Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients
Abstract
1. Introduction
2. Adult Patients with Congenital Heart Disease: A Special Population with Special Needs
2.1. General Aspects of a Lifelong Chronic Condition
2.2. Follow-Up in ACHD Patients
2.3. Lifelong Continuous Surveillance in ACHD: Lessons Learnt from COVID-19
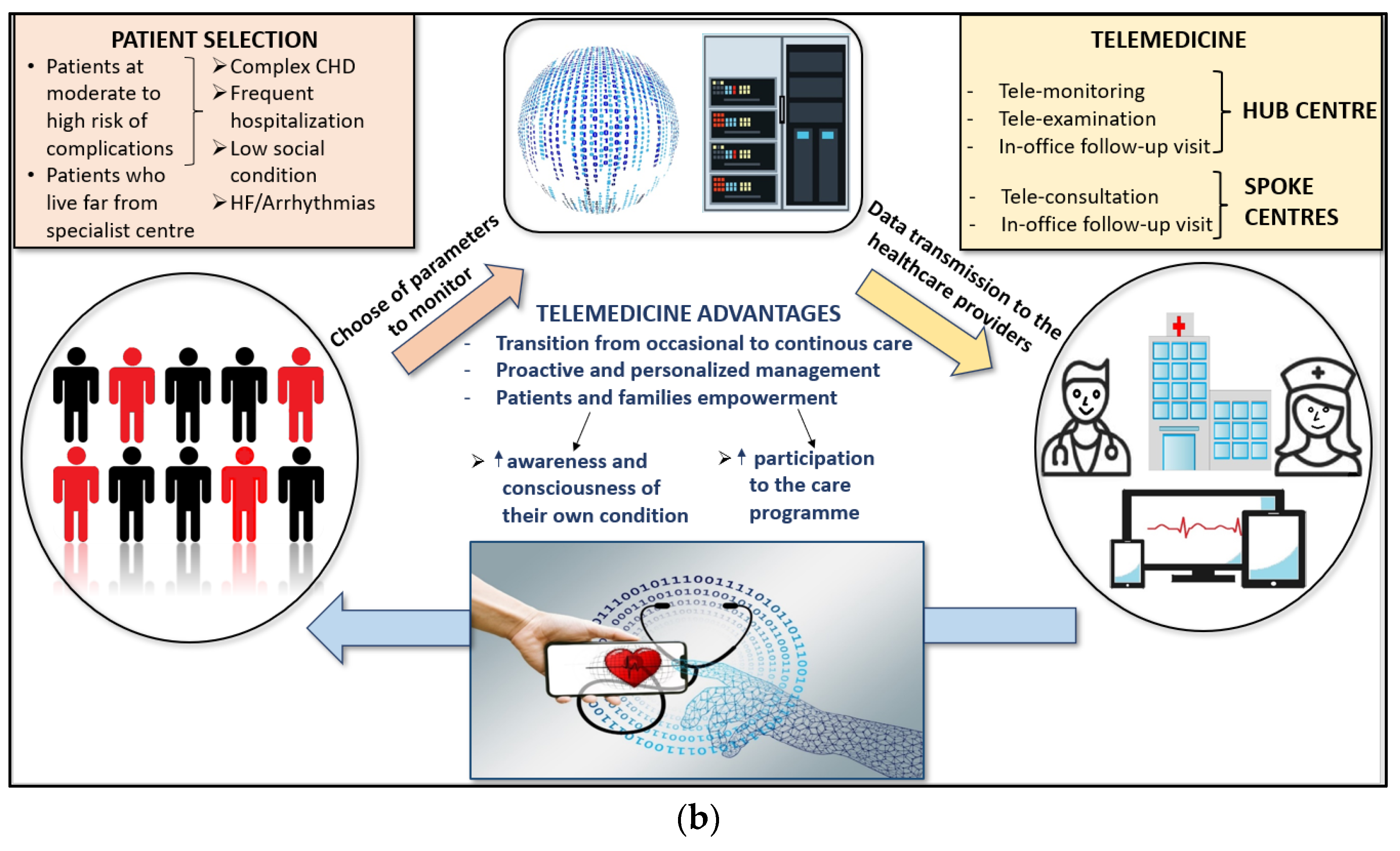
3. Impact of Telemedicine on Health Care
- Tele-examination: a medical procedure in which a doctor interacts remotely with a patient, eliciting a complete clinical history and performing a virtual physical examination;
- Tele-consultation: a remote consulting activity between a clinician and a patient that allows for the diagnosis or treatment of patients without their physical presence;
- Healthcare remote cooperation: assistance provided by a doctor or other healthcare professional to another engaged in a health act (e.g., guidance given during an emergency aid);
- Tele-monitoring: this technique involves the remote monitoring of clinical parameters using wearable, insertable, or close-proximity medical devices.
- Remote care of patients with HF, improving their clinical outcome and reducing the risk of recurrent hospitalizations and cardiovascular death [28];
- The heart logic is a multisensory algorithm combining first and third heart sounds, respiration rate, nocturnal HR, thoracic impedance, weight, and physical activity (sensibility 70%, specificity 85%, and a median lead time before HFE of 34 days) [37].
- The algorithm developed by D’Onofrio et al. includes temporal trends of diurnal and nocturnal HR, ventricular extrasystoles, atrial tachyarrhythmia burden, physical activity, and thoracic impedance (65.5% of first post-implant HF hospitalization prediction, a median alerting time of 42 days, and one false alert every 17 months) [38].
- Recently, multisensory non-invasive remote monitoring of physiological data by a chest-applied temporary patch has been shown to accurately predict hospitalization for HF exacerbation with 76–88% sensitivity, 85% specificity, and a median duration between initial alert and readmission of 6.5 days [39].
4. Telemedicine in the Care of Adult Patients with CHD
5. Discussion
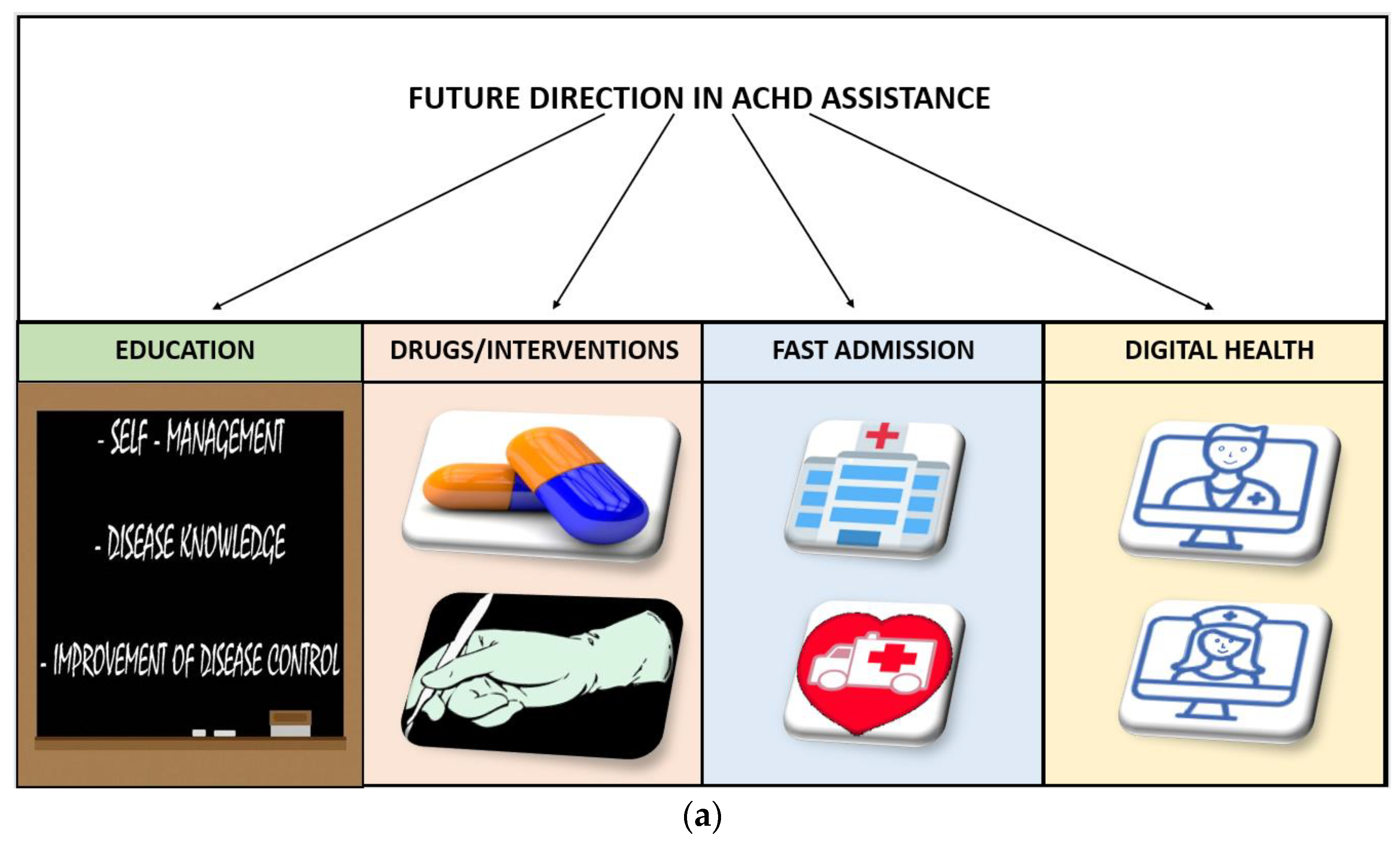
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brida, M.; Gatzoulis, M.A. Adult congenital heart disease: Past, present and future. Acta Paediatr. 2019, 108, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Preminger, T.J. Telemedicine in pediatric cardiology: Pros and cons. Curr. Opin. Pediatr. 2022, 34, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Selamet Tierney, E.S. Telehealth in Pediatric Heart Transplant Patients: Exercise, Nutrition, and Parental Imaging. Pediatr. Clin. N. Am. 2020, 67, 635–639. [Google Scholar] [CrossRef]
- Alvarez, P.; Sianis, A.; Brown, J.; Ali, A.; Briasoulis, A. Chronic disease management in heart failure: Focus on telemedicine and remote monitoring. Rev. Cardiovasc. Med. 2021, 22, 403–413. [Google Scholar] [CrossRef]
- MacKinnon, G.E.; Brittain, E.L. Mobile Health Technologies in Cardiopulmonary Disease. Chest 2020, 157, 654–664. [Google Scholar] [CrossRef]
- Khurrum, M.; Asmar, S.; Joseph, B. Telemedicine in the ICU: Innovation in the Critical Care Process. J. Intensive Care Med. 2021, 36, 1377–1384. [Google Scholar] [CrossRef]
- Makkar, A.; Milsten, J.; McCoy, M.; Szyld, E.G.; Lapadula, M.C.; Ganguly, A.; DeShea, L.A.; Ponniah, U. Tele-Echocardiography for Congenital Heart Disease Screening in a Level II Neonatal Intensive Care Unit with Hybrid Telemedicine System. Telemed. J. E Health 2021, 27, 1136–1142. [Google Scholar] [CrossRef]
- Thamman, R.; Janardhanan, R. Cardiac rehabilitation using telemedicine: The need for tele cardiac rehabilitation. Rev. Cardiovasc. Med. 2020, 21, 497–500. [Google Scholar]
- Liu, A.; Diller, G.P.; Moons, P.; Daniels, C.J.; Jenkins, K.J.; Marelli, A. Changing epidemiology of congenital heart disease: Effect on outcomes and quality of care in adults. Nat. Rev. Cardiol. 2022, 20, 126–137. [Google Scholar] [CrossRef]
- Ntiloudi, D.; Giannakoulas, G.; Parcharidou, D.; Panagiotidis, T.; Gatzoulis, M.A.; Karvounis, H. Adult congenital heart disease: A paradigm of epidemiological change. Int. J. Cardiol. 2016, 218, 269–274. [Google Scholar] [CrossRef]
- Billett, J.; Cowie, M.R.; Gatzoulis, M.A.; Vonder Muhll, I.F.; Majeed, A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: Cross-sectional, population-based study with case control analysis. Heart 2008, 94, 1194–1199. [Google Scholar] [CrossRef]
- Oechslin, E.N.; Harrison, D.A.; Connelly, M.S.; Webb, G.D.; Siu, S.C. Mode of death in adults with congenital heart disease. Am. J. Cardiol. 2000, 86, 1111–1116. [Google Scholar] [CrossRef]
- Fusco, F.; Scognamiglio, G.; Guarguagli, S.; Merola, A.; Palma, M.; Barracano, R.; Borrelli, N.; Correra, A.; Grimaldi, N.; Colonna, D.; et al. Prognostic Relevance of Thyroid Disorders in Adults with Congenital Heart Disease. Am. J. Cardiol. 2022, 166, 107–113. [Google Scholar] [CrossRef]
- Ramage, K.; Grabowska, K.; Silversides, C.; Quan, H.; Metcalfe, A. Association of Adult Congenital Heart Disease With Pregnancy, Maternal, and Neonatal Outcomes. JAMA Netw. Open. 2019, 2, e193667. [Google Scholar] [CrossRef]
- Chowdhury, D.; Johnson, J.N.; Baker-Smith, C.M.; Jaquiss, R.D.B.; Mahendran, A.K.; Curren, V.; Bhat, A.; Patel, A.; Marshall, A.C.; Fuller, S.; et al. Health Care Policy and Congenital Heart Disease: 2020 Focus on Our 2030 Future. J. Am. Heart Assoc. 2021, 10, e020605. [Google Scholar] [CrossRef]
- Goossens, E.; Fieuws, S.; Van Deyk, K.; Luyckx, K.; Gewillig, M.; Budts, W.; Moons, P. Effectiveness of structured education on knowledge and health behaviors in patients with congenital heart disease. J. Pediatr. 2015, 166, 1370–1376.e1. [Google Scholar] [CrossRef]
- Moscatelli, S.; Borrelli, N.; Sabatino, J.; Leo, I.; Avesani, M.; Montanaro, C.; Di Salvo, G. Role of Cardiovascular Imaging in the Follow-Up of Patients with Fontan Circulation. Children 2022, 9, 1875. [Google Scholar] [CrossRef]
- Broberg, C.S.; Kovacs, A.H.; Sadeghi, S.; Rosenbaum, M.S.; Lewis, M.J.; Carazo, M.R.; Rodriguez, F.H., 3rd; Halpern, D.G.; Feinberg, J.; Galilea, F.A.; et al. COVID-19 in Adults With Congenital Heart Disease. J. Am. Coll. Cardiol. 2021, 77, 1644–1655. [Google Scholar] [CrossRef]
- Fusco, F.; Scognamiglio, G.; Merola, A.; Palma, M.; Correra, A.; Borrelli, N.; Barracano, R.; Grimaldi, N.; Colonna, D.; Romeo, E.; et al. Coronavirus disease 2019 in patients with Fontan circulation. Int. J. Cardiol. Congenit. Heart Dis. 2021, 3, 100126. [Google Scholar] [CrossRef]
- Scognamiglio, G.; Fusco, F.; Merola, A.; Palma, M.; Correra, A.; Sarubbi, B. Caring for adults with CHD in the era of coronavirus disease 2019 pandemic: Early experience in an Italian tertiary centre. Cardiol. Young 2020, 30, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- NHSX. Information Governance. Available online: https://www.nhsx.nhs.uk/covid-19-response/dataand-information-governance/information-governance (accessed on 15 December 2022).
- NHS Digital. Approved Video Consultation Systems. Available online: https://digital.nhs.uk/services/future-gp-it-systems-and-services/approved-econsultation-systems (accessed on 15 May 2020).
- Benziger, C.P.; Huffman, M.D.; Sweis, R.N.; Stone, N.J. The Telehealth Ten: A Guide for a Patient-Assisted Virtual Physical Examination. Am. J. Med. 2021, 134, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Mabo, P.; Victor, F.; Bazin, P.; Ahres, S.; Babuty, D.; Da Costa, A.; Binet, D.; Daubert, J.C.; COMPAS Trial Investigators. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur. Heart J. 2012, 33, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, F.J.; Osca Asensi, J.; Romero, R.; Fernández Lozano, I.; Larrazabal, J.M.; Martínez Ferrer, J.; Ortiz, R.; Pombo, M.; Tornés, F.J.; Moradi Kolbolandi, M. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: A long-term randomized trial (RM-ALONE). Eur. Heart J. 2019, 40, 1837–1846. [Google Scholar] [CrossRef]
- Perl, S.; Stiegler, P.; Rotman, B.; Prenner, G.; Lercher, P.; Anelli-Monti, M.; Sereinigg, M.; Riegelnik, V.; Kvas, E.; Kos, C.; et al. Socio-economic effects and cost saving potential of remote patient monitoring (SAVE-HM trial). Int. J. Cardiol. 2013, 169, 402–407. [Google Scholar] [CrossRef]
- Dierckx, R.; Inglis, S.C.; Clark, R.A.; Prieto-Merino, D.; Cleland, J.G. Telemedicine in heart failure: New insights from the Cochrane meta-analyses. Eur. J. Heart Fail. 2017, 19, 304–306. [Google Scholar] [CrossRef]
- Papaccioli, G.; Bassi, G.; Lugi, C.; Parente, E.; D’Andrea, A.; Proietti, R.; Imbalzano, E.; Al Turki, A.; Russo, V. Smartphone and new tools for afillation diagnosis: Evidence for clinical applicability. Minerva Cardiol. Angiol. 2022, 70, 616–627. [Google Scholar] [CrossRef]
- Ricci, R.P.; Morichelli, L.; Santini, M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace 2009, 11, 54–61. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and CRT therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Ricci, R.P.; Morichelli, L.; Quarta, L.; Porfili, A.; Magris, B.; Giovene, L.; Torcinaro, S.; Gargaro, A. Effect of daily remote monitoring on pacemaker longevity: A retrospective analysis. Heart Rhythm 2015, 12, 330–337. [Google Scholar] [CrossRef]
- Burri, H.; Sticherling, C.; Wright, D.; Makino, K.; Smala, A.; Tilden, D. Cost-consequence analysis of daily continuous remote monitoring of implantable cardiac defibrillator and resynchronization devices in the UK. Europace 2013, 15, 1601–1608. [Google Scholar] [CrossRef]
- Varma, N.; Epstein, A.E.; Irimpen, A.; Schweikert, R.; Love, C.; TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010, 122, 325–332. [Google Scholar] [CrossRef]
- Ricci, R.P.; Morichelli, L.; Gargaro, A.; Laudadio, M.T.; Santini, M. Home monitoring in patients with implantable cardiac devices: Is there a potential reduction of stroke risk? Results from a computer model tested through monte carlo simulations. J. Cardiovasc. Electrophysiol. 2009, 20, 1244–1251. [Google Scholar] [CrossRef]
- Whellan, D.J.; Ousdigian, K.T.; Al-Khatib, S.M.; Pu, W.; Sarkar, S.; Porter, C.B.; Pavri, B.B.; Connor, C.M.; PARTNERS Study Investigators. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: Results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J. Am. Coll. Cardiol. 2010, 55, 1803–1810. [Google Scholar]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results From the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- Onofrio, A.; Solimene, F.; Calò, L.; Calvi, V.; Viscusi, M.; Melissano, D.; Russo, V.; Rapacciuolo, A.; Campana, A.; Caravati, F.; et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: Results from the SELENE HF study. Europace 2022, 24, 234–244. [Google Scholar] [CrossRef]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization: The LINK-HF Multicenter Study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef]
- Angermann, C.E.; Assmus, B.; Anker, S.D.; Asselbergs, F.W.; Brachmann, J.; Brett, M.E.; Brugts, J.J.; Ertl, G.; Ginn, G.; Hilker, L.; et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur. J. Heart Fail. 2020, 22, 1891–1901. [Google Scholar] [CrossRef]
- Shavelle, D.M.; Desai, A.S.; Abraham, W.T.; Bourge, R.C.; Raval, N.; Rathman, L.D.; Heywood, J.T.; Jermyn, R.A.; Pelzel, J.; Jonsson, O.T.; et al. Lower Rates of Heart Failure and All-Cause Hospitalizations During Pulmonary Artery Pressure-Guided Therapy for Ambulatory Heart Failure: One-Year Outcomes From the CardioMEMS Post-Approval Study. Circ. Heart Fail. 2020, 13, e006863. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Nachman, D.; Gepner, Y.; Goldstein, N.; Kabakov, E.; Ishay, A.B.; Littman, R.; Azmon, Y.; Jaffe, E.; Eisenkraft, A. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci. Rep. 2020, 10, 16116. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Berson, A.S.; Robbins, C.; Jamieson, M.J.; Prisant, L.M.; Roccella, E.; Sheps, S.G. National standard for measurement of resting and ambulatory blood pressures with automated sphygmomanometers. Hypertension 1993, 21, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Janelle, G.M.; Gravenstein, N. An accuracy evaluation of the T-Line Tensymeter (continuous noninvasive blood pressure management device) versus conventional invasive radial artery monitoring in surgical patients. Anesth. Analg. 2006, 102, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kikuya, M.; Hara, A.; Hirose, T.; Obara, T.; Metoki, H.; Asayama, K.; Inoue, R.; Ohkubo, T.; Totsune, K.; et al. Validation of the FM-800 ambulatory blood pressure monitor according to the Association for the Advancement of Medical Instrumentation criteria and the International Protocol. Clin. Exp. Hypertens. 2010, 32, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Brien, E.; Atkins, N.; Stergiou, G.; Karpettas, N.; Parati, G.; Asmar, R.; Imai, Y.; Wang, J.; Mengden, T.; Shennan, A.; et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010, 15, 23–38. [Google Scholar] [CrossRef]
- Dvir, A.; Goldstein, N.; Rapoport, A.; Balmor, R.G.; Nachman, D.; Merin, R.; Fons, M.; Ben Ishay, A.; Eisenkraft, A. MHA Comparing Cardiac Output Measurements Using a Wearable, Wireless, Noninvasive Photoplethysmography-Based Device to Pulse Contour Cardiac Output in the General ICU: A Brief Report. Crit. Care Explor. 2022, 10, e0624. [Google Scholar] [CrossRef]
- Joosten, A.; Desebbe, O.; Suehiro, K.; Murphy, L.S.; Essiet, M.; Alexander, B.; Fischer, M.O.; Barvais, L.; Van Obbergh, L.; Maucort-Boulch, D.; et al. Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: A systematic review and meta-analysis. Br. J. Anaesth. 2017, 118, 298–310. [Google Scholar] [CrossRef]
- McNamara, H.; Barclay, P.; Sharma, V. Accuracy and precision of the ultrasound cardiac output monitor (USCOM 1A) in pregnancy: Comparison with three-dimensional transthoracic echocardiography. Br. J. Anaesth. 2014, 113, 669–676. [Google Scholar] [CrossRef]
- Nachman, D.; Constantini, K.; Poris, G.; Wagnert-Avraham, L.; Gertz, S.D.; Littman, R.; Kabakov, E.; Eisenkraft, A.; Gepner, Y. Wireless, non-invasive, wearable device for continuous remote monitoring of hemodynamic parameters in a swine model of controlled hemorrhagic shock. Sci. Rep. 2020, 10, 17684. [Google Scholar] [CrossRef]
- Proesmans, T.; Mortelmans, C.; Van Haelst, R.; Verbrugge, F.; Vandervoort, P.; Vaes, B. Mobile Phone-Based Use of the Photoplethysmography Technique to Detect afillation in Primary Care: Diagnostic Accuracy Study of the FibriCheck App. JMIR Mhealth. Uhealth. 2019, 7, e12284. [Google Scholar] [CrossRef]
- Chan, P.H.; Wong, C.K.; Poh, Y.C.; Pun, L.; Leung, W.W.; Wong, Y.F.; Pun, L.; Leung, W.W.; Wong, Y.; Wong, M.M.; et al. Diagnostic Performance of a Smartphone-Based Photoplethysmographic Application for Atrial Fibrillation Screening in a Primary Care Setting. J. Am. Heart Assoc. 2016, 5, e003428. [Google Scholar] [CrossRef]
- Mutke, M.R.; Brasier, N.; Raichle, C.; Ravanelli, F.; Doerr, M.; Eckstein, J. Comparison and Combination of Single-Lead ECG and Photoplethysmography Algorithms for WearableBased Atrial Fibrillation Screening. Telemed. J. E-Health 2021, 27, 296–302. [Google Scholar] [CrossRef]
- William, A.D.; Kanbour, M.; Callahan, T.; Bhargava, M.; Varma, N.; Rickard, J.; Saliba, W.; Wolski, K.; Hussein, A.; Lindsay, B.D.; et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: The iREAD Study. Heart Rhythm 2018, 15, 1561–1565. [Google Scholar] [CrossRef]
- Yan, B.P.; Lai, W.; Fong, A.H.T.; Lai, P.S.; Cheng, O.W.; Chan, C.K.Y.; To, O.T.L.; Chan, W.M.; Poh, Y.C.; Poh, M.Z. Validation of a deep convolutional network for detecting atrial fibrillation with a wrist-worn wearable device. Heart Rhythm 2018, 15 (Suppl. 1), S362. [Google Scholar]
- Dorr, M.; Nohturfft, V.; Brasier, N.; Bosshard, E.; Djurdjevic, A.; Gross, S.; Raichle, C.J.; Rhinisperger, M.; Stöckli, R.; Eckstein, J. The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin. Electrophysiol. 2019, 5, 199–208. [Google Scholar] [CrossRef]
- Desteghe, L.; Raymaekers, Z.; Lutin, M.; Vijgen, J.; Dilling-Boer, D.; Koopman, P.; Schurmans, J.; Vanduynhoven, P.; Dendale, P.; Heidbuchel, H. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017, 19, 29–39. [Google Scholar]
- Conroy, T.; Guzman, J.H.; Hall, B.; Tsouri, G.; Couderc, J.-P. Detection of atrial fibrillation using an earlobe photoplethysmographic sensor. Physiol. Meas. 2017, 38, 1906–1918. [Google Scholar] [CrossRef]
- Lopez Perales, C.R.; Van Spall, H.G.C.; Maeda, S.; Jimenez, A.; Laţcu, D.G.; Milman, A.; Kirakoya-Samadoulougou, F.; Mamas, M.A.; Muser, D.; Casado Arroyo, R. Mobile health applications for the detection of atrial fibrillation: A systematic review. Europace 2021, 23, 11–28. [Google Scholar] [CrossRef]
- Varma, N.; Cygankiewicz, I.; Turakhia, M.; Heidbuchel, H.; Hu, Y.; Chen, L.Y.; Couderc, J.P.; Cronin, E.M.; Estep, J.D.; Grieten, L.; et al. 2021 ISHNE/HRS/EHRA/APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals: From the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society. Ann. Noninvasive Electrocardiol. 2021, 26, e12795. [Google Scholar]
- Muhlestein, J.B.; Anderson, J.L.; Bethea, C.F.; Severance, H.W.; Mentz, R.J.; Barsness, G.W.; Barbagelata, A.; Albert, D.; Le PA-, V.T.; Bunch, T.J.; et al. Feasibility of combining serial smartphone single-lead electrocardiograms for the diagnosis of ST-elevation myocardial infarction: Smartphone ECG for STEMI Diagnosis. Am. Heart J. 2020, 221, 125–135. [Google Scholar] [CrossRef]
- Vardas, P.E.; Asselbergs, F.W.; van Smeden, M.; Friedman, P. The year in cardiovascular medicine 2021: Digital health and innovation. Eur. Heart J. 2022, 43, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Schuuring, M.J.; Kauw, D. How to initiate eHealth in congenital heart disease patients? Eur. Heart J. Digit. Health 2020, 1, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Engelfriet, P.; Kaemmerer, H.; Meijboom, F.J.; Oechslin, E.; Mulder, B.J. Expert Committee of Euro Heart Survey on Adult Congenital Heart Disease. Delivery of care for adult patients with congenital heart disease in Europe: Results from the Euro Heart Survey. Eur. Heart J. 2006, 27, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Brida, M.; Gatzoulis, M.A.; Diller, G.P.; Roos-Hesselink, J.W.; Dimopoulos, K.; Behringer, W.; Möckel, M.; Giamberti, A.; Galletti, L.; et al. Emergency department management of patients with adult congenital heart disease: A consensus paper from the ESC Working Group on Adult Congenital Heart Disease, the European Society for Emergency Medicine (EUSEM), the European Association for Cardio-Thoracic Surgery (EACTS), and the Association for Acute Cardiovascular Care (ACVC). Eur. Heart J. 2021, 42, 2527–2535. [Google Scholar]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. 2015, 10, CD007228. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Guédon-Moreau, L.; Kouakam, C.; Klug, D.; Marquié, C.; Brigadeau, F.; Boulé, S.; Blangy, H.; Lacroix, D.; Clémenty, J.; Sadoul, N.; et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: A substudy of the ECOST trial. J. Cardiovasc. Electrophysiol. 2014, 25, 763–770. [Google Scholar] [CrossRef]
- Shameer, K.; Johnson, K.W.; Glicksberg, B.S.; Dudley, J.T.; Sengupta, P.P. Machine learning in cardiovascular medicine: Are we there yet? Heart 2018, 104, 1156–1164. [Google Scholar] [CrossRef]
- Zengin, E.; Sinning, C.; Blaum, C.; Blankenberg, S.; Rickers, C.; von Kodolitsch, Y.; Kirchhof, P.; Drury, N.E.; Stoll, V.M. Heart failure in adults with congenital heart disease: A narrative review. Cardiovasc. Diagn. Ther. 2021, 11, 529–537. [Google Scholar] [CrossRef]
- Kauw, D.; Koole, M.A.C.; Winter, M.M.; Dohmen, D.A.J.; Tulevski, I.I.; Blok, S.; Somsen, G.A.; Schijven, M.P.; Vriend, J.W.J.; Robbers-Visser, D.; et al. Advantages of mobile health in the management of adult patients with congenital heart disease. Int. J. Med. Inform. 2019, 132, 104011. [Google Scholar] [CrossRef]
- Ding, E.Y.; Marcus, G.M.; McManus, D.D. Emerging technologies for identifying atrial fibrillation. Circ. Res. 2020, 127, 128–142. [Google Scholar] [CrossRef]
- Verheugt, C.L.; Uiterwaal, C.S.; van der Velde, E.T.; Meijboom, F.J.; Pieper, P.G.; van Dijk, A.P.; Vliegen, H.W.; Grobbee, D.E.; Mulder, B.J. Mortality in adult congenital heart disease. Eur. Heart J. 2010, 31, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- de Waure, C.; Cadeddu, C.; Gualano, M.R.; Ricciardi, W. Telemedicine for the reduction of myocardial infarction mortality: A systematic review and a meta-analysis of published studies. Telemed. J. E-Health 2012, 18, 323–328. [Google Scholar] [CrossRef]
- Ashwood, J.S.; Mehrotra, A.; Cowling, D.; Uscher-Pines, L. Direct-To-Consumer Telehealth May Increase Access To Care But Does Not Decrease Spending. Health Aff. 2017, 36, 485–491. [Google Scholar] [CrossRef]
- Alkilany, R.; Tarabichi, Y.; Hong, R. Telemedicine Visits During COVID-19 Improved Clinic Show Rates. ACR Open Rheumatol. 2022, 4, 136–141. [Google Scholar] [CrossRef]
- Marelli, A.J.; Ionescu-Ittu, R.; Mackie, A.S.; Guo, L.; Dendukuri, N.; Kaouache, M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014, 130, 749756. [Google Scholar] [CrossRef]
- Sabatino, J.; Moscatelli, S.; Rustamova, Y.; Kotlar, I.; Avesani, M.; Brida, M.; Gök, G.; Borrelli, N.; Marchenko, O.; Calvieri, C.; et al. Women’s perspective on the COVID-19 pandemic: Walking into a post-peak phase. Int. J. Cardiol. 2021, 323, 29–33. [Google Scholar] [CrossRef]
- Satou, G.M.; Rheuban, K.; Alverson, D.; Lewin, M.; Mahnke, C.; Marcin, J.; Martin, G.R.; Mazur, L.S.; Sahn, D.J.; Shah, S.; et al. Telemedicine in Pediatric Cardiology: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e648–e678. [Google Scholar] [CrossRef]
- Webb, C.L.; Waugh, C.L.; Grigsby, J.; Busenbark, D.; Berdusis, K.; Sahn, D.J.; Sable, C.A.; American Society of Echocardiography Telemedicine Collaborators’ Group. Impact of telemedicine on hospital transport, length of stay, and medical outcomes in infants with suspected heart disease: A multicenter study. J. Am. Soc. Echocardiogr. 2013, 26, 1090–1098. [Google Scholar]
- Trocchio, G.; Parodi, A.; Bellotti, P.; Pescatori, R.; Castelli, R.; Ameri, P.; Pentimalli, F.; De Caro, E.; Con la Collaborazione del Consiglio Direttivo della Sezione Regionale Liguria della Società Italiana di Telemedicina (SIT). Un nuovo percorso di cura integrato con la telemedicina per la gestione del paziente adulto con cardiopatia congenita [An up-to-date telemedicine integrated clinical pathway for adult patients with congenital heart disease]. G. Ital. Cardiol. 2022, 23, 90–99. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrelli, N.; Grimaldi, N.; Papaccioli, G.; Fusco, F.; Palma, M.; Sarubbi, B. Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients. Int. J. Environ. Res. Public Health 2023, 20, 5775. https://doi.org/10.3390/ijerph20105775
Borrelli N, Grimaldi N, Papaccioli G, Fusco F, Palma M, Sarubbi B. Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients. International Journal of Environmental Research and Public Health. 2023; 20(10):5775. https://doi.org/10.3390/ijerph20105775
Chicago/Turabian StyleBorrelli, Nunzia, Nicola Grimaldi, Giovanni Papaccioli, Flavia Fusco, Michela Palma, and Berardo Sarubbi. 2023. "Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients" International Journal of Environmental Research and Public Health 20, no. 10: 5775. https://doi.org/10.3390/ijerph20105775
APA StyleBorrelli, N., Grimaldi, N., Papaccioli, G., Fusco, F., Palma, M., & Sarubbi, B. (2023). Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients. International Journal of Environmental Research and Public Health, 20(10), 5775. https://doi.org/10.3390/ijerph20105775






