Effects of an Exercise Program and Cold-Water Immersion Recovery in Patients with Rheumatoid Arthritis (RA): Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
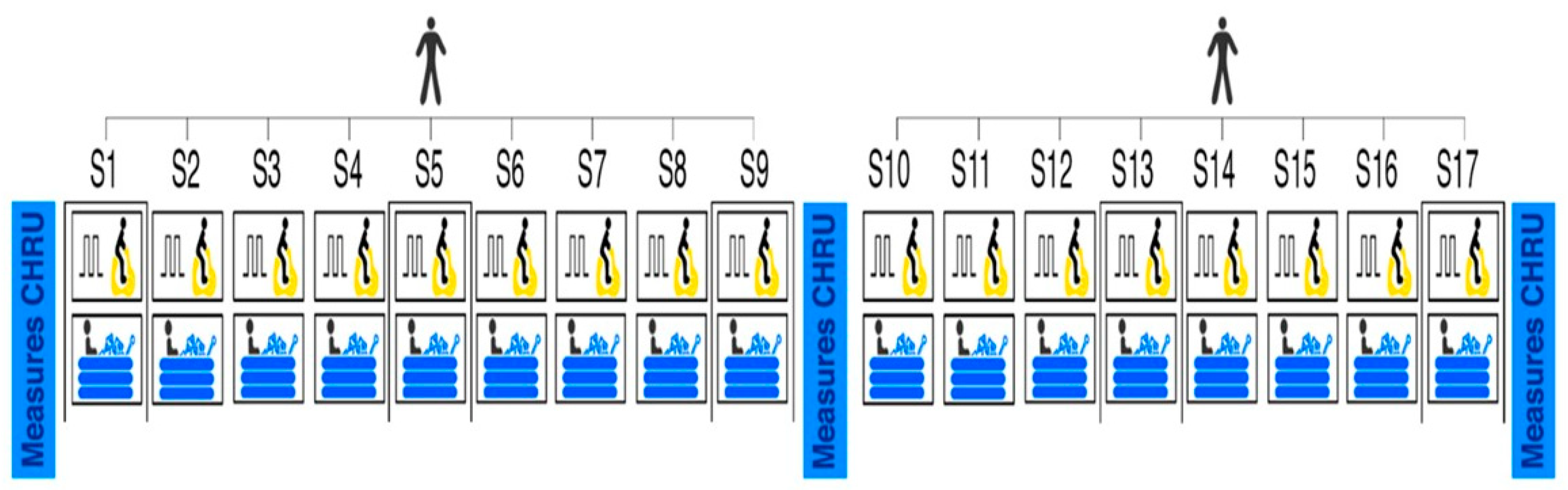
2.2. Intervention
2.2.1. Interval Exercise Program
2.2.2. Water Cold Immersion
2.3. Measures
2.3.1. Adherence by Assiduity
2.3.2. Tolerance
2.3.3. Safety
2.3.4. Effectiveness
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of Cardiovascular Mortality in Patients with Rheumatoid Arthritis: A Meta-Analysis of Observational Studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.C.; Libby, P. Cardiovascular Disease in Patients with Chronic Inflammation: Mechanisms Underlying Premature Cardiovascular Events in Rheumatologic Conditions. Eur. Heart J. 2015, 36, 482–489. [Google Scholar] [CrossRef]
- Lee, J.; Dunlop, D.; Ehrlich-Jones, L.; Semanik, P.; Song, J.; Manheim, L.; Chang, R.W. Public Health Impact of Risk Factors for Physical Inactivity in Adults with Rheumatoid Arthritis. Arthritis Care Res. 2012, 64, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Hollan, I.; Meroni, P.L.; Ahearn, J.M.; Cohen Tervaert, J.W.; Curran, S.; Goodyear, C.S.; Hestad, K.A.; Kahaleh, B.; Riggio, M.; Shields, K.; et al. Cardiovascular Disease in Autoimmune Rheumatic Diseases. Autoimmun. Rev. 2013, 12, 1004–1015. [Google Scholar] [CrossRef]
- Wang, P.; Huang, L.; Xu, Q.; Xu, L.; Deng, F.-Y.; Lei, S.-F. Assessment of Aortic Stiffness in Patients with Rheumatoid Arthritis Using Pulse Wave Velocity: An Update Meta-Analysis. Arch. Med. Res. 2019, 50, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Puttevils, D.; De Vusser, P.; Geusens, P.; Dens, J. Increased Cardiovascular Risk in Patients with Rheumatoid Arthritis: An Overview. Acta Cardiol. 2014, 69, 111–118. [Google Scholar] [CrossRef]
- Pinto, A.J.; Roschel, H.; de Sá Pinto, A.L.; Lima, F.R.; Pereira, R.M.R.; Silva, C.A.; Bonfá, E.; Gualano, B. Physical Inactivity and Sedentary Behavior: Overlooked Risk Factors in Autoimmune Rheumatic Diseases? Autoimmun. Rev. 2017, 16, 667–674. [Google Scholar] [CrossRef]
- Thomsen, T.; Beyer, N.; Aadahl, M.; Hetland, M.L.; Løppenthin, K.; Midtgaard, J.; Esbensen, B.A. Sedentary Behaviour in Patients with Rheumatoid Arthritis: A Qualitative Study. Int. J. Qual. Stud. Health Well-Being 2015, 10, 28578. [Google Scholar] [CrossRef] [PubMed]
- Global Recommendations on Physical Activity for Health; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-159997-9.
- Peres, D.; Tordi, N.; Sagawa, Y.; Wendling, D.; Prati, C. THU0248 Association of Kinesiophobia, Aerobic Exercise, Functional Impairment and Disease Activity of Patients with Rheumatoid Arthritis and Spondyloarthritis. Ann. Rheum. Dis. 2018, 77, 344. [Google Scholar] [CrossRef]
- Guillot, X.; Tordi, N.; Prati, C.; Verhoeven, F.; Pazart, L.; Wendling, D. Cryotherapy Decreases Synovial Doppler Activity and Pain in Knee Arthritis: A Randomized-Controlled Trial. Jt. Bone Spine 2017, 84, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Tordi, N.; Mourot, L.; Demougeot, C.; Dugué, B.; Prati, C.; Wendling, D. Cryotherapy in Inflammatory Rheumatic Diseases: A Systematic Review. Expert Rev. Clin. Immunol. 2014, 10, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Martin, H.; Seguin-Py, S.; Maguin-Gaté, K.; Moretto, J.; Totoson, P.; Wendling, D.; Demougeot, C.; Tordi, N. Local Cryotherapy Improves Adjuvant-Induced Arthritis through down-Regulation of IL-6/IL-17 Pathway but Independently of TNFα. PLoS ONE 2017, 12, e0178668. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.; Sagawa, Y.; Dugué, B.; Domenech, S.C.; Tordi, N.; Prati, C. The Practice of Physical Activity and Cryotherapy in Rheumatoid Arthritis: Systematic Review. Eur. J. Phys. Rehabil. Med. 2017, 53, 775–787. [Google Scholar] [CrossRef] [PubMed]
- American College of Rheumatology Subcommittee on Rheumatoid Arthritis. Guidelines Guidelines for the Management of Rheumatoid Arthritis: 2002 Update. Arthritis Rheum. 2002, 46, 328–346. Available online: https://pubmed.ncbi.nlm.nih.gov/11840435/ (accessed on 8 May 2023). [CrossRef] [PubMed]
- Ottawa Panel. Ottawa Panel Evidence-Based Clinical Practice Guidelines for Therapeutic Exercises in the Management of Rheumatoid Arthritis in Adults. Phys. Ther. 2004, 84, 934–972. Available online: https://pubmed.ncbi.nlm.nih.gov/15449978/ (accessed on 8 May 2023).
- Gimenez, M.; Servera, E.; Salinas, W. Square-Wave Endurance Exercise Test (SWEET) for Training and Assessment in Trained and Untrained Subjects. I. Description and Cardiorespiratory Responses. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 359–368. [Google Scholar] [CrossRef]
- Arends, S.; Hofman, M.; Kamsma, Y.P.; van der Veer, E.; Houtman, P.M.; Kallenberg, C.G.; Spoorenberg, A.; Brouwer, E. Daily Physical Activity in Ankylosing Spondylitis: Validity and Reliability of the IPAQ and SQUASH and the Relation with Clinical Assessments. Arthritis Res. Ther. 2013, 15, R99. [Google Scholar] [CrossRef]
- Borg, G. Perceived Exertion as an Indicator of Somatic Stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef]
- Kersten, P.; Küçükdeveci, A.A.; Tennant, A. The Use of the Visual Analogue Scale (VAS) in Rehabilitation Outcomes. J. Rehabil. Med. 2012, 44, 609–610. [Google Scholar] [CrossRef]
- Fries, J.F.; Spitz, P.; Kraines, R.G.; Holman, H.R. Measurement of Patient Outcome in Arthritis. Arthritis Rheum. 1980, 23, 137–145. [Google Scholar] [CrossRef]
- Matcham, F.; Scott, I.C.; Rayner, L.; Hotopf, M.; Kingsley, G.H.; Norton, S.; Scott, D.L.; Steer, S. The Impact of Rheumatoid Arthritis on Quality-of-Life Assessed Using the SF-36: A Systematic Review and Meta-Analysis. Semin. Arthritis Rheum. 2014, 44, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Bailey, M.A.; Griffin, K.J.; Scott, D.J.A. Pulse Wave Velocity and the Non-Invasive Methods Used to Assess It: Complior, SphygmoCor, Arteriograph and Vicorder. Vascular 2012, 20, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhao, D.; Pan, Y.; Ding, W.; Wei, Q.; Li, H.; Gao, P.; Mi, J. Validation of Omron HBP-1300 Professional Blood Pressure Monitor Based on Auscultation in Children and Adults. BMC Cardiovasc. Disord. 2016, 16, 9. [Google Scholar] [CrossRef]
- Fleming, T.R. One-Sample Multiple Testing Procedure for Phase II Clinical Trials. Biometrics 1982, 38, 143–151. [Google Scholar] [CrossRef] [PubMed]
- A’Hern, R.P. Sample Size Tables for Exact Single-Stage Phase II Designs. Stat. Med. 2001, 20, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Scarvell, J.; Elkins, M.R. Aerobic Exercise Is Beneficial for People with Rheumatoid Arthritis. Br. J. Sport. Med. 2011, 45, 1008–1009. [Google Scholar] [CrossRef]
- Veldhuijzen van Zanten, J.J.C.S.; Rouse, P.C.; Hale, E.D.; Ntoumanis, N.; Metsios, G.S.; Duda, J.L.; Kitas, G.D. Perceived Barriers, Facilitators and Benefits for Regular Physical Activity and Exercise in Patients with Rheumatoid Arthritis: A Review of the Literature. Sports Med. 2015, 45, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; Addy, K.; Campbell, S.; Wilkinson, P. Primary Prevention of Cardiovascular Disease: Updated Review of Contemporary Guidance and Literature. JRSM Cardiovasc. Dis. 2020, 9, 2048004020949326. [Google Scholar] [CrossRef]
- Abitteboul, Y.; Rougé Bugat, M.E.; Le Naoures, H.; Lassoued, S.; Oustric, S.; Riviere, D. Efficacite d’un programme d’entraînement individualise base sur la mesure directe du VO2max chez les malades porteurs de maladies chroniques; le protocole PEP’C. Sci. Sport. 2020, 35, 12–19. [Google Scholar] [CrossRef]
- Gimenez, M. Exercise Training in Patients with Chronic Airways Obstruction. Eur. Respir. J. Suppl. 1989, 7, 611s–617s. [Google Scholar]
- Mettauer, B.; Lampert, E.; Schnedecker, B.; Frey, M.; Geny, B.; Eisenman, B.; Hoppeler, H.; Lonsdorfer, J. A Short Endurance Training Program Increases the Physical Fitness of Heart Transplant Recipients. Sci. Sport. 1993, 8, 25–26. [Google Scholar] [CrossRef]
- Tordi, N.; Dugue, B.; Klupzinski, D.; Rasseneur, L.; Rouillon, J.D.; Lonsdorfer, J. Interval Training Program on a Wheelchair Ergometer for Paraplegic Subjects. Spinal Cord. 2001, 39, 532–537. [Google Scholar] [CrossRef]
- Yeung, S.S.; Ting, K.H.; Hon, M.; Fung, N.Y.; Choi, M.M.; Cheng, J.C.; Yeung, E.W. Effects of Cold Water Immersion on Muscle Oxygenation During Repeated Bouts of Fatiguing Exercise. Medicine 2016, 95, e2455. [Google Scholar] [CrossRef]
- Van Hout, M.J.; Dekkers, I.A.; Westenberg, J.J.; Schalij, M.J.; Widya, R.L.; de Mutsert, R.; Rosendaal, F.R.; de Roos, A.; Jukema, J.W.; Scholte, A.J.; et al. Normal and Reference Values for Cardiovascular Magnetic Resonance-Based Pulse Wave Velocity in the Middle-Aged General Population. J. Cardiovasc. Magn. Reson. 2021, 23, 46. [Google Scholar] [CrossRef]
- Byram, K.W.; Oeser, A.M.; Linton, M.F.; Fazio, S.; Stein, C.M.; Ormseth, M.J. Exercise Is Associated with Increased Small HDL Particle Concentration and Decreased Vascular Stiffness in Rheumatoid Arthritis. J. Clin. Rheumatol. 2018, 24, 417–421. [Google Scholar] [CrossRef]
- Tordi, N.; Mourot, L.; Colin, E.; Regnard, J. Intermittent versus Constant Aerobic Exercise: Effects on Arterial Stiffness. Eur. J. Appl. Physiol. 2010, 108, 801–809. [Google Scholar] [CrossRef]
- Vogel, T.; Leprêtre, P.-M.; Brechat, P.-H.; Lonsdorfer-Wolf, E.; Kaltenbach, G.; Lonsdorfer, J.; Benetos, A. Effect of a Short-Term Intermittent Exercise-Training Programme on the Pulse Wave Velocity and Arterial Pressure: A Prospective Study among 71 Healthy Older Subjects. Int. J. Clin. Pract. 2013, 67, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Veldhuijzen van Zanten, J.J.C.S.; Treharne, G.J.; Panoulas, V.F.; Douglas, K.M.J.; Koutedakis, Y.; Kitas, G.D. Rheumatoid Arthritis, Cardiovascular Disease and Physical Exercise: A Systematic Review. Rheumatology 2008, 47, 239–248. [Google Scholar] [CrossRef] [PubMed]
| Age (years) | 55.1 (11.9) | |
| Female | 17 | |
| Body Mass Index (kg·m−2) | 25.5 (4.7) | |
| Work Status—working, n | 12 | |
| Tabaco consumption, n (active/ex-smoker/non-smoker) | 2/5/11 | |
| Chronicity of disease (years) | 12.6 (9.8) | |
| Questionnaire SQUASH | 5513.5 (2591.0) | |
| Tolerance measures | Baseline | S9 |
| BORG (points) | 11.6 (1.7) | 12 (1.4) |
| VAS-temperature (points) | ||
| First minute | 2.9 (1.1) | 2.6 (1.1) |
| Fifth minute | 1.9 (0.7) | 1.7 (0.7) |
| Tenth minute | 1.7 (0.8) | 1.4 (0.6) |
| Fifteenth minute | 1.6 (0.8) | 1.4 (0.6) |
| Safety measures | ||
| Echography (0–64 points) | 6.4 (3.8) | 4.3 (2.9) |
| Painful Joints (n) | 4.3 (5.0) | 1.7 (2.8) |
| Swollen Joints (n) | 0.9 (2.9) | 0.7 (1.0) |
| Pain (0–100 points) | 42.2 (24.4) | 38.8 (15.2) |
| Questionnaires | ||
| HAQ | 0.7 (0.5) | 0.6 (0.6) |
| SF-36 physical composite | 58 (32.9) | 58.4 (33.1) |
| SF-36 mental composite | 59.8 (22.9) | 64.1 (24.5) |
| Effectiveness measures | ||
| Pulse Wave Velocity (m·s−1) | 8.9 (1.2) | 7.0 (0.8) ** |
| Systolic Blood Pressure (mmHg) | 120.9 (14.2) | 118.2 (13.1) |
| Diastolic Blood Pressure (mmHg) | 68.5 (8.3) | 68.6 (10.5) |
| Heart Rate (bpm) | 70.2 (8.4) | 66.6 (5.5) * |
| Tolerance Measures | Baseline | S9 | S17 |
|---|---|---|---|
| BORG (points) | 10.9 (1.5) | 12.1 (1.6) | 11.8 (2.1) |
| VAS-temperature (points) | |||
| First minute | 3 (0.8) | 2.7 (1.1) | 2 (1.0) |
| Fifth minute | 1.9 (0.7) | 1.7 (0.6) | 1.3 (0.6) |
| Tenth minute | 1.7 (0.8) | 1.5 (0.5) | 1.1 (0.3) |
| Fifteenth minute | 1.6 (0.8) | 1.5 (0.5) | 1.1 (0.3) |
| Safety measures | |||
| Echography (0–64 points) | 7.9 (4.2) | 4.9 (3.2) | 3.6 (2.3) |
| Painful Joints (n) | 3.0 (3.5) | 2.2 (3.4) | 1.3 (3.1) |
| Swollen Joints (n) | 0.7 (1) | 0.5 (0.9) | 0.5 (1.0) |
| Pain (0–100 points) | 43.6 (20) | 40.5 (18.1) | 39.5 (22.3) |
| Questionnaires | |||
| HAQ | 0.7 (0.5) | 0.8 (0.6) | 0.6 (0.4) |
| SF-36 physical composite | 61.4 (32) | 56.6 (35.4) | 64.1 (33.4) |
| SF-36 mental composite | 59.1 (25) | 58.7 (23.9) | 59.8 (26.2) |
| Effectiveness measures | Baseline | S9 | S17 |
| Pulse Wave Velocity (m·s−1) | 9.1 (0.9) | 7.0 (0.8) * | 6.9 (0.7) * |
| Systolic Blood Pressure (mmHg) | 124.9 (12.1) | 121.1 (13.2) | 122 (15) |
| Diastolic Blood Pressure (mmHg) | 70.1 (7.8) | 69.8 (12.3) | 72.2 (9.7) |
| Heart Rate (bpm) | 71.5 (8.5) | 66.8 (6.5) | 68.3 (8.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, D.; Prati, C.; Mourot, L.; Demartino, A.M.; Sagawa, Y., Jr.; Tordi, N. Effects of an Exercise Program and Cold-Water Immersion Recovery in Patients with Rheumatoid Arthritis (RA): Feasibility Study. Int. J. Environ. Res. Public Health 2023, 20, 6128. https://doi.org/10.3390/ijerph20126128
Peres D, Prati C, Mourot L, Demartino AM, Sagawa Y Jr., Tordi N. Effects of an Exercise Program and Cold-Water Immersion Recovery in Patients with Rheumatoid Arthritis (RA): Feasibility Study. International Journal of Environmental Research and Public Health. 2023; 20(12):6128. https://doi.org/10.3390/ijerph20126128
Chicago/Turabian StylePeres, Daniele, Clément Prati, Laurent Mourot, Amanda Magalhães Demartino, Yoshimasa Sagawa, Jr., and Nicolas Tordi. 2023. "Effects of an Exercise Program and Cold-Water Immersion Recovery in Patients with Rheumatoid Arthritis (RA): Feasibility Study" International Journal of Environmental Research and Public Health 20, no. 12: 6128. https://doi.org/10.3390/ijerph20126128
APA StylePeres, D., Prati, C., Mourot, L., Demartino, A. M., Sagawa, Y., Jr., & Tordi, N. (2023). Effects of an Exercise Program and Cold-Water Immersion Recovery in Patients with Rheumatoid Arthritis (RA): Feasibility Study. International Journal of Environmental Research and Public Health, 20(12), 6128. https://doi.org/10.3390/ijerph20126128








