vs. 0 | OR | Parental occupation exposure |
| Nikkilä et al., 2020 [32] | Finland | 1990–2011 | 2–7 | Childhood leukemia | 1093/3279 | 37 | OR | Age, parental occupation |
| Pedersen et al., 2014 [31] | France | 1968–1991 | <15 | Childhood leukemia | 879/1621 | ≥42 | RR | Socioeconomic status, maternal age, birth order |
| Raaschou-Nielsen et al., 2007 [33] | Denmark | 1968–1994 | <14 | Childhood leukemia | 860/1720 | <260 | RR | Age and sex |
| Steinbuch et al., 1999 [16] | USA | 1989–1993 | <15 | Childhood leukemia | 173/254 | >100 vs. <37 | OR | Sex, age, maternal education, family income, and maternal race |
| Cartwright et al., 2002 [67] | UK | 1992–1996 | <14 | Childhood leukemia | 2226/3773 | >200 vs. <24 | OR | Age and sex |
| Vaclavik Brauner et al., 2010 [12] | Denmark | 1986–1994 | <20 | Childhood leukemia | 985/1969 | >200 vs. <20 | OR | Birth order, mother’s age, and electromagnetic fields |
| Human Cancer | Types of Estimate Size Effect | No. of Studies | No. of Cases | No. of Controls | Pooled Estimated Size Effect | I2 | p Value |
|---|---|---|---|---|---|---|---|
| Lung cancer | OR | 39 | 30,884 | 51,759 | 1.38 [1.19; 1.60] | 90 | <0.00001 |
| RR | 3 | 1794 | 2251 | 0.85 [0.26; 2.74] | 86 | 0.0006 | |
| Childhood leukemia | OR | 8 | 12,053 | 36,804 | 1.43 [1.19; 1.72] | 0.00 | 0.51 |
| RR | 5 | 50,127 | 46,360 | 1.15 [0.92; 1.45] | 52 | 0.08 |
| Subgroup Analysis | Lung Cancer | Childhood Leukemia | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | OR (95% CI) | I2 (%) | p Value | No of Studies | OR (95% CI) | I2 (%) | p Value | |
| All studies | 39 | 1.38 [1.19; 1.60] | 90 | <0.00001 | 8 | 1.43 [1.19;1.71] | 0.00 | 0.51 |
| Study population | ||||||||
| North America | 11 | 1.16 [0.95; 1.41] | 69.55 | <0.0001 | 1 | 1.89 [0.25; 14.17] | – | – |
| Europe | 20 | 1.56 [1.37; 1.78] | 42.28 | 0.025 | 6 | 1.36 [1.12; 1.64] | 0.00 | 0.739 |
| Asia | 8 | 1.24 [0.89; 1.71] | 93.78 | <0.0001 | – | – | – | – |
| Africa | – | – | – | – | 1 | 2.64 [1.30; 5.18] | – | – |
| Period of study (years) | ||||||||
| <10 | 32 | 1.36 [1.16; 1.59] | 90.78 | <0.0001 | 6 | 1.49 [1.19; 1.86] | 12.41 | 0.336 |
| 10–20 | 6 | 1.84 [1.57; 2.15] | 0.00 | 0.545 | – | – | – | – |
| >20 | 1 | 1.13 [0.83; 1.55] | – | – | 2 | 1.30 [0.95; 1.79] | 0.00 | 0.714 |
| Level of radon exposure (Bq/m3) | ||||||||
| <100 | – | – | – | – | 4 | 1.44 [0.97; 1.78] | 18.05 | 0.301 |
| 100–150 | 23 | 1.21 [1.13; 1.28] | 63 | <0.0001 | 2 | 1.48 [0.65; 3.35] | 0.00 | 0.831 |
| ≥200 | 16 | 1.37 [1.09; 1.72] | 91 | <0.00001 | 2 | 1.56 [1.02; 2.39] | 42.35 | 0.188 |
| Smoking status | ||||||||
| Nonsmoker | 8 | 1.21 [1.12; 1.31] | 80 | <0.0001 | – | – | – | – |
| Smokers | 31 | 1.38 [1.20; 1.57] | 83 | <0.00001 | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoc, L.T.N.; Park, D.; Lee, Y.-C. Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case–Control Studies. Int. J. Environ. Res. Public Health 2023, 20, 97. https://doi.org/10.3390/ijerph20010097
Ngoc LTN, Park D, Lee Y-C. Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case–Control Studies. International Journal of Environmental Research and Public Health. 2023; 20(1):97. https://doi.org/10.3390/ijerph20010097
Chicago/Turabian StyleNgoc, Le Thi Nhu, Duckshin Park, and Young-Chul Lee. 2023. "Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case–Control Studies" International Journal of Environmental Research and Public Health 20, no. 1: 97. https://doi.org/10.3390/ijerph20010097
APA StyleNgoc, L. T. N., Park, D., & Lee, Y.-C. (2023). Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case–Control Studies. International Journal of Environmental Research and Public Health, 20(1), 97. https://doi.org/10.3390/ijerph20010097








