Achilles Tendon Repair after Tenorraphy Imaging and the Doughnut Metaphor
Abstract
1. Introduction
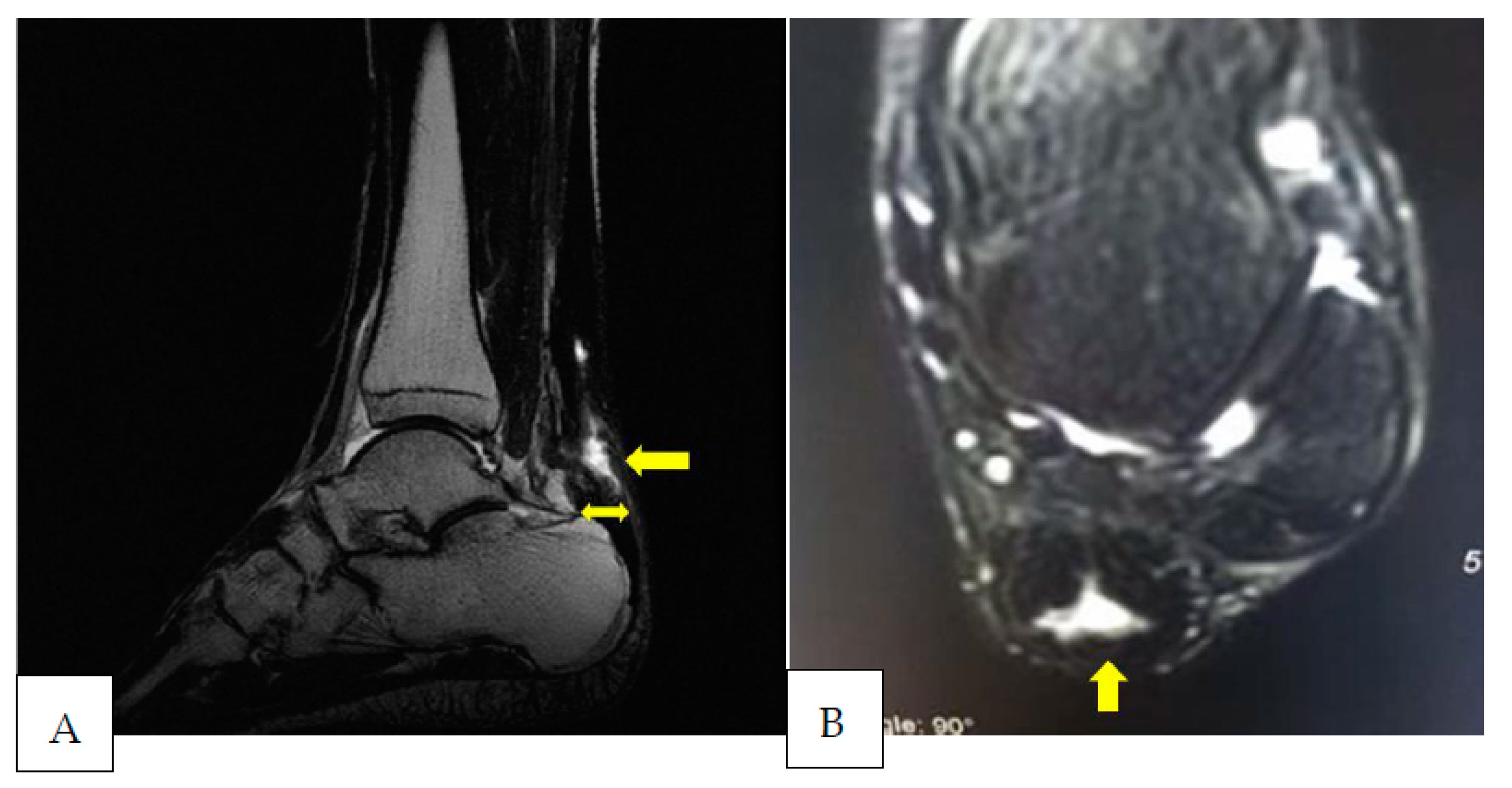
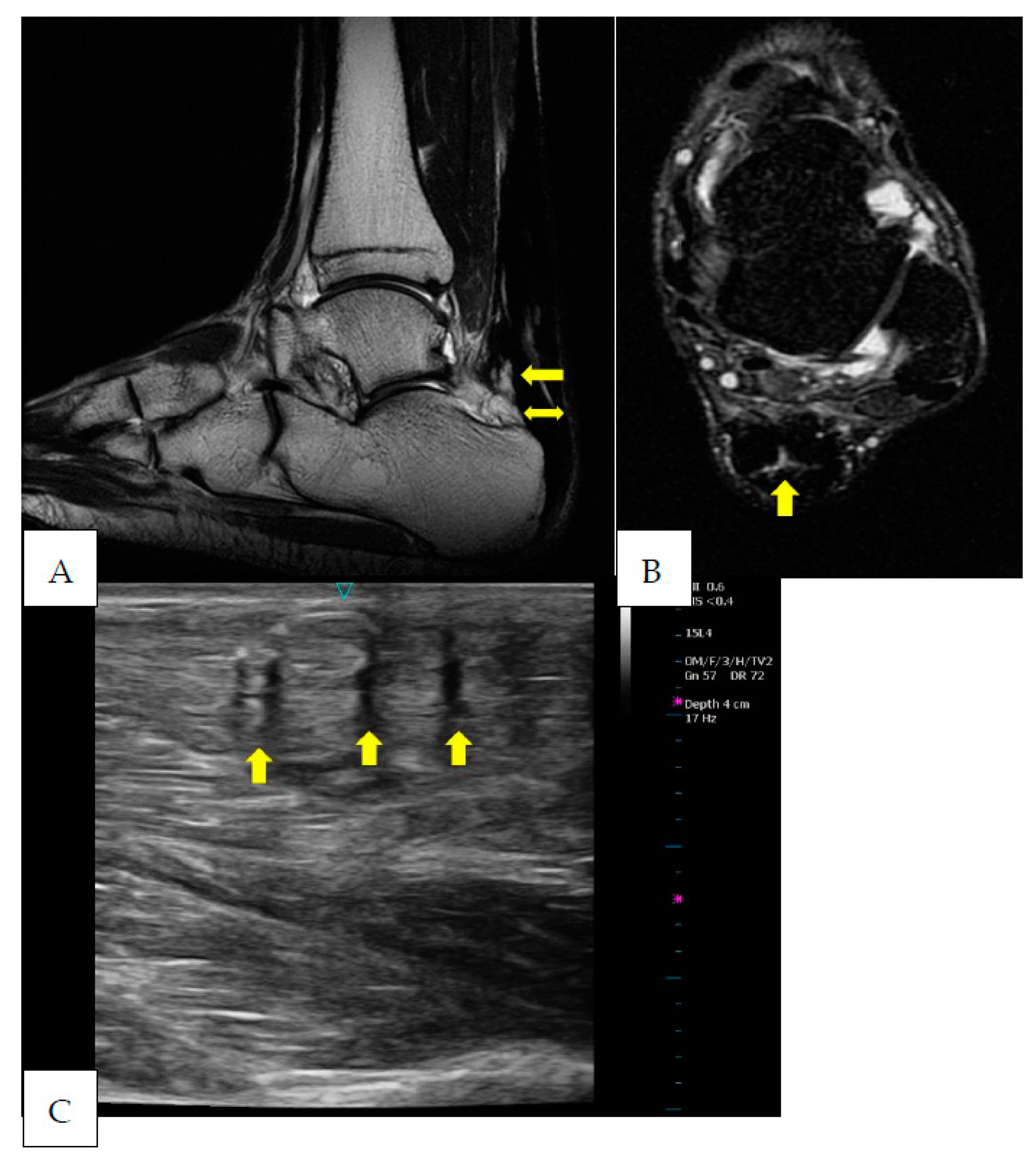
2. Case Presentation
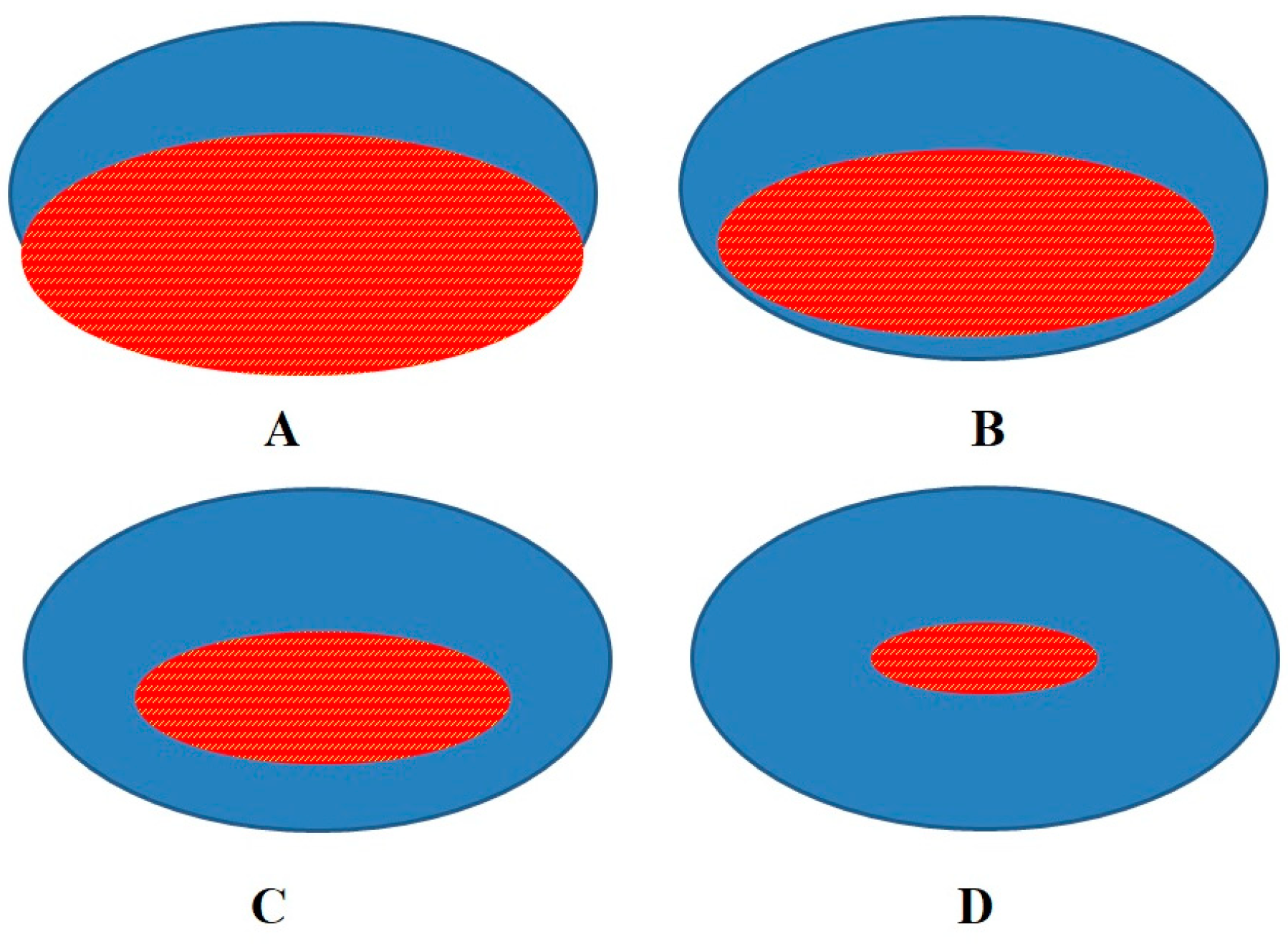
3. Discussion
4. Limitation of the Study and Future Implications
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarantino, D.; Palermi, S.; Sirico, F.; Corrado, B. Achilles Tendon Rupture: Mechanisms of Injury, Principles of Rehabilitation and Return to Play. J. Funct. Morphol. Kinesiol. 2020, 5, 95. [Google Scholar] [CrossRef]
- Masood, T.; Kalliokoski, K.; Bojsen-Møller, J.; Finni, T. Muscle-tendon glucose uptake in Achilles tendon rupture and tendinopathy before and after eccentric rehabilitation: Comparative case reports. Phys. Ther. Sport 2016, 21, 14–19. [Google Scholar] [CrossRef]
- Longo, U.G.; Petrillo, S.; Maffulli, N.; Denaro, V. Acute achilles tendon rupture in athletes. Foot Ankle Clin. 2013, 18, 319–338. [Google Scholar] [CrossRef]
- Bisciotti, G.N.; Eirale, C.; Corsini, A.; Baudot, C.; Saillant, G.; Chalabi, H. Return to football training and competition after lockdown caused by the COVID-19 pandemic: Medical recommendations. Biol. Sport 2020, 37, 313–319. [Google Scholar] [CrossRef]
- Rouvillain, J.-L.; Navarre, T.; Labrada-Blanco, O.; Garron, E.; Daoud, W. Percutaneous suture of acute Achilles tendon rupture. A study of 60 cases. Acta Orthop. Belg. 2010, 76, 237–242. [Google Scholar]
- Lin, T.W.; Cardenas, L.; Soslowsky, L.J. Biomechanics of tendon injury and repair. J. Biomech. 2004, 37, 865–877. [Google Scholar] [CrossRef]
- Quinn, S.F.; Murray, W.T.; Clark, R.A.; Cochran, C.F. Achilles tendon: MR imaging at 1.5 T. Radiology 1987, 3, 767–770. [Google Scholar] [CrossRef]
- Gold, G.E.; Pauly, J.M.; Macovski, A.; Herfkens, R.J. MR spectroscopic imaging of collagen: Tendons and knee menisci. Magn. Reason. Med. 1995, 5, 647–654. [Google Scholar] [CrossRef]
- Weinreb, J.H.; Sheth, C.; Apostolakos, J.; McCarthy, M.B.; Barden, B.; Cote, M.P.; Mazzocca, A.D. Tendon structure, disease, and imaging. Muscles Ligaments Tendons J. 2014, 4, 66–73. [Google Scholar] [CrossRef]
- Fullerton, G.D.; Cameron, I.L.; Ord, V.A. Orientation of tendons in the magnetic field and its effecton T2 relaxation times. Radiology 1985, 2, 433–435. [Google Scholar] [CrossRef]
- Koblik, P.D.; Freeman, D.M. Short echo time magnetic resonance imaging of tendon. Investig. Radiol. 1993, 12, 1095–1100. [Google Scholar] [CrossRef]
- Cheung, Y.; Rosenberg, Z.S.; Magee, T.; Chinitz, L. Normal anatomy and pathologic conditions of ankle tendons: Current imaging techniques. Radiographics 1992, 3, 429–444. [Google Scholar] [CrossRef]
- Erickson, S.J.; Fitzgerald, S.W.; Quinn, S.F.; Carrera, G.F.; Black, K.P.; Lawson, T.L. Long bicipital tendon of the shoulder: Normal anatomy and pathologic findings on MR imaging. Am. J. Roentgenol. 1992, 5, 1091–1096. [Google Scholar] [CrossRef]
- Davies, S.G.; Baudouin, C.J.; King, J.B.; Perry, J.D. Ultrasound, computed tomography and magnetic resonance imaging in patellar tendinitis. Clin. Radiol. 1991, 1, 52–56. [Google Scholar] [CrossRef]
- Khan, K.M.; Bonar, F.; Desmond, P.M.; Cook, J.L.; Young, D.A.; Visentini, P.J.; Wark, J.D. Patellar tendinosis (jumper’s knee): Findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology 1996, 3, 821–827. [Google Scholar] [CrossRef]
- Yoshioka, H.; Anno, I.; Niitsu, M.; Takahashi, H.; Matsumoto, K.; Itai, Y. MRI of muscle strain injuries. J. Comput. Assist. Tomogr. 1994, 3, 454–460. [Google Scholar] [CrossRef]
- Masciocchi, C.; Catalucci, A.; Barile, A. Ankle impingement syndromes. Eur. J. Radiol. 1998, 27, S70–S73. [Google Scholar] [CrossRef]
- Nakamura, T.; Yabe, Y.; Horiuchi, Y. Fat suppression magnetic resonance imaging of the triangular fibrocartilage complex. Comparison with spin echo, gradient echo pulse sequences and histology. J. Hand Surg. Br. 1999, 1, 22–26. [Google Scholar] [CrossRef]
- Guichoux, F.; Vuillemin, V.; Morvan, G.; Zins, M.; Thevenin, F.; Guerini, H.; Omoumi, P.; Drape, J.L. Fat suppression with Dixon techniques in musculoskeletal magnetic resonance imaging: A pictorial review. Semin. Musculoskelet. Radiol. 2015, 19, 335–347. [Google Scholar] [CrossRef]
- Miquel, A.; Molina, V.; Phan, C. Ultrasound of the Achilles tendon after percutaneous repair. J. Radiol. 2009, 90, 305–309. [Google Scholar] [CrossRef]
- Bruce, R.K.; Hale, T.L.; Gilbert, S.K. Ultrasonography evaluation for ruptured Achilles tendon. J. Am. Podiatry Assoc. 1982, 1, 15–17. [Google Scholar]
- Maffulli, N.; Dymond, N.P.; Regine, R. Surgical repair of ruptured Achilles tendon in sportsmen and sedentary patients: A longitudinal ultrasound assessment. Int. J. Sport Med. 1990, 1, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Zappia, M.; Oliva, F.; Luca, B.; Maffulli, N. Post-operative MRI and US appearance of the Achilles tendons. J. Ultrasound. 2020, 23, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Martinoli, C.; Derchi, L.E.; Pastorino, C.; Bertolotto, M.; Silvestri, E. Analysis of echotexture of tendons with US. Radiology 1993, 186, 839–843. [Google Scholar] [CrossRef]
- Mascarenhas, S. A Narrative Review of the Classification and Use of Diagnostic Ultrasound for Conditions of the Achilles Tendon. Diagnostics 2020, 10, 944. [Google Scholar] [CrossRef]
- Wang, J.H. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013, 27, 2074–2079. [Google Scholar] [CrossRef]
- Barfod, K.W. Achilles tendon rupture; assessment of nonoperative treatment. Dan Med. J. 2014, 61, B4837. [Google Scholar]
- Libby, W.F.; Berger, R.; Mead, J.F.; Alexander, G.V.; Ross, J.F. Replacement rates for human tissue from atmospheric radiocarbon. Science 1964, 146, 1170–1172. [Google Scholar] [CrossRef]
- Lynnerup, N.; Kjeldsen, H.; Heegaard, S.; Jacobsen, C.; Heinemeier, J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE 2008, 3, e1529. [Google Scholar] [CrossRef]
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? Br. J. Sport Med. 2016, 50, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Betsch, M.; Dirrichs, T.; Schrading, S.; Tingart, M.; Michalik, R.; Quack, V. Eccentric and Isometric Exercises in Achilles Tendinopathy Evaluated by the VISA-A Score and Shear Wave Elastography. Sport Health 2020, 12, 373–381. [Google Scholar] [CrossRef]
- Bisciotti, G.N.; Volpi, P.; Alberti, G.; Aprato, A.; Artina, M.; Auci, A.; Bait, C.; Belli, A.; Bellistri, G.; Bettinsoli, P.; et al. Italian consensus statement (2020) on return to play after lower limb muscle injury in football (soccer). BMJ Open Sport Exerc. Med. 2019, 5, e000505. [Google Scholar] [CrossRef]
- Karjalainen, P.T.; Ahovuo, J.; Pihlajamäki, H.K.; Soila, K.; Aronen, H.J. Postoperative MR imaging and ultrasonography of surgically repaired Achilles tendon ruptures. Acta Radiol. 1996, 37, 639–646. [Google Scholar] [CrossRef]
- Karjalainen, P.T.; Aronen, H.J.; Pihlajamäki, H.K.; Soila, K.; Paavonen, T.; Bostman, O.M. Magnetic resonance imaging during healing of surgically repaired Achilles tendon ruptures. Am. J. Sport Med. 1997, 25, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kellam, J.F.; Hunter, G.A.; Mcelwain, J.P. Review of the operative treatment of Achilles tendon rupture. Clin. Orthop. Relat. Res. 1985, 201, 80–83. [Google Scholar] [CrossRef]
- Beskin, J.L.; Sanders, R.A.; Hunter, S.C.; Hughston, J.C. Surgical repair of Achilles tendon ruptures. Am. J. Sport Med. 1987, 15, 1–8. [Google Scholar] [CrossRef]
- Langberg, H.; Skovgaard, D.; Petersen, L.J.; Bulow, J.; Kjaer, M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J. Physiol. 1999, 521, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Babraj, J.A.; Cuthbertson, D.J.; Smith, K.; Langberg, H.; Miller, B.; Krogsgaard, M.R.; Kjaer, M.; Rennie, M.J. Collagen synthesis in human musculoskeletal tissues and skin. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E864–E869. [Google Scholar] [CrossRef]
- Miller, B.F.; Olesen, J.L.; Hansen, M.; Døssing, S.; Crameri, R.M.; Welling, R.J.; Langberg, H.; Flyvbjerg, A.; Kjaer, M.; Babraj, J.A.; et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 2005, 567, 1021–1033. [Google Scholar] [CrossRef]
- Rosager, S.; Aagaard, P.; Dyhre-Poulsen, P.; Neergaard, K.; Kjaer, M.; Magnusson, S.P. (2002) Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand. J. Med. Sci. Sport 2002, 12, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Couppé, C.; Kongsgaard, M.; Aagaard, P.; Hansen, P.; Bojsen-Moller, J.; Kjaer, M.; Magnusson, S.P. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J. Appl. Physiol. 2008, 105, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Connell, D.A.; Schneider-Kolsky, M.E.; Hoving, J.L.; Malara, F.; Buchbinder, R.; Koulouris, G.; Burke, F.; Bass, C. Longitudinal study comparing sonographic and MRI assessments of acute and healing hamstring injuries. AJR Am. J. Roentgenol. 2004, 183, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, J.L.; Silder, A.; Sherry, M.A.; Tuite, M.J.; Heiderscheit, B.C. Hamstring strength and morphology progression after return to sport from injury. Med. Sci. Sport Exerc. 2013, 45, 448–454. [Google Scholar] [CrossRef]
- Reurink, G.; Brilman, E.G.; de Vos, R.J.; Maas, M.; Moen, M.H.; Weir, A.; Tol, J.L. Magnetic resonance imaging in acute hamstring injury: Can we provide a return to play prognosis? Sport Med. 2015, 45, 133–146. [Google Scholar] [CrossRef]
- Slavotinek, J.P. Muscle injury: The role of imaging in prognostic assignment and monitoring of muscle repair. Knee Surgery Sports Traumatol. Arthrosc. 2010, 14, 194–200. [Google Scholar] [CrossRef]
- Müller, S.A.; Todorov, A.; Heisterbach, P.E.; Martin, I.; Majewski, M. Tendon healing: An overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sport Traumatol Arthrosc. 2015, 23, 2097–2105. [Google Scholar] [CrossRef]
- Liu, W.; Zhuang, H.; Shao, D.; Wang, L.; Shi, M. High-Frequency Color Doppler Ultrasound in Diagnosis, Treatment, and Rehabilitation of Achilles Tendon Injury. Med. Sci. Monit. 2017, 23, 5752–5759. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisciotti, G.N.; Bisciotti, A.; Auci, A.; Bisciotti, A.; Eirale, C.; Corsini, A.; Volpi, P. Achilles Tendon Repair after Tenorraphy Imaging and the Doughnut Metaphor. Int. J. Environ. Res. Public Health 2023, 20, 5985. https://doi.org/10.3390/ijerph20115985
Bisciotti GN, Bisciotti A, Auci A, Bisciotti A, Eirale C, Corsini A, Volpi P. Achilles Tendon Repair after Tenorraphy Imaging and the Doughnut Metaphor. International Journal of Environmental Research and Public Health. 2023; 20(11):5985. https://doi.org/10.3390/ijerph20115985
Chicago/Turabian StyleBisciotti, Gian Nicola, Andrea Bisciotti, Alessio Auci, Alessandro Bisciotti, Cristiano Eirale, Alessandro Corsini, and Piero Volpi. 2023. "Achilles Tendon Repair after Tenorraphy Imaging and the Doughnut Metaphor" International Journal of Environmental Research and Public Health 20, no. 11: 5985. https://doi.org/10.3390/ijerph20115985
APA StyleBisciotti, G. N., Bisciotti, A., Auci, A., Bisciotti, A., Eirale, C., Corsini, A., & Volpi, P. (2023). Achilles Tendon Repair after Tenorraphy Imaging and the Doughnut Metaphor. International Journal of Environmental Research and Public Health, 20(11), 5985. https://doi.org/10.3390/ijerph20115985





