SARS-CoV-2 Survival on Surfaces. Measurements Optimisation for an Enthalpy-Based Assessment of the Risk
Abstract
:1. Introduction
2. Materials and Methods
- Temperature: is generally most investigated by holding relative humidity constant and is believed to be responsible for stabilising the lipidic layer at low temperatures and high humidity [17]. Furthermore, low temperatures and low relative humidity favour the survival and transmission of certain influenza viruses [18,19], and are associated with an increased occurrence of respiratory tract infections.
- UV light: action on viruses is well known in the literature, and sterilisation by UV light is a commonly used process. Although several studies have already demonstrated the effect of UV on SARS-CoV-2 [23,24,25,26], controlling the exposure of samples to light during experiments is not always carried out, which becomes an additional confounding factor and a source of data dispersion. This circumstance affects the validity of comparing the results of different experiments.
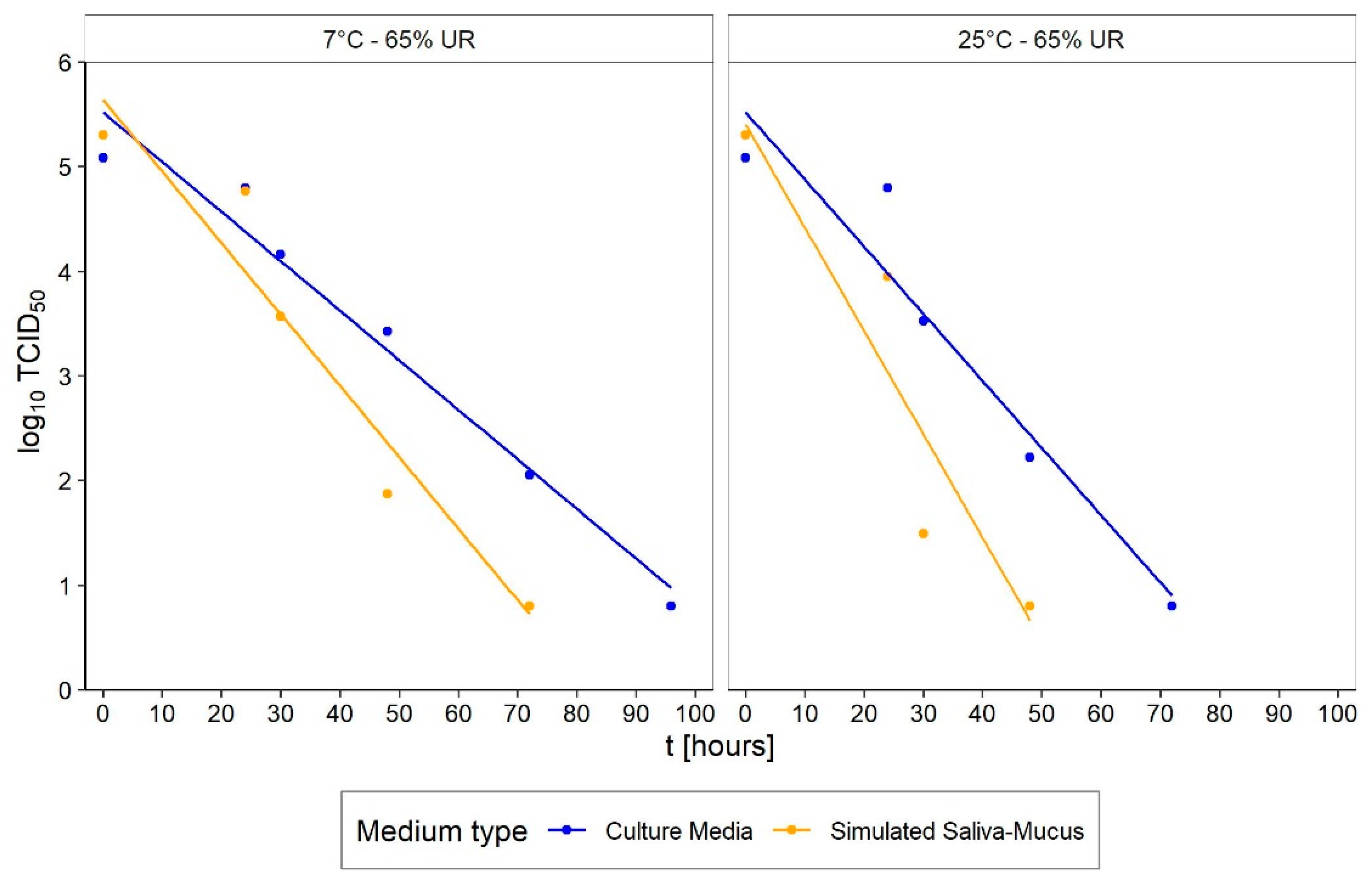
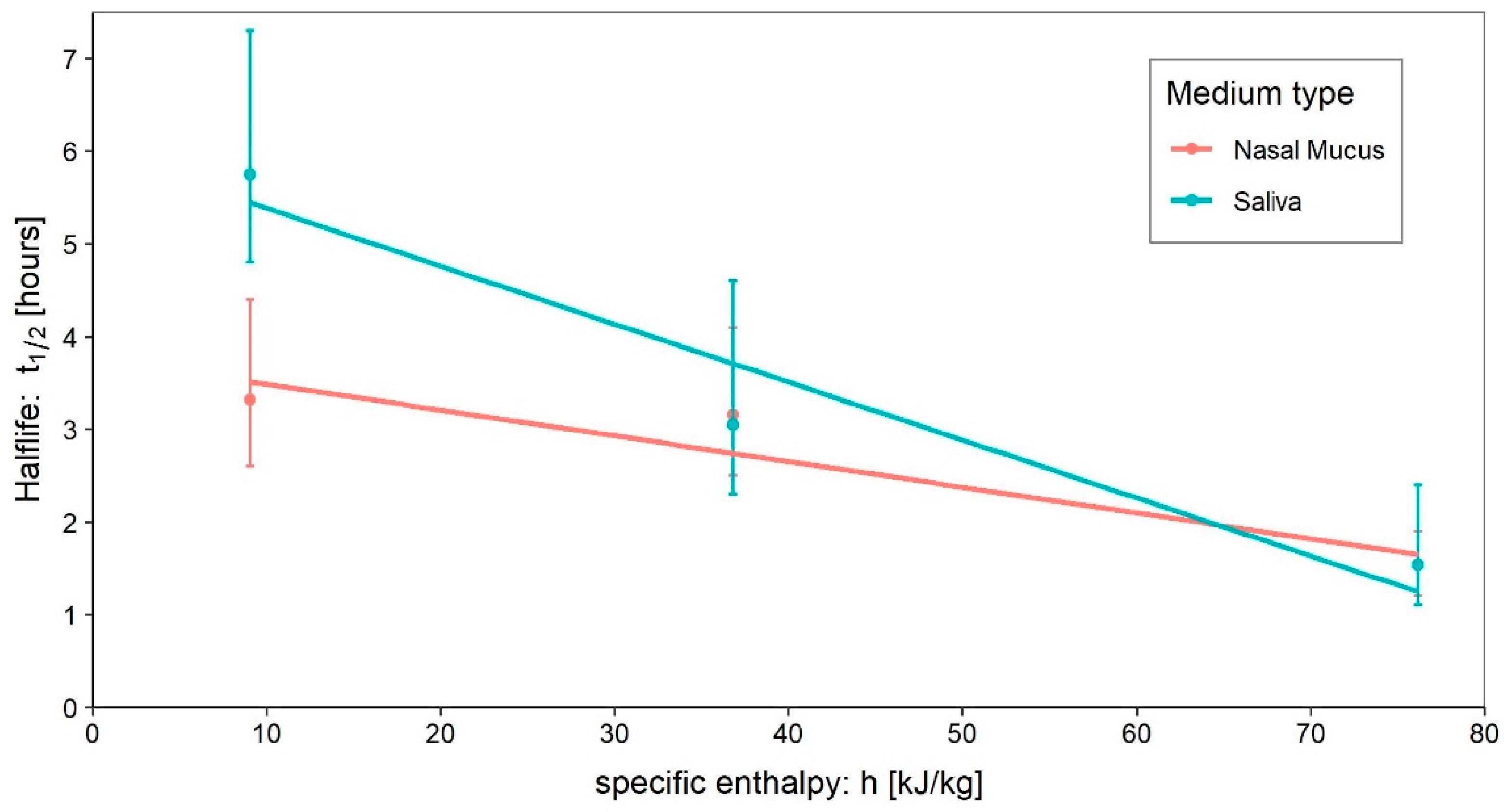
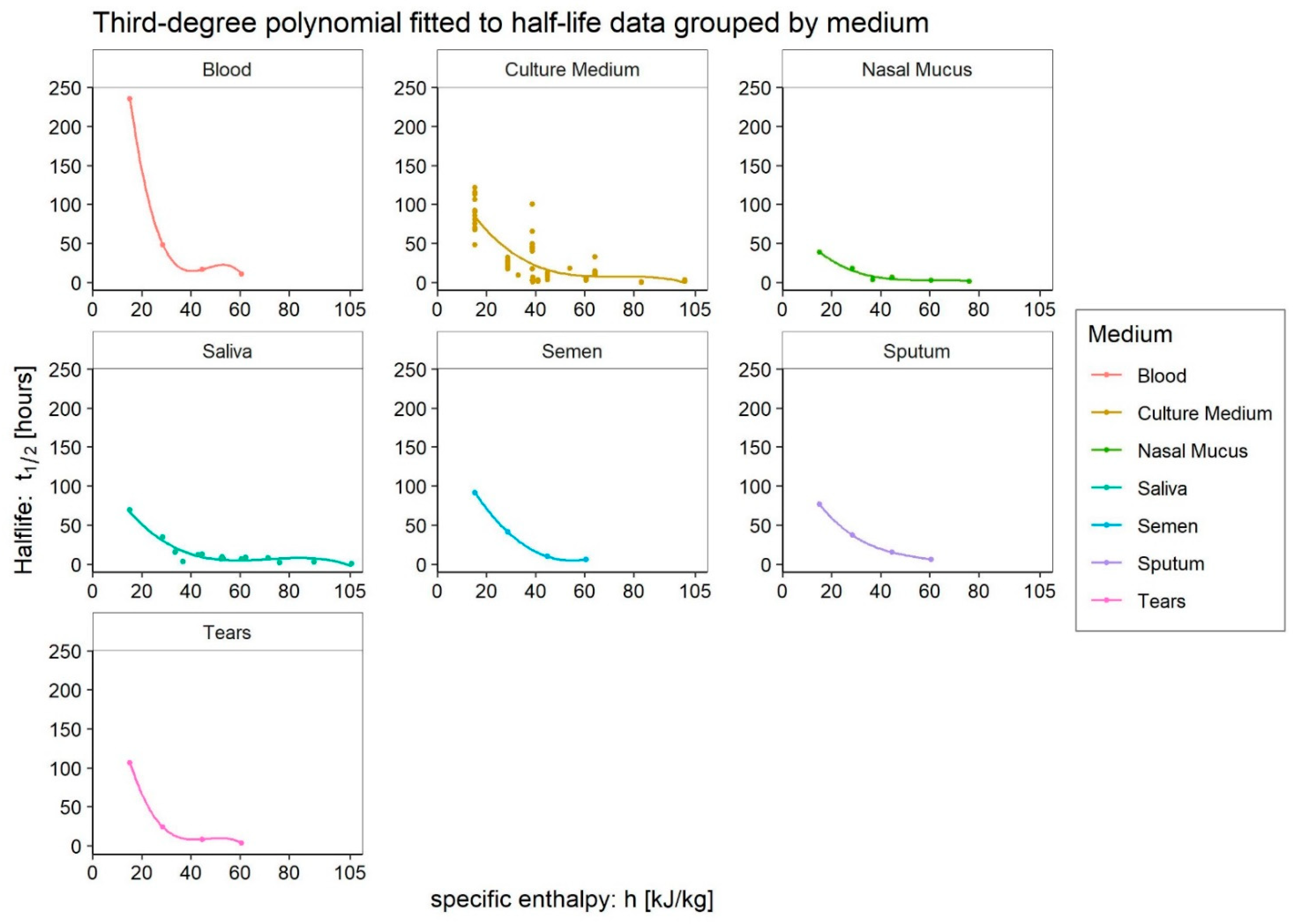
- Medium: different media, or the variation in their composition, is another confounding factor [26,27,28]. The protein composition of the medium, for example, alters the ability of the virus to proliferate and survive, as demonstrated by Pastorino et al. [29] (Figure 1). This constitutes a further obstacle when comparing data from different experiments. We can perceive this dependence appropriately by visualising the data from the work of Szpiro et al. [30] (Figure 2) and Matson et al. [31] (Figure 3), the latter expressly given as a function of specific enthalpy.
- Pollutants: the opinion of the scientific literature is now converging on the established role of pollutants in the survival and transmission capacity of viruses [32,33]. However, this role is especially significant when studying the survival of viruses in aerosols; it does not appear to be relevant—as was also the case in the present study—for the survival of SARS-CoV-2 on surfaces.
3. Results
4. Discussion
4.1. Culture Medium
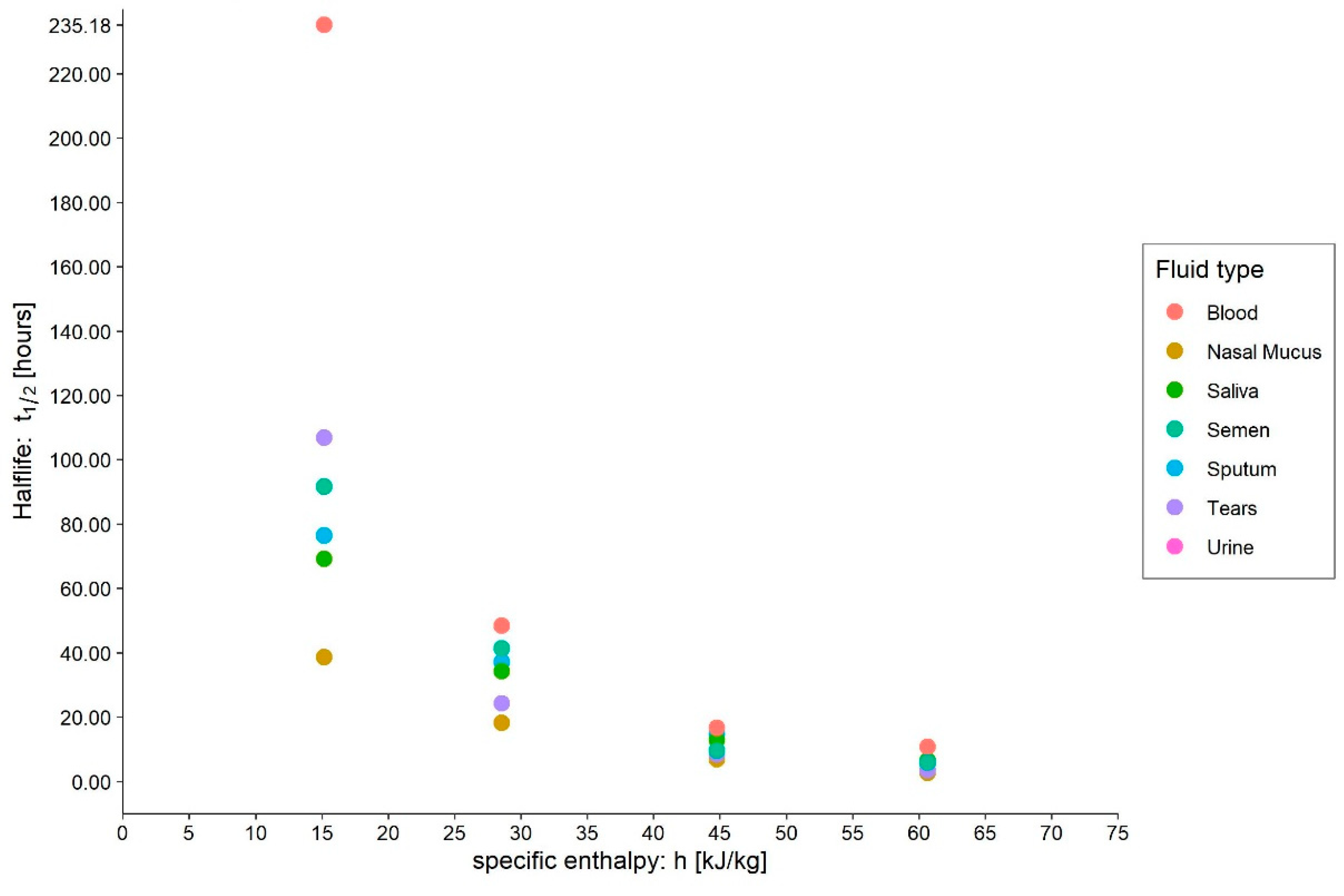
4.2. Blood
4.3. Tears
4.4. Sputum
4.5. Saliva
4.6. Nasal Mucus
4.7. Semen
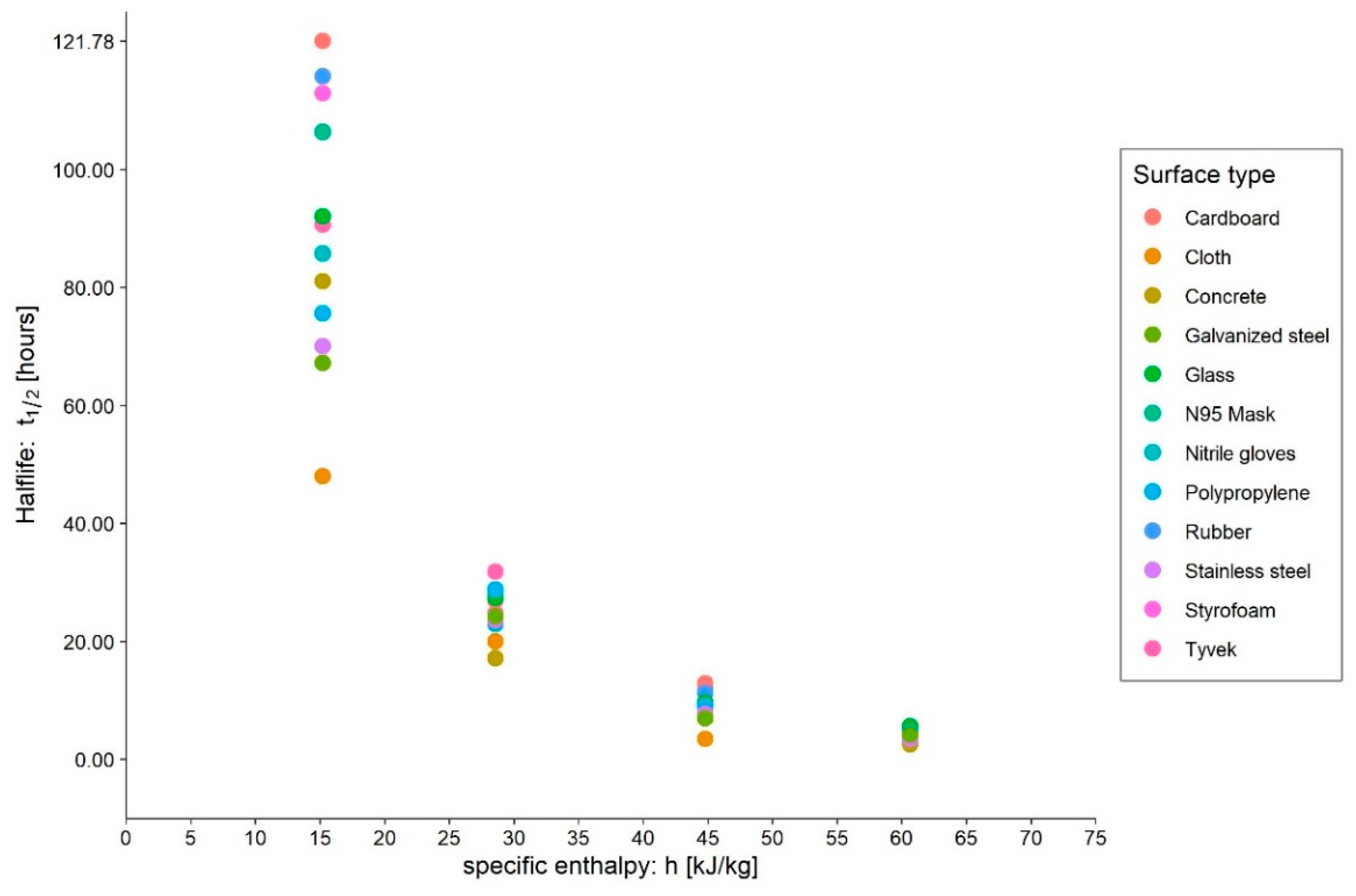
4.8. Very Low Enthalpies
4.9. Criticism of Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spena, A.; Palombi, L.; Corcione, M.; Carestia, M.; Spena, V.A. On the Optimal Indoor Air Conditions for SARS-CoV-2 Inactivation. An Enthalpy-Based Approach. Int. J. Environ. Res. Public Health 2020, 17, 6083. [Google Scholar] [CrossRef] [PubMed]
- Spena, A.; Palombi, L.; Corcione, M.; Quintino, A.; Carestia, M.; Spena, V.A. Predicting SARS-CoV-2 Weather-Induced Seasonal Virulence from Atmospheric Air Enthalpy. Int. J. Environ. Res. Public Health 2020, 17, 9059. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Appl. Environ. Microbiol. 2010, 76, 2712–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, K.; Yanagi, U.; Kagi, N.; Kim, H.; Ogata, M.; Hayashi, M. Environmental factors involved in SARS-CoV-2 transmission: Effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020, 25, 66. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.; Perera, M.R.; Hui, K.P.; Yen, H.L.; Chan, M.C.; Peiris, M.; Poon, L.L. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Deng, Y.; Liu, S.; Zhang, D.; Li, H.; Wang, X.; Jia, L.; Han, J.; Bei, Z.; et al. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J. Hosp. Infect. 2021, 107, 105–107. [Google Scholar] [CrossRef]
- Grinchuk, P.S.; Fisenko, K.I.; Fisenko, S.P.; Danilova-Tretiak, S.M. Isothermal Evaporation Rate of Deposited Liquid Aerosols and the SARS-CoV-2 Coronavirus Survival. Aerosol Air Qual. Res. 2021, 21, 200428. [Google Scholar] [CrossRef]
- Kasloff, S.B.; Leung, A.; Strong, J.E.; Funk, D.; Cutts, T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021, 11, 984. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- REALM Project-Test 1 Results Available|OCLC. Available online: https://www.oclc.org/realm/news/20200622-round-1-test-results-now-available.html (accessed on 22 November 2022).
- REALM Project-Test 2 Results. Available online: https://www.oclc.org/realm/news/20200720-test2-results-available.html (accessed on 22 November 2022).
- REALM Project-Test 3 Results. Available online: https://www.oclc.org/realm/news/20200818-test-3-results-available.html (accessed on 22 November 2022).
- Akers, T.G. Some aspects of the airborne inactivation of viruses. In Airborne Transmission and Airborne Infection; Hers, J.F.P., Winkler, K.C., Eds.; John Wiley & Sons: New York, NY, USA, 1973. [Google Scholar]
- de Jong, J.C. Decay mechanism of polio and EMC viruses in aerosols. In Third International Symposium on Aerobiology; Academic Press, Inc.: London, UK, 1970; pp. 210–245. [Google Scholar]
- Morawska, L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 2006, 16, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershenson, A.; Gierasch, L.M. Protein folding in the cell: Challenges and progress. Curr. Opin. Struct. Biol. 2011, 21, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ijaz, M.K.; Brunner, A.H.; Sattar, S.A.; Nair, R.C.; Johnson-Lussenburg, C.M. Survival Characteristics of Airborne Human Coronavirus 229E. J. Gen. Virol. 1985, 66, 2743–2748. [Google Scholar] [CrossRef]
- Ikäheimo, T.M.; Jaakkola, K.; Jokelainen, J.; Saukkoriipi, A.; Roivainen, M.; Juvonen, R.; Vainio, O.; Jaakkola, J.J. A Decrease in Temperature and Humidity Precedes Human Rhinovirus Infections in a Cold Climate. Viruses 2016, 8, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.E.; Dougherty, E.; McArthur, C.; Huang, Q.S.; Baker, M.G. Cold, dry air is associated with influenza and pneumonia mortality in Auckland, New Zealand. Influenza Other Respir. Viruses 2016, 10, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, W.; Huang, J.; Gamber, M.; Wu, J.; He, G. Indirect Virus Transmission in Cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Marr, L.C. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl. Environ. Microbiol. 2012, 78, 6781–6788. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Elankumaran, S.; Marr, L.C. Relationship between Humidity and Influenza A Viability in Droplets and Implications for Influenza’s Seasonality. PLoS ONE 2012, 7, e46789. [Google Scholar] [CrossRef] [Green Version]
- Ratnesar-Shumate, S.; Williams, G.; Green, B.; Krause, M.; Holland, B.; Wood, S.; Bohannon, J.; Boydston, J.; Freeburger, D.; Hooper, I.; et al. Simulated Sunlight Rapidly Inactivates SARS-CoV-2 on Surfaces. J. Infect. Dis. 2020, 222, 214–222. [Google Scholar] [CrossRef]
- Raiteux, J.; Eschlimann, M.; Marangon, A.; Rogée, S.; Dadvisard, M.; Taysse, L.; Larigauderie, G. Inactivation of SARS-CoV-2 by Simulated Sunlight on Contaminated Surfaces. Microbiol. Spectr. 2021, 9, e00333-21. [Google Scholar] [CrossRef]
- Herman, J.; Biegel, B.; Huang, L. Inactivation times from 290 to 315 nm UVB in sunlight for SARS coronaviruses CoV and CoV-2 using OMI satellite data for the sunlit Earth. Air Qual. Atmos. Health 2021, 14, 217–233. [Google Scholar] [CrossRef]
- American Society of Heating Refrigerating Air Conditioning Engineers. ASHRAE Positions on Infectious Aerosols. 2022. Available online: www.ashrae.org (accessed on 10 January 2023).
- Kwon, T.; Gaudreault, N.N.; Richt, J.A. Seasonal Stability of SARS-CoV-2 in Biological Fluids. Pathogens 2021, 10, 540. [Google Scholar] [CrossRef]
- Bueckert, M.; Gupta, R.; Gupta, A.; Garg, M.; Mazumder, A. Infectivity of SARS-CoV-2 and Other Coronaviruses on Dry Surfaces: Potential for Indirect Transmission. Materials 2020, 13, 5211. [Google Scholar] [CrossRef]
- Pastorino, B.; Touret, F.; Gilles, M.; De Lamballerie, X.; Charrel, R.N. Prolonged Infectivity of SARS-CoV-2 in Fomites. Emerg. Infect. Dis. 2020, 26, 2256–2257. [Google Scholar] [CrossRef]
- Szpiro, L.; Pizzorno, A.; Durimel, L.; Julien, T.; Traversier, A.; Bouchami, D.; Marie, Y.; Rosa-Calatrava, M.; Terrier, O.; Moules, V. Role of interfering substances in the survival of coronaviruses on surfaces and their impact on the efficiency of hand and surface disinfection. medRxiv 2020. [Google Scholar] [CrossRef]
- Matson, M.J.; Yinda, C.K.; Seifert, S.N.; Bushmaker, T.; Fischer, R.J.; van Doremalen, N.; Lloyd-Smith, J.O.; Munster, V.J. Effect of Environmental Conditions on SARS-CoV-2 Stability in Human Nasal Mucus and Sputum. Emerg. Infect. Dis. 2020, 26, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Krishnamoorthy, P.; Gangamma, S.; Raut, A.A.; Kumar, H. Particulate matter (PM10) enhances RNA virus infection through modulation of innate immune responses. Environ. Pollut. 2020, 266, 115148. [Google Scholar] [CrossRef] [PubMed]
- Groulx, N.; Urch, B.; Duchaine, C.; Mubareka, S.; Scott, J.A. The Pollution Particulate Concentrator (PoPCon): A platform to investigate the effects of particulate air pollutants on viral infectivity. Sci. Total Environ. 2018, 628–629, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Harbourt, D.E.; Haddow, A.D.; Piper, A.E.; Bloomfield, H.; Kearney, B.J.; Fetterer, D.; Gibson, K.; Minogue, T. Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. PLOS Negl. Trop. Dis. 2020, 14, e0008831. [Google Scholar] [CrossRef]
- American Society of Heating Refrigerating Air Conditioning Engineers. ASHRAE Handbook of Fundamentals; Chapter 6-Psychrometrics; ASHRAE: Atlanta, GA, USA, 2005. [Google Scholar]
- Hyland, R.W.; Wexter, A. Formulations for the thermodynamic properties of the saturated phases of H2O from 173.15 K to 473.15 K. ASHRAE Trans. 1983, 89, 500–519. [Google Scholar]
- Wulff, N.H.; Tzatzaris, M.; Young, P.J. Monte Carlo simulation of the Spearman-Kaerber TCID50. J. Clin. Bioinform. 2012, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Kratzel, A.; Steiner, S.; Todt, D.; V’Kovski, P.; Brueggemann, Y.; Steinmann, J.; Steinmann, E.; Thiel, V.; Pfaender, S. Temperature-dependent surface stability of SARS-CoV-2. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; Freeburger, D.; et al. SARS-CoV-2 is rapidly inactivated at high temperature. Environ. Chem. Lett. 2021, 19, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Gaudreault, N.N.; Richt, J.A. Environmental Stability of SARS-CoV-2 on Different Types of Surfaces under Indoor and Seasonal Climate Conditions. Pathogens 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Zhang, L.; Wang, S.; Bai, T.; Xiang, Y.; Long, E. Systematic review of the effects of environmental factors on virus inactivation: Implications for coronavirus disease 2019. Int. J. Environ. Sci. Technol. 2021, 18, 2865–2878. [Google Scholar] [CrossRef]
- Cappi, R.; Casini, L.; Tosi, D.; Roccetti, M. Questioning the seasonality of SARS-COV-2: A Fourier spectral analysis. BMJ Open 2022, 12, e061602. [Google Scholar] [CrossRef]
- Lu, L.; Quintela, I.; Lin, C.; Lin, T.; Lin, C.; Wu, V.C.H.; Lin, C. A review of epidemic investigation on cold-chain food-mediated SARS-CoV-2 transmission and food safety consideration during COVID-19 pandemic. J. Food Saf. 2021, 41, e12932. [Google Scholar] [CrossRef]
- Chi, Y.; Zheng, S.; Liu, C.; Wang, Q. Transmission of SARS-CoV-2 on cold-chain food overpacks: A new challenge. J. Glob. Health 2021, 11, 3071. [Google Scholar] [CrossRef]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Wardzala, C.L.; Wood, A.M.; Belnap, D.M.; Kramer, J.R. Mucins Inhibit Coronavirus Infection in a Glycan-Dependent Manner. ACS Central Sci. 2022, 8, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracely, E.J. Half-Life Defined as the Time Until Half of the Log Titer Is Not Informative and Should Be Replaced With Segments: Comment on Hirose et al., 2020. Clin. Infect. Dis. 2021, 73, e853–e854. [Google Scholar] [CrossRef]
- Hirose, R.; Ikegaya, H.; Naito, Y.; Watanabe, N.; Yoshida, T.; Bandou, R.; Daidoji, T.; Itoh, Y.; Nakaya, T. Survival of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Influenza Virus on Human Skin: Importance of Hand Hygiene in Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, e4329–e4335. [Google Scholar] [CrossRef] [PubMed]

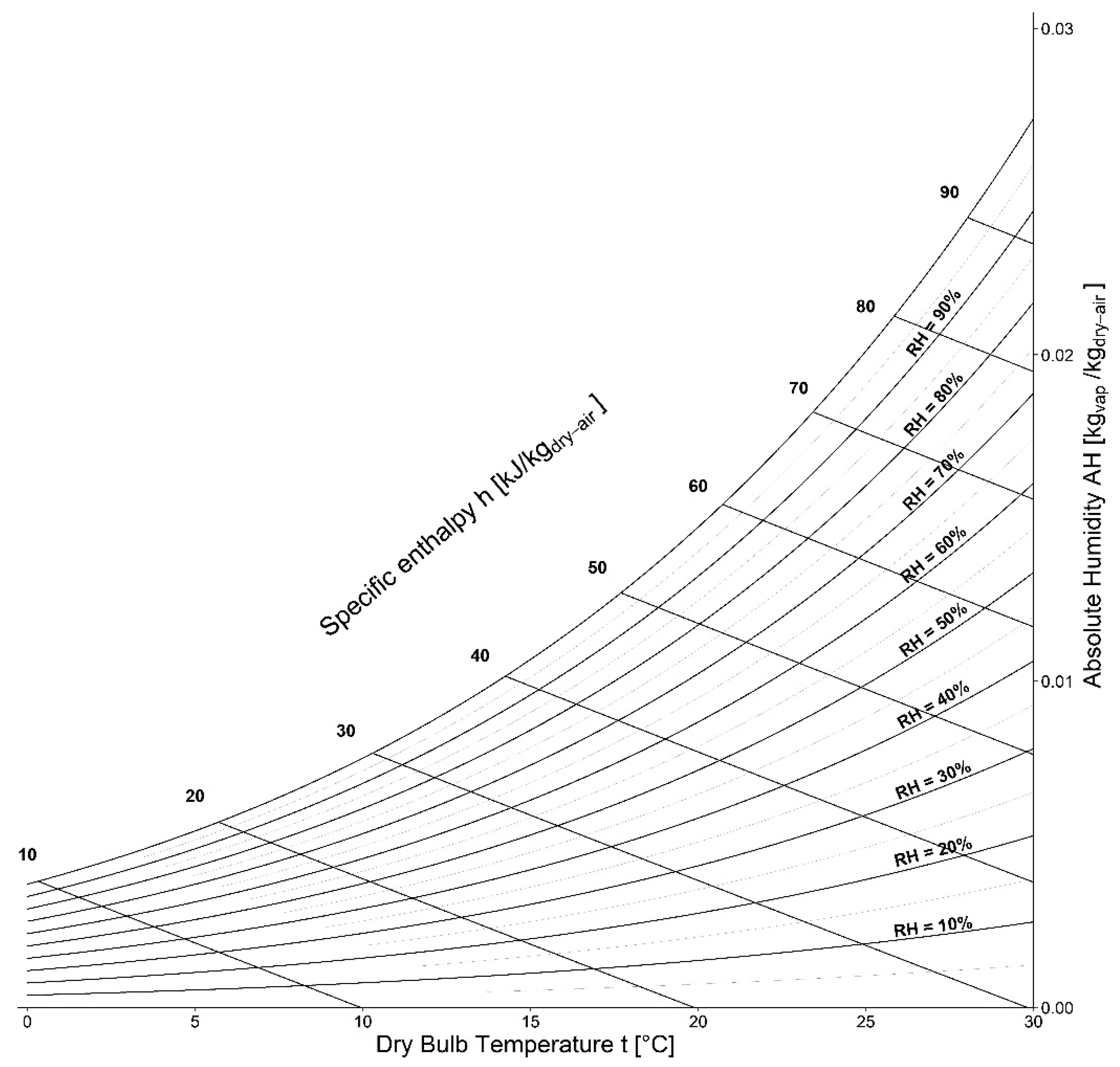

| Strain | Medium | T | RH | h | |
|---|---|---|---|---|---|
| [°C] | [%] | [kJ/kgdry-air] | |||
| Harbourt et al. [34] | USA-WA1/2020 | CM EMEM w/10% FBS | 4 | 45 | 9.69 |
| 22 | 45 | 40.95 | |||
| 37 | 45 | 83.16 | |||
| Kratzel et al. [38] | Munchen-1.1/2020/929 | CM w/0.3% BSA | 4 | 35 | 8.43 |
| 20 | 35 | 33.00 | |||
| 30 | 35 | 53.89 | |||
| Pastorino et al. [29] | BavPat1/2020 | CM w/5% FBS 1 | 20 | 50 | 38.58 |
| Van Doremalen et al. [39] | nCoV-WA1-2020 Tor2 | CM DMEM w/10% FBS | 22 | 40 | 38.84 |
| Matson et al. [31] | USA-WA1/2020 | Nasal Mucus–Sputum | 4 | 40 | 9.06 |
| 21 | 40 | 36.82 | |||
| 27 | 85 | 76.19 | |||
| Biryukov et al. [40] | USA-WA1/2020 | Simulated Saliva | 24 | 20 | 33.54 |
| 24 | 40 | 43.06 | |||
| 24 | 60 | 52.68 | |||
| 24 | 80 | 62.42 | |||
| 28 | 40 | 52.28 | |||
| 35 | 20 | 53.17 | |||
| 35 | 40 | 71.53 | |||
| 35 | 60 | 90.32 | |||
| Biryukov et al. [41] | USA-WA1/2020 | Simulated Saliva | 54.5 | 20 | 105.60 |
| Kwon et al. [27,42] | USA-WA1/2020 | CM DMEM w/5% FBS Biological fluids | 21 | 60 | 44.78 |
| 25 | 70 | 60.67 | |||
| 13 | 66 | 28.58 | |||
| 5 | 75 | 15.20 | |||
| Riddell et al. [43] | Betacoronavirus/Australia/SA01/2020 | CM DMEM w/Penicillin, Streptomycin, Fungizone, 10% FCS and 1% BSA | 20 | 50 | 38.58 |
| 30 | 50 | 64.27 | |||
| 40 | 50 | 100.91 |
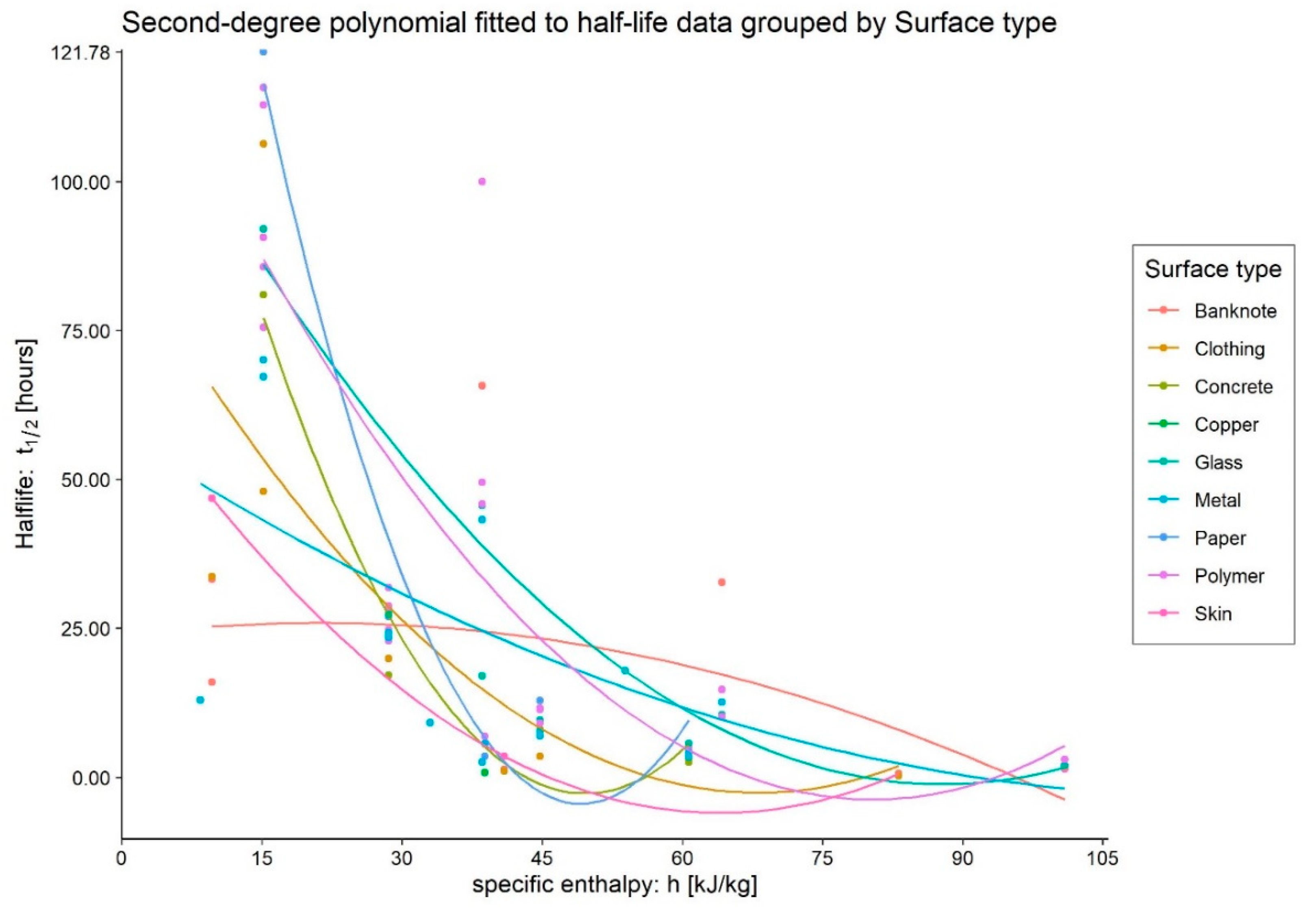
| Medium | 2nd-Degree Polynomial | 3rd-Degree Polynomial | ||
|---|---|---|---|---|
| before | after | |||
| p-Value | R2 | p-Value | R2 | |
| Blood | * 0.205 | 0.96 | nd | nd |
| Culture medium | 2.7 × 10−10 | 0.40 | 8.0 × 10−15 | 0.57 |
| Nasal mucus | * 0.513 | 0.28 | ** 0.053 | 0.96 |
| Saliva | 0.051 | 0.37 | 2.7 × 10−6 | 0.92 |
| Semen | 0.011 | 1 | nd | nd |
| Sputum | ** 0.057 | 1 | nd | nd |
| Tears | * 0.196 | 0.96 | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spena, A.; Palombi, L.; Carestia, M.; Spena, V.A.; Biso, F. SARS-CoV-2 Survival on Surfaces. Measurements Optimisation for an Enthalpy-Based Assessment of the Risk. Int. J. Environ. Res. Public Health 2023, 20, 6169. https://doi.org/10.3390/ijerph20126169
Spena A, Palombi L, Carestia M, Spena VA, Biso F. SARS-CoV-2 Survival on Surfaces. Measurements Optimisation for an Enthalpy-Based Assessment of the Risk. International Journal of Environmental Research and Public Health. 2023; 20(12):6169. https://doi.org/10.3390/ijerph20126169
Chicago/Turabian StyleSpena, Angelo, Leonardo Palombi, Mariachiara Carestia, Vincenzo Andrea Spena, and Francesco Biso. 2023. "SARS-CoV-2 Survival on Surfaces. Measurements Optimisation for an Enthalpy-Based Assessment of the Risk" International Journal of Environmental Research and Public Health 20, no. 12: 6169. https://doi.org/10.3390/ijerph20126169







