A Review of the Role of Pollen in COVID-19 Infection
Abstract
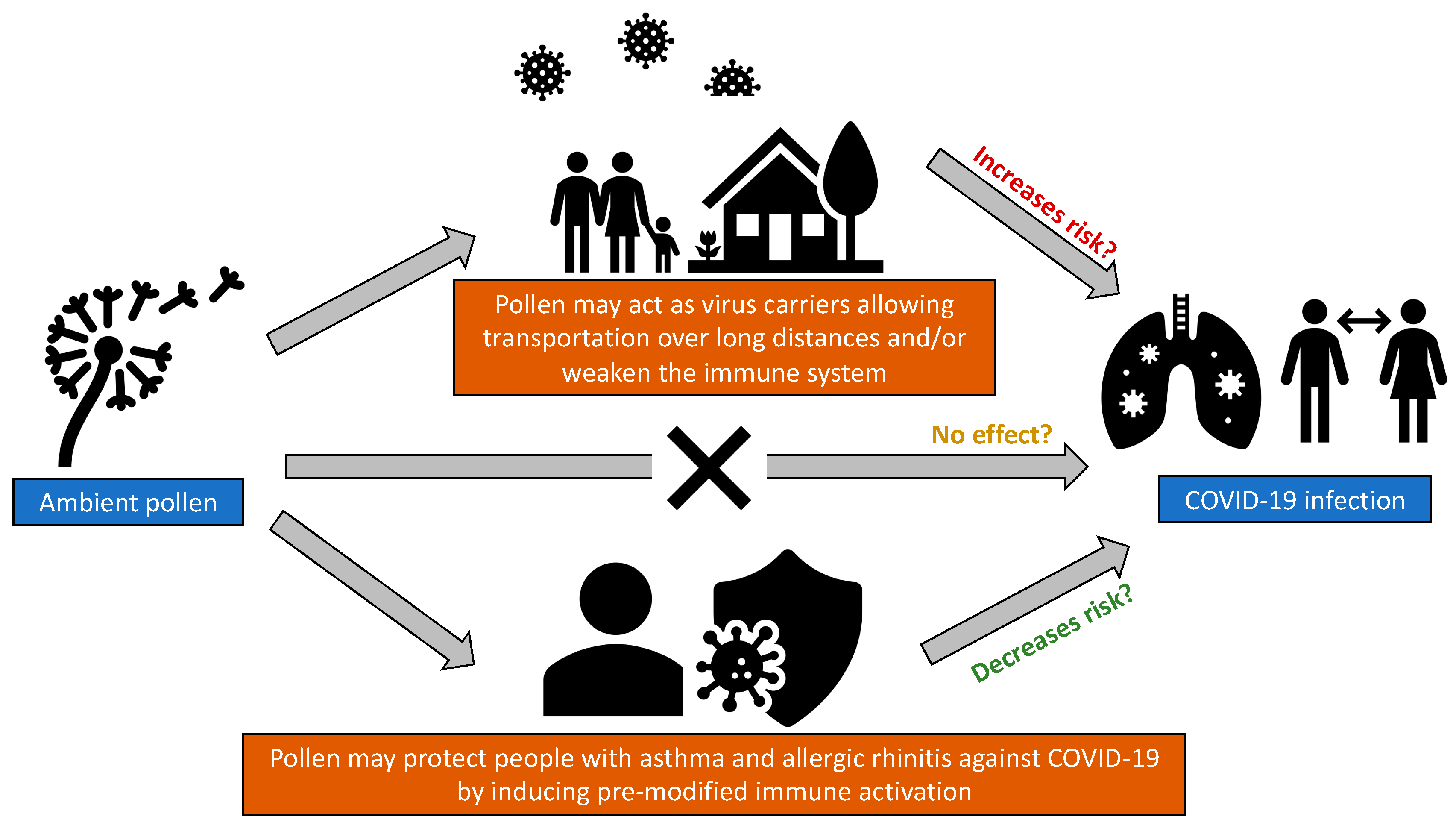
:1. Introduction
2. Aims of This Review and Search Strategy
3. Role of Pollen in the Risk of COVID-19 Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brindisi, G.; De Vittori, V.; De Nola, R.; Pignataro, E.; Anania, C.; De Castro, G.; Cinicola, B.; Gori, A.; Cicinelli, E.; Zicari, A.M. Updates on Children with Allergic Rhinitis and Asthma during the COVID-19 Outbreak. J. Clin. Med. 2021, 10, 2278. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Trecca, E.; Fortunato, F.; Iannuzzi, L.; Ronca, G.; Quaranta, N.; Cassano, M. COVID-19 lockdown and seasonal allergic rhinitis: Our experience in 40 patients. Acta Bio-Med. Atenei Parm. 2021, 92, e2021215. [Google Scholar] [CrossRef]
- Sánchez-García, S.; Ruiz-Hornillos, J.; Bernaola, M.; Habernau-Mena, A.; Lasa, E.M.; Contreras, J.; Candón-Morillo, R.; Antón-Rodríguez, C.; Escudero, C. Effect of the SARS-CoV-2 pandemic on the control and severity of pediatric asthma. Allergol. Immunopathol. 2022, 50, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Nozu, K.; Ishiko, S.; Kondo, A.; Ninchoji, T.; Nagano, C.; Takeda, H.; Unzaki, A.; Ishibashi, K.; Morioka, I.; et al. Impact of the State of Emergency during the COVID-19 Pandemic in 2020 on Asthma Exacerbations among Children in Kobe City, Japan. Int. J. Environ. Res. Public Health 2021, 18, 11407. [Google Scholar] [CrossRef]
- Bergmann, K.-C.; Kugler, S.; Zuberbier, T.; Becker, S. Face masks suitable for preventing COVID-19 and pollen allergy. A study in the exposure chamber. Allergo J. Int. 2021, 30, 176–182. [Google Scholar] [CrossRef]
- Liccardi, G.; Martini, M.; Bilò, M.B.; Milanese, M.; Rogliani, P. Use of face masks and allergic rhinitis from ragweed: Why mention only total pollen count and not air pollution levels? Int. Forum Allergy Rhinol. 2021, 12, 886–888. [Google Scholar] [CrossRef]
- Mengi, E.; Kara, C.O.; Alptürk, U.; Topuz, B. The effect of face mask usage on the allergic rhinitis symptoms in patients with pollen allergy during the COVID-19 pandemic. Am. J. Otolaryngol. 2022, 43, 103206. [Google Scholar] [CrossRef]
- Sözener, Z.; Öztürk, B.; Aydın, Ö.; Demirel, Y.S.; Pınar, N.M.; Bavbek, S.; Sin, B.A.; Mungan, D. Coincidence of pollen season and coronavirus disease 2019 pandemic: Less time outdoors—Lesser allergy symptoms in 2020. Asia Pac. Allergy 2021, 11, e16. [Google Scholar] [CrossRef]
- Liu, D.T.; Phillips, K.M.; Speth, M.M.; Besser, G.; Mueller, C.A.; Sedaghat, A.R. Portable HEPA Purifiers to Eliminate Airborne SARS-CoV-2: A Systematic Review. Otolaryngol. Head Neck Surg. 2021, 166, 615–622. [Google Scholar] [CrossRef]
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. USA 2020, 117, 18984–18990. [Google Scholar] [CrossRef]
- Dondi, A.; Betti, L.; Carbone, C.; Dormi, A.; Paglione, M.; Rinaldi, M.; Scotto, F.; Poluzzi, V.; Fabi, M.; Lanari, M. Understanding the environmental factors related to the decrease in Pediatric Emergency Department referrals for acute asthma during the SARS-CoV-2 pandemic. Pediatr. Pulmonol. 2022, 57, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ščevková, J.; Vašková, Z.; Sepšiová, R.; Kováč, J. Seasonal variation in the allergenic potency of airborne grass pollen in Bratislava (Slovakia) urban environment. Environ. Sci. Pollut. Res. Int. 2021, 28, 62583–62592. [Google Scholar] [CrossRef] [PubMed]
- Idrose, N.S.; Dharmage, S.C.; Lowe, A.J.; Lambert, K.A.; Lodge, C.J.; Abramson, M.J.; Douglass, J.A.; Newbigin, E.J.; Erbas, B. A systematic review of the role of grass pollen and fungi in thunderstorm asthma. Environ. Res. 2020, 181, 108911. [Google Scholar] [CrossRef]
- Idrose, N.S.; Tham, R.C.A.; Lodge, C.J.; Lowe, A.J.; Bui, D.; Perret, J.L.; Vicendese, D.; Newbigin, E.; Tang, M.; Aldakheel, F.; et al. Is short-term exposure to grass pollen adversely associated with lung function and airway inflammation in the community? Allergy 2020, 76, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Idrose, N.S.; Vicendese, D.; Peters, R.L.; Koplin, J.J.; Douglass, J.A.; Walters, E.H.; Perret, J.L.; Lowe, A.J.; Tang, M.L.; Newbigin, E.J.; et al. Children With Food Allergy Are at Risk of Lower Lung Function on High-Pollen Days. J. Allergy Clin. Immunol. Pract. 2022, 10, 2144–2153.e10. [Google Scholar] [CrossRef]
- Idrose, N.S.; Walters, E.H.; Zhang, J.; Vicendese, D.; Newbigin, E.J.; Douglass, J.A.; Erbas, B.; Lowe, A.J.; Perret, J.L.; Lodge, C.J.; et al. Outdoor pollen-related changes in lung function and markers of airway inflammation: A systematic review and meta-analysis. Clin. Exp. Allergy 2021, 51, 636–653. [Google Scholar] [CrossRef] [PubMed]
- Idrose, N.S.; Lodge, C.J.; Peters, R.L.; Douglass, J.A.; Koplin, J.J.; Lowe, A.J.; Perrett, K.P.; Tang, M.L.K.; Newbigin, E.J.; Abramson, M.J.; et al. The role of short-term grass pollen exposure in food skin-prick test reactivity, food allergy, and eczema flares in children. Pediatr. Allergy Immunol. 2022, 33, e13862. [Google Scholar] [CrossRef]
- Douglass, J.A.; Lodge, C.; Chan, S.; Doherty, A.; Tan, J.A.; Jin, C.; Stewart, A.; Southcott, A.M.; Gillman, A.; Lee, J.; et al. Thunderstorm asthma in seasonal allergic rhinitis: The TAISAR study. J. Allergy Clin. Immunol. 2022, 149, 1607–1616. [Google Scholar] [CrossRef]
- Zarei, A.; Ramazani, A.; Rezaei, A.; Moradi, S. Screening of honey bee pollen constituents against COVID-19: An emerging hot spot in targeting SARS-CoV-2-ACE-2 interaction. Nat. Prod. Res. 2022, 37, 974–980. [Google Scholar] [CrossRef]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Zhang, H.; Luo, H.; Zhu, L.; Jiang, P.; et al. Small Molecules Blocking the Entry of Severe Acute Respiratory Syndrome Coronavirus into Host Cells. J. Virol. 2004, 78, 11334–11339. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phytother. Res. 2021, 35, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Damialis, A.; Gilles, S.; Sofiev, M.; Sofieva, V.; Kolek, F.; Bayr, D.; Plaza, M.; Leier-Wirtz, V.; Kaschuba, S.; Ziska, L.; et al. Higher airborne pollen concentrations correlated with increased SARS-CoV-2 infection rates, as evidenced from 31 countries across the globe. Proc. Natl. Acad. Sci. USA 2021, 118, e2019034118. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, M.J.; Kroes, A.C.; Hoogeveen, E.K. Environmental factors and mobility predict COVID-19 seasonality in the Netherlands. Environ. Res. 2022, 211, 113030. [Google Scholar] [CrossRef] [PubMed]
- Dbouk, T.; Drikakis, D. On pollen and airborne virus transmission. Phys. Fluids 2021, 33, 063313. [Google Scholar] [CrossRef]
- Shah, R.B.; Shah, R.D.; Retzinger, D.G.; Retzinger, A.C.; Retzinger, D.A.; Retzinger, G.S. Competing Bioaerosols May Influence the Seasonality of Influenza-Like Illnesses, including COVID-19. The Chicago Experience. Pathogens 2021, 10, 1204. [Google Scholar] [CrossRef] [PubMed]
- Moral de Gregorio, A.J.; Guzmán Rodríguez, R.; Senent Sánchez, C.J.; Lemus Calderón, J.A.; Valero Santiago, A. Is environmental pollen concentration a risk factor for SARS-CoV-2 infection? J. Investig. Allergol. Clin. Immunol. 2021, 32, 79–80. [Google Scholar] [CrossRef]
- Dunker, S.; Hornick, T.; Szczepankiewicz, G.; Maier, M.; Bastl, M.; Bumberger, J.; Treudler, R.; Liebert, U.; Simon, J.-C. No SARS-CoV-2 detected in air samples (pollen and particulate matter) in Leipzig during the first spread. Sci. Total Environ. 2021, 755 Pt 1, 142881. [Google Scholar] [CrossRef]
- Takabayashi, T.; Yoshida, K.; Imoto, Y.; Schleimer, R.P.; Fujieda, S. Regulation of the Expression of SARS-CoV-2 Receptor Angiotensin-Converting Enzyme 2 in Nasal Mucosa. Am. J. Rhinol. Allergy 2022, 36, 115–122. [Google Scholar] [CrossRef]
- Jackson, D.J.; Busse, W.W.; Bacharier, L.B.; Kattan, M.; O’Connor, G.T.; Wood, R.A.; Visness, C.; Durham, S.; Larson, D.; Esnault, S.; et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020, 146, 203–206.e3. [Google Scholar] [CrossRef]
- Hoogeveen, M. Pollen likely seasonal factor in inhibiting flu-like epidemics. A Dutch study into the inverse relation between pollen counts, hay fever and flu-like incidence 2016–2019. Sci. Total Environ. 2020, 727, 138543. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, M.; Schürch, C.M. Expression of SARS-CoV-2 entry receptors in the respiratory tract of healthy individuals, smokers and asthmatics. Respir. Res. 2020, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Goyal, A.; Mor, S. Does airborne pollen influence COVID-19 outbreak? Sustain. Cities Soc. 2021, 70, 102887. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.J.; Doherty, A.; Bui, D.S.; Cassim, R.; Lowe, A.J.; Agusti, A.; Russell, M.A.; Dharmage, S.C. Is asthma associated with COVID-19 infection? A UK Biobank analysis. ERJ Open Res. 2021, 7, 00309–02021. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Bai, W.; Hou, J.; Ma, T.; Zeng, X.; Zhang, L.; An, T. The source and transport of bioaerosols in the air: A review. Front. Environ. Sci. Eng. 2021, 15, 44. [Google Scholar] [CrossRef]
- Singh, R.; Levitt, A.L.; Rajotte, E.G.; Holmes, E.C.; Ostiguy, N.; Vanengelsdorp, D.; Lipkin, W.I.; Depamphilis, C.W.; Toth, A.L.; Cox-Foster, D.L. RNA Viruses in Hymenopteran Pollinators: Evidence of Inter-Taxa Virus Transmission via Pollen and Potential Impact on Non-Apis Hymenopteran Species. PLoS ONE 2010, 5, e14357. [Google Scholar] [CrossRef]
- Hoogeveen, M.J.; van Gorp, E.C.M.; Hoogeveen, E.K. Can pollen explain the seasonality of flu-like illnesses in the Netherlands? Sci. Total Environ. 2021, 755 Pt 2, 143182. [Google Scholar] [CrossRef]
- Gilles, S.; Blume, C.; Wimmer, M.; Damialis, A.; Meulenbroek, L.; Gökkaya, M.; Bergougnan, C.; Eisenbart, S.; Sundell, N.; Lindh, M.; et al. Pollen exposure weakens innate defense against respiratory viruses. Allergy 2020, 75, 576–587. [Google Scholar] [CrossRef]
- Betsch, C.; Sprengholz, P. The human factor between airborne pollen concentrations and COVID-19 disease dynamics. Proc. Natl. Acad. Sci. USA 2021, 118, e2107239118. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Bui, D.S.; Cassim, R.; Russell, M.A.; Doherty, A.; Lowe, A.J.; Agusti, A.; Dharmage, S.C.; Lodge, C.J. Lung Function Levels Influence the Association between Obesity and Risk of COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Vasquez Reyes, M. The Disproportional Impact of COVID-19 on African Americans. Health Hum. Rights 2020, 22, 299–307. [Google Scholar] [PubMed]
- Mena, G.E.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 372, eabg5298. [Google Scholar] [CrossRef] [PubMed]

| Author (Publication Year) | Country/Countries | Main Findings | Pollen Levels | Meteorological Factors | Study Period | Stringency Index | Statistical Methods |
|---|---|---|---|---|---|---|---|
| Positive association—pollen is associated with an increased risk of infection | |||||||
| Damialis et al. (2021) [23] | 31 countries across 5 continents (South Korea, Sweden, Ukraine, Australia, Belgium, France, Germany, Hungary, Italy, Latvia, Netherlands, Russia, South Africa, Spain, Switzerland, UK, USA, Argentina, Canada, Croatia, Czech Republic, Denmark, Finland, Greece, Lithuania, Poland, Portugal, Serbia, Slovakia, Slovenia, Turkey) | Pollen concentrations were associated with an increased risk of COVID-19 infection, with a lagged effect of up to 4 days. Pollen concentrations accounted for, on average, 44% of the infection rate variability. | Cumulative pollen concentration of 1201 grains/m3 over 4 days | Temperature and humidity were found to be significantly positively correlated with infection rates, indicating that they may act in synergy with pollen. | 4 days (10 to 14 March 2020) | Mean values: South Korea (55.56), Sweden (26.11) Ukraine (34.44), Australia (19.44), Belgium (26.48), France (45.09), Germany (32.87), Hungary (43.15), Italy (81.85), Latvia (26.29), Netherlands (38.89), Russia (27.13), South Africa (13.89), Spain (50.09), Switzerland (28.33), UK (11.85), USA (27.59), Argentina (22.22), Canada (17.04), Croatia (27.04), Czech Republic (48.15), Denmark (47.22), Finland (30.00), Greece (40.55), Lithuania (no data until 20 March 2020 at 81.48), Poland (36.48), Portugal (40.10), Serbia (22.78), Slovakia (46.85), Slovenia (25.74), Turkey (23.15, no data on 10 March 2020). | Correlation analysis |
| Hoogeveen et al. (2022) [24] | Netherlands | A combined model of hay fever incidence, temperature, solar radiation, and mobility to indoor recreational locations accounted for 87.5% of the variance in the reproduction number of COVID-19 (Rt). The authors did not include daily pollen concentrations into this combined model due to homoscedasticity issues, but pollen and hay fever were moderately strongly correlated. | Mean of 69.2 grains/m3 | Temperature was only associated with Rt if mobility trends and pollen dispersion/maturation were taken into account. Humidity, in general, was associated with Rt and seasonal allergens. | 7 months (17 February 2020 to 21 September 2020) | Mean (SD): 57.22 (18.12) | Backward stepwise multiple linear regression |
| Dbouk et al. (2021) [25] | Middle East | Pollen can increase the transmission rate by acting as a carrier. | Not relevant | The wind speed, temperature, and humidity were set to be 4 km/h, 22 °C, and 50%, respectively. | Not relevant | Not relevant | Computational multiphysics, multiscale modeling, and simulations |
| Shah et al. (2021) [26] | USA | Peak weed pollen count preceded the peak of COVID-19 presentations. | Graph showed a range of 0–200 grains/m3. | Meteorological data were not available. | 4 months (18 July 2020 to 18 November 2020) | Mean (SD): 65.48 (2.66) | Nonlinear least squares regression |
| No association—pollen is not associated with risk of infection | |||||||
| Moral de Gregorio et al. (2021) [27] | Spain | No evidence of a relationship between daily ambient pollen concentrations and daily COVID-19 cases. | Not stated | Meteorological data were not available. | 1 year (1 March 2020 to 28 February 2021) | Mean (SD): 67.08 (13.01) | Spearman correlation |
| Dunker et al. (2021) [28] | Germany | No virus particles were found in ambient pollen during the pandemic. | Graph showed a range of 0–1800 grains/m3. | Meteorological data were not available. | 4 months (1 Jan 2020 to 21 May 2020) | Mean (SD): 47.60 (29.11) | Purified pollen was tested for the presence of the virus using RT-PCR and virus-induced cytopathic effects (CPE) on Vero cells |
| Takabayashi et al. (2022) [29] | Japan | Mean levels of ACE2 expression in patients with seasonal allergic rhinitis induced by Japanese cedar pollen (JCP) did not significantly increase during the JCP season. | Not relevant | Meteorological data were not available. | Not relevant | Not relevant | Kruskal–Wallis analysis of variance with Dunnett post hoc testing and the Mann–Whitney U-test |
| Protective association—pollen is associated with a reduced risk of infection | |||||||
| Jackson et al. (2020) [30] | USA | In the URECA cohort, consisting of children, the authors showed that allergic sensitisation and increased levels of type-2 biomarkers such as IgE and IL-13 can downregulate ACE2 expression (i.e., a COVID-19 entry receptor) in airway cells, irrespective of asthma. In another cohort, consisting of adults with mild asthma who were not on controller therapy, segmental allergen bronchoprovocation to ragweed pollen led to significantly lower ACE2 expression in the lower airway epithelium. | Not relevant | Meteorological data were not available | Not relevant | Not relevant | Weighted linear mixed effects model |
| Hoogeveen et al. (2021) [31] | Netherlands | Pollen had inverse correlations with changes in flu-like incidence, which included the COVID-19 pandemic period. | Mean of 349 grains/m3 | Temperature was associated with pollen levels, but not flu-like incidence. Humidity and solar radiation were both associated with pollen levels and flu-like incidence. | 4 years (4 Jan 2016 to 3 May 2020) COVID data were available from 27 Feb 2020 to 3 May 2020. | Mean (SD): 60.75 (27.51) | Linear regression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrose, N.S.; Zhang, J.; Lodge, C.J.; Erbas, B.; Douglass, J.A.; Bui, D.S.; Dharmage, S.C. A Review of the Role of Pollen in COVID-19 Infection. Int. J. Environ. Res. Public Health 2023, 20, 5805. https://doi.org/10.3390/ijerph20105805
Idrose NS, Zhang J, Lodge CJ, Erbas B, Douglass JA, Bui DS, Dharmage SC. A Review of the Role of Pollen in COVID-19 Infection. International Journal of Environmental Research and Public Health. 2023; 20(10):5805. https://doi.org/10.3390/ijerph20105805
Chicago/Turabian StyleIdrose, Nur Sabrina, Jingwen Zhang, Caroline J. Lodge, Bircan Erbas, Jo A. Douglass, Dinh S. Bui, and Shyamali C. Dharmage. 2023. "A Review of the Role of Pollen in COVID-19 Infection" International Journal of Environmental Research and Public Health 20, no. 10: 5805. https://doi.org/10.3390/ijerph20105805
APA StyleIdrose, N. S., Zhang, J., Lodge, C. J., Erbas, B., Douglass, J. A., Bui, D. S., & Dharmage, S. C. (2023). A Review of the Role of Pollen in COVID-19 Infection. International Journal of Environmental Research and Public Health, 20(10), 5805. https://doi.org/10.3390/ijerph20105805







