Abstract
Coking wastewater is a typical high-strength organic wastewater, for which it is difficult to meet discharging standards with a single biological treatment. In this study, effective advanced treatment of coking wastewater was achieved by coagulation with freshly prepared polyaluminum silicate sulfate (PASS). The performance advantage was determined through comparison with commercial coagulants including ferric chloride, polyferric sulfate, aluminum sulfate and polyaluminum chloride. Both single-factor and Taguchi experiments were conducted to determine the optimal conditions for coagulation with CODCr and UV254 as indicators. A dosage of 7 mmol/L PASS, flocculation velocity of 75 r/min, flocculation time of 30 min, pH of 7, and temperature of 20 °C could decrease the CODCr concentration from 196.67 mg/L to 59.94 mg/L. Enhanced coagulation could further help to remove the organic compounds, including pre-oxidation with ozonation, adsorption with activated carbon, assistant coagulation with polyacrylamide and secondary coagulation. UV spectrum scanning and gas chromatography-mass spectrometry revealed that the coagulation process effectively removed the majority of organic compounds, especially the high molecular weight alkanes and heterocyclic compounds. Coagulation with PASS provides an effective alternative for the advanced treatment of coking wastewater.
1. Introduction
Coking wastewater is a high-strength industrial wastewater generated from coal coking, coal gas purification, and by-product recovery processes of the coking industry [1]. It usually contains dozens of inorganic pollutants (ammonium, sulfate, cyanide, thiocyanate, etc.) and organic contaminants, such as phenolic compounds, polycyclic aromatic hydrocarbons (PAHs), nitrogen- and sulfur-containing heterocyclic compounds, etc., [2]. Most of these constituents are persistent, toxic, mutative, and carcinogenic, which can cause respiratory diseases, reproductive diseases, genetic diseases and so on [3]. Therefore, coking wastewater poses a severe threat to the environment and human health. Especially in China, where coal is a major energy source, hundred million tons of coking wastewater (1.8 × 108 t/a) is generated each year [4]. Therefore, efficient treatment of coking wastewater has become an urgent problem.
Conventional coking wastewater treatment procedures include pretreatment and biological treatment [5,6]. Activated sludge process is the most frequently adopted biological treatment, which can effectively remove ammonia and short-chain fatty acids [7]. However, it is difficult for refractory organics such as PAHs and heterocyclic compounds to achieve a high removal efficiency, although some new biological methods have been developed and employed, including powdered activated carbon treatment (PACT) process, photosynthetic bacteria method, combined hydrolytic acidification, and aerobic treatment [2,7,8,9,10]. As a result, it is difficult to meet the discharge requirements using conventional coking wastewater treatment, hence post-treatment becomes a focus for current researchers [11]. Common advanced treatment methods include catalytic ozonation, active carbon adsorption, ion exchange and reverse osmosis, which usually require a high-cost investment and operation [12,13,14,15,16].
As an advanced treatment method, coagulation is advantageous due to its merits of low cost and easy operation [17]. Aluminum and ferric salts are the most frequently used coagulants, which are efficient in the removal of color, suspended soils and some organic compounds in the wastewater industry [18,19,20]. The coagulation process efficiently removes these pollutants by decreasing or neutralizing the electric charge or zeta potential, which facilitates the aggregation of colloidal and fine suspended materials to form microflocs [21,22]. Polymeric flocculants could further bring together the microflocs to form large agglomerations through physical mixing and binding actions [23,24]. However, the traditional aluminum and ferric coagulants still have the disadvantages of large dosage and dissatisfactory performance, especially when treating industrial wastewater for organic compound removal [25,26]. A relatively new inorganic polymer coagulant, polyaluminum silicate sulfate (PASS), was developed in the 1990s and has attracted wide attention as the introduction of silicate components significantly improved the contaminants removal capability, ascribed to the large floc size, strong adsorption bridging and wide pH adaption ability [27,28,29].
This research aims to: (i) prepare a polymeric coagulant polyaluminum silicate sulfate and to determine its effectiveness in the advanced treatment of coking wastewater through comparison with commercial aluminum and ferric chloride; (ii) to further evaluate the optimal conditions of removing organic compounds represented by CODCr and UV254 by single factor and Taguchi experiments; (iii) to finally explore the removal mechanisms through UV scanning and GC-MS detection of the wastewater before and after treatment by polyaluminum silicate sulfate.
2. Materials and Methods
2.1. Wastewater
The raw coking wastewater used for the coagulation tests was collected from the effluent of a lab-scale biological anaerobic and aerobic filter treating coking wastewater, which was originally taken from a coking plant in Tangshan, Hebei province. The average CODCr and UV254 value of the effluent was 196.67 mg/L and 3.358 cm−1, respectively. The temperature and pH averaged at 20 °C and 8.32, respectively. Notably, only the removal of organic compounds was emphasized, as the ammonium had been degraded to as low as 5.12 mg/L by the biological process [30,31].
2.2. Preparation of PASS Coagulants
The polyaluminum silicate sulfate (PASS) used in this study was prepared in the laboratory using the modified method with an Al/Si ratio of 5 [32]. In addition, commercial coagulants including ferric chloride (FC), polyferric sulfate (PFS), aluminum sulfate (AS), and polyaluminum chloride (PAC) were also employed and conducted in the coagulation experiments parallelly with PASS.
2.3. Jar Tests
The jar tests were carried out in a six-paddle agitation apparatus to determine the coagulation performance at varied dosage and conditions. Firstly, 150 mL coking wastewater in a beaker was mixed with the coagulant at a high speed (230 r/min) for 30 s, followed by a slow mixing (75 r/min) for 15 min. After settling for 30 min, the supernatant was sampled for determining the CODCr and UV254 values. The dosage of PASS and the commercial coagulants was expressed by the molar concentration (in mmol/L) of active component (aluminum or ferrum) [33]. At the optimal dosage of PASS, single-factor experiment at varied flocculation intensity, flocculation time, settling time, pH value and temperature were conducted to determine the optimal coagulation conditions with CODCr and UV254 values as the main indicators [34,35]. Taguchi L16 experiment at four levels of varied factors was further conducted to explore the optimized coagulation conditions.
2.4. Enhanced Coagulation Experiment
A series of enhanced coagulation experiments were conducted to explore the potential enhancement on the coagulation efficiencies, such as pre-oxidation, active carbon adsorption, assisted coagulation by polyacrylamide, and secondary coagulation. In the pre-oxidation, KMnO4, NaClO, O3 was used as the oxidizer and reacted for 20 min prior to the jar-test coagulation. In the adsorption, activated carbon at a dosage of 0~500 mg/L was added to the coking wastewater prior to the PASS addition. In the assisted coagulation, polyacrylamide dosed at 0~5 mg/L was supplemented to the reaction system at the beginning of the slow mixing step. In the secondary coagulation, the collected supernatant after coagulation was implemented to a repetitive coagulation with PASS dosage at 0~8 mmol/L. When each enhanced coagulation experiment was completed after settling for 30 min, the supernatant was sampled and CODCr and UV254 values were determined.
2.5. Analytical Methods
The supernatant was filtrated with a 0.45 μm filter membrane before analysis. CODCr concentration and UV254 were determined by the potassium dichromate oxidation method and UV absorption value at 254 nm, respectively. The scan of the wastewater was conducted by UV–vis spectrophotometer with wavelengths ranging from 190~600 nm.
Gas chromatography-mass spectrometry (GCMS) analysis was carried out to analyze the removal performance of organic compounds by the coagulation. Briefly, 200 mL coking wastewater in a 500 mL separating funnel was extracted three times with 10 mL dichloromethane at neutral, alkaline and acidic conditions, respectively. The extracted liquid was condensed into 1 mL with high-purity nitrogen gas and 2 μL was injected to analysis by 6890 N/5973 GCMS system (Agilent Corporation, Santa Clara, CA, USA) with a modified condition referring to a previous study [33]. A BP-5MS capillary column with an inner diameter of 0.20 mm and a length of 12 m was used in the separation system with high-purity helium gas as the carrier gas. Inlet temperature was maintained at 280 °C. The temperature control program included an initial temperature of 40 °C for 15 min, followed by increasing to 280 °C with an increment of 10 °C/min and maintaining for 5 min. Electron ionization with the electron energy and electron multiplier voltage at 70 eV and 1200 V, respectively. Full scan mode ranged from 50 to 500 amu. The organic compounds peaking at different retention times were identified through matching their mass spectrum with the standard mass spectral library NIST 11.
3. Results and Discussion
3.1. Coagulation Performance of PASS
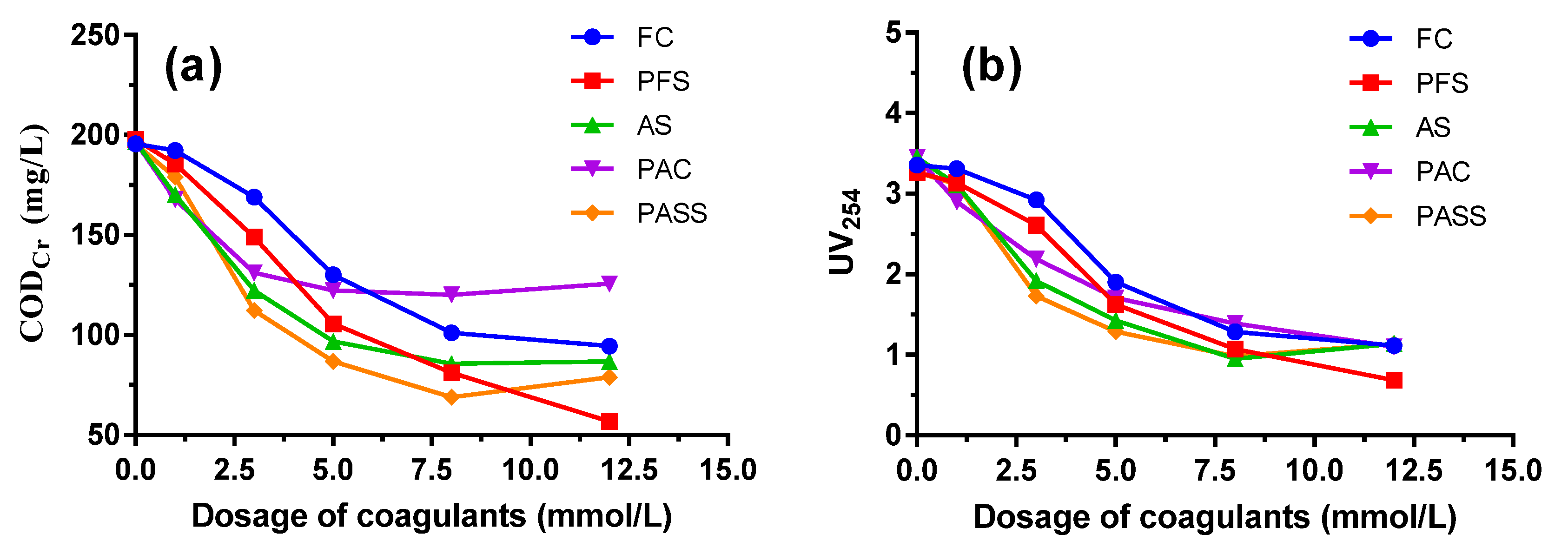
As can be seen from Figure 1, CODCr and UV254 values obviously decreased with increasing coagulant dosage in the beginning, and the trend slowed down later on. An increasing tendency even emerged as coagulant dosage increased to higher than 8 mmol/L. At a small dosage of coagulants, the removal efficiency of organic compounds was relatively low due to the difficulty of flocs formation or sedimentation of small flocs. With the dosage increasing, flocs grow bigger and the precipitation also gets faster, which speeds up the removal efficiency. At a low dosage of coagulants, the main coagulation mechanisms are charge neutralization and adsorption bridging, while at high dosage adsorption bridging as well as net capturing and precipitation play a vital role in organics removal [21,36].
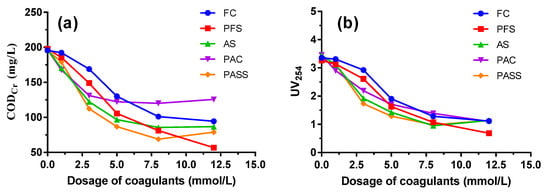
Figure 1.
CODCr (a) and UV254 (b) removal performance by polyaluminum silicate sulfate (PASS) and commercial coagulants including ferric chloride (FC), polyferric sulfate (PFS), aluminum sulfate (AS), and polyaluminum chloride (PAC).
Compared to the commercial coagulants, PASS showed the best coagulation performance at a dosage of 3~8 mmol/L. It could be seen that when the dosage of PASS was 5 mmol/L, the CODCr value in the discharged water reached 86.67 mg/L, meeting the standard of the Integrated Wastewater Discharge Standard of China (GB 8978-1996). PASS also achieved the best removal efficiency of UV254 at the dosage of 3~5 mmol/L. As UV254 could represent the content of high weight molecular organic compounds containing C=C and C=O, such as humic and aromatic compounds, it is suitable to be used to reflect the concentration of refractory organics in coking wastewater, which usually correlates well to CODCr values. The dosage of 5 mmol/L was recommended to be the application dosage, taking financial costs into consideration, and was chosen as the optimal value for the following jar test in this study.
3.2. Optimal Factors Affecting Coagulation Performance
3.2.1. Effect of Flocculation Speed and Time
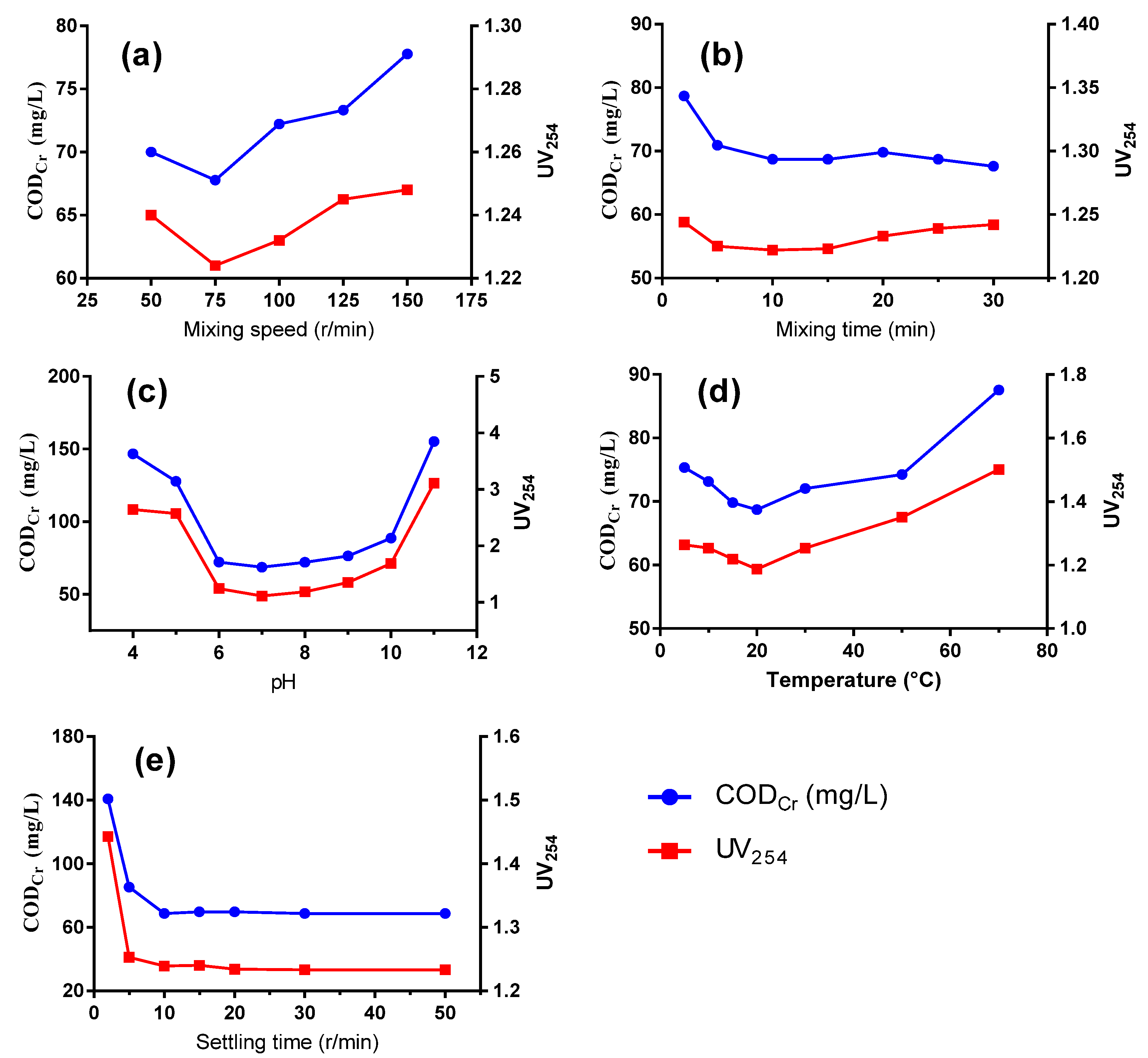
As shown in Figure 2, operation conditions posed a significant impact on the removal of organic compounds, including mixing speed, mixing time, pH, temperature and settling time. As the mixing speed increased, the removal efficiency reached a high of 75 r/min and decreased at higher speeds (Figure 2a). Low flocculation speed would result in insufficient contact between flocs and organic compounds, while too high a speed would cause vigorous stirring and shear force that would not only hinder the formation of large flocs but also break newly formed flocs. Generally, the CODCr concentration decreases when extending flocculation time, as time is needed for floc formation and agglomeration. However, as time progressed longer than 10 min, the removal efficiency of CODCr and UV254 no longer exhibited a downward trend (Figure 2b).
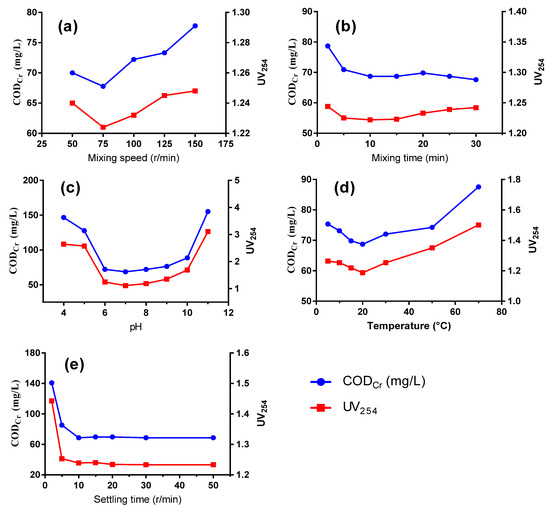
Figure 2.
CODCr and UV254 removal performance by PASS at varied mixing speeds (a), mixing time (b), pH (c), temperature (d), and settling time (e).
3.2.2. Effect of pH Value
Optimal coagulation performance could be realized under neutral conditions, while an acidic or alkaline condition is not suitable for the removal of organics (Figure 2c). When the pH increased to around 7, the hydrolysis of PASS was promoted with the production of Al(OH)3, which is an amphoteric compound that presents in different forms as the pH varies. Under neutral conditions, [Al(OH)2(H2O)4]+ and [AI(OH)3(H2O)3] are the primary forms, when the aluminum hydroxide flocs from the hydrolysis of aluminum-based compounds reach the highest concentration, thereby performing well the roles of compressing the double electric layers and charge neutralization. The zero-potential point of PASS was pH 5.0. Under neutral conditions, the hydrolyzed polysilicate sol-gel performed well at adsorption bridging and the net trapping effect, effectively achieving contaminant removal efficiency together with Al(OH)3. Moreover, the ζ potential of the organics in wastewater approaches 0 under neutral or weak acidic conditions, leading to a decreasing solubility as the number of organics in the ionic state reduces while that in the molecular state increases [18]. Therefore, more organics would be absorbed into the abundant aluminum hydroxide flocs and be efficiently removed after co-precipitation.
3.2.3. Impact of Temperature
As can be seen from Figure 2d, the optimal water temperature was 20 °C, while a higher or a lower temperature impedes the removal efficiency of the organic compounds. At a low temperature, the water viscosity increases, which results in weaker Brownian movement and weaker anisotropic flocculation. Moreover, the efficient hydrolysis of the coagulant is inhibited, which poses an impact on the destabilization of colloidal particles and hinders the adsorption process. Too high a temperature causes petty flocs and makes precipitation difficult due to the increased kinetic energy of molecular motion. In addition, a high temperature causes high moisture content of the sludge, increasing the disposal cost.
3.2.4. Impact of Settling Time
As depicted in Figure 2e, CODCr and UV254 values decreased more sharply with the increase of sedimentation time. Nonetheless, the measured performance remained relatively stable after 10 min when a clear sludge-water interface formed and the residual suspended particles in the supernatant would hardly settle. Finally, a relatively longer time of 30 min was chosen for optimal sedimentation to ensure a dense sludge and a stable removal efficiency.
3.3. Optimal Results by Taguchi Experiments
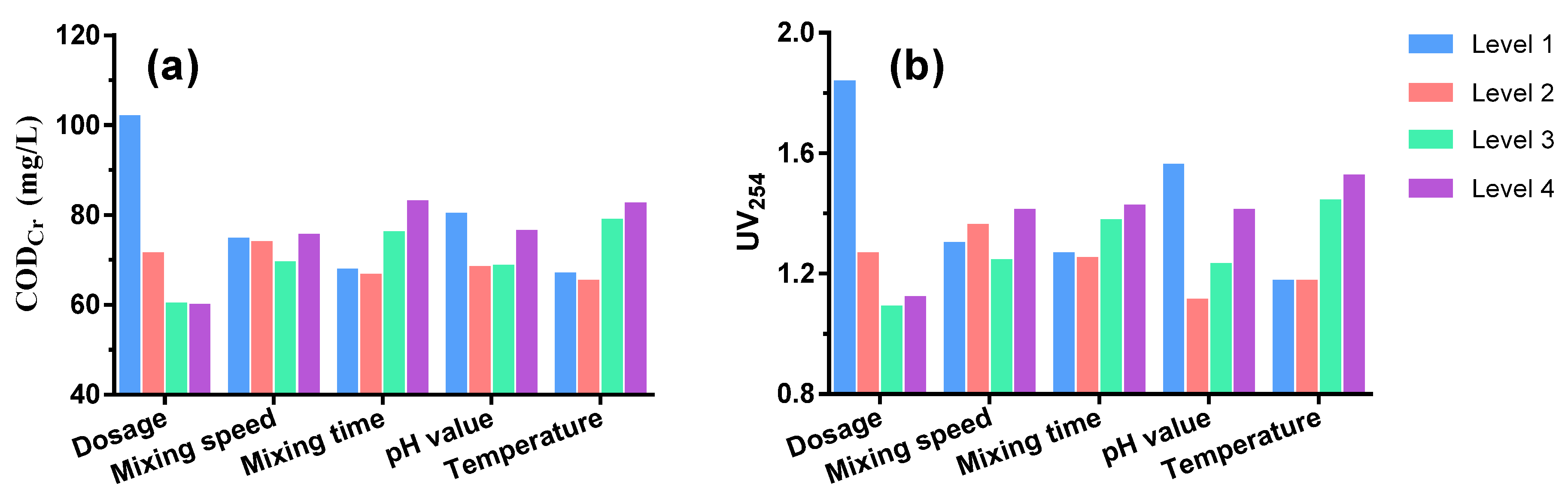
The optimal conditions were determined by a Taguchi L16 experiment and range analysis (Table 1). The order of impact on CODCr removal efficiency was as follows: dosage > temperature > flocculation time > pH value > mixing speed. The optimal condition for CODCr removal included a coagulant dosage of 9 mmol/L, temperature of 20 °C, flocculation time of 10 min, pH value of 7 and mixing speed of 90 r/min (Figure 3). For UV254 removal efficiency, the order of impact could be seen as follows: dosage > pH > temperature > flocculation time > mixing speed. The optimal conditions were similar to those for CODCr except for a temperature of 15 °C. Taking into consideration both CODCr and UV254, the optimal conditions were chosen as: coagulant dosage of 7 mmol/L, mixing speed of 90 r/min, flocculation time of 10 min, pH value of 7, and temperature of 20 °C. Under these conditions, CODCr could be reduced to as low as 59.94 mg/L, meeting the standard of make-up water in industrial recirculating cooling systems (≤60 mg/L) as required by the Code for Design of Industrial Recirculating Cooling Water Treatment (GB/T 50050-2017).

Table 1.
Taguchi L16 experiment design and the corresponding results.
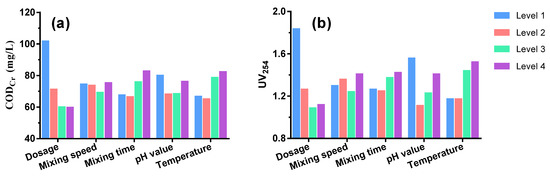
Figure 3.
Taguchi L16 experiment results of CODCr (a) and UV254 (b) removal performance by PASS at four levels of varied factors.
3.4. Enhanced Coagulation Results
3.4.1. Pre-Oxidation
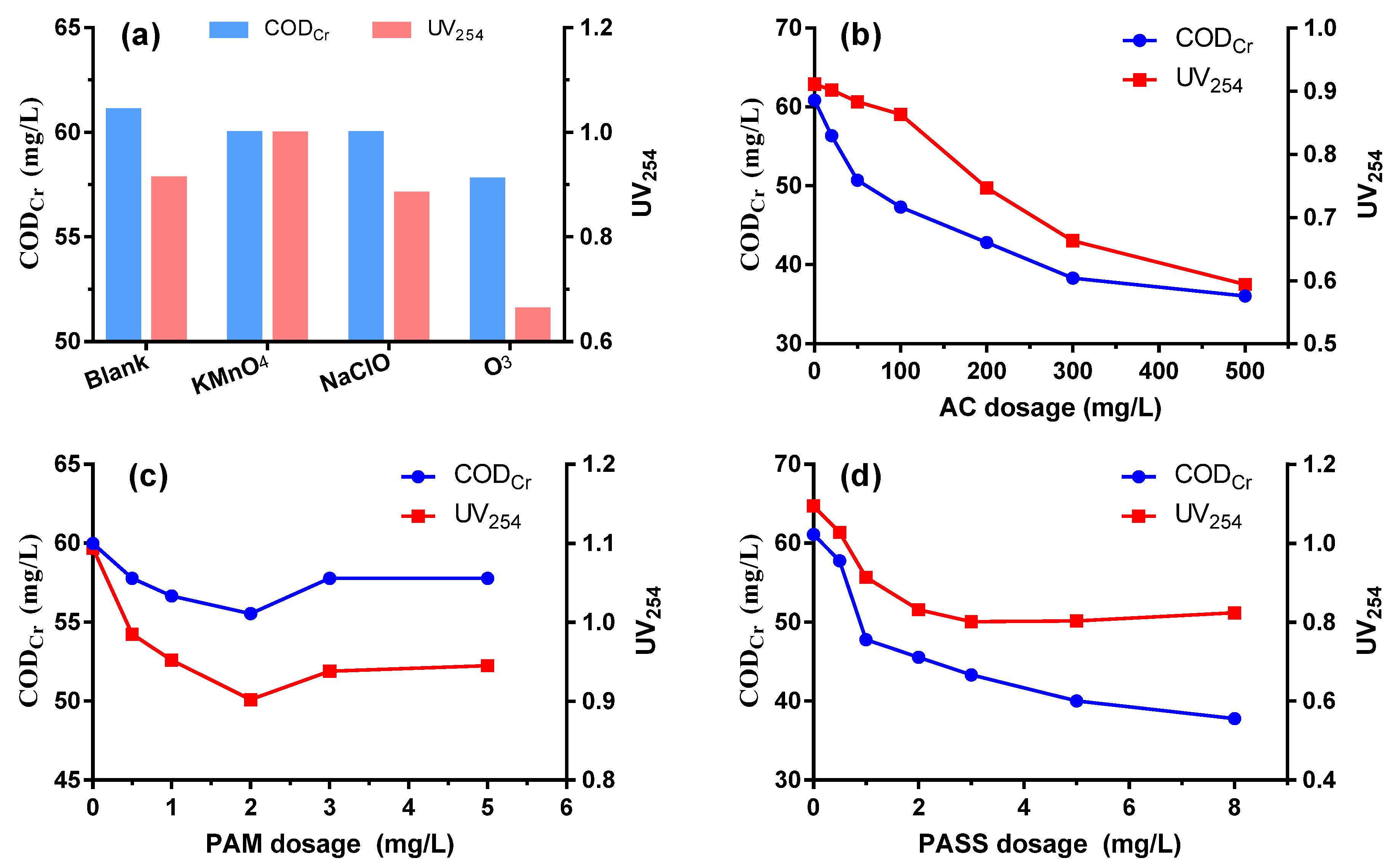
The potential enhancement of the coagulation performance with the help of other physico-chemical methods was investigated, which is of significance especially for wastewater that is notoriously difficult to purify by a single coagulation process. As shown in Figure 4a, compared to the blank, pre-oxidation by KMnO4 and NaClO did not realize any improvement of CODCr removal; KMnO4 even contributes to some UV254 increase due to its own colority. Only pre-oxidation by ozone had obvious benefit to the removal of organic compounds, especially in achieving a high UV254 removal due to its efficient decoloration performance.
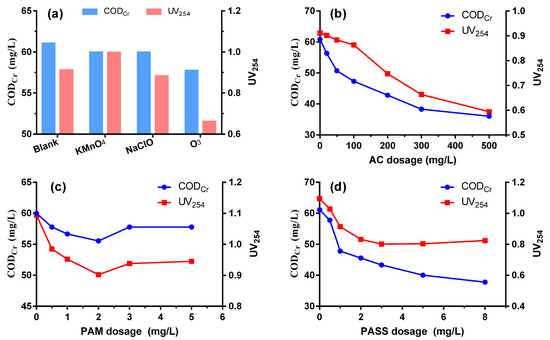
Figure 4.
CODCr and UV254 removal performance enhanced by pre-oxidation (a), adsorption (b), flocculation aid (c) and secondary coagulation (d).
3.4.2. Activated Carbon Adsorption
A significant enhancement of CODCr and UV254 removal was realized by synergetic active carbon adsorption. CODCr could be reduced to 50.70 mg/L at a dosage of 50 mg/L and further decreased with the increase of activated carbon addition (Figure 4b). On one hand, the gigantic surface of activated carbon powder has a strong absorption for dissolved organics, especially low molecular weight compounds, forming a complementation effect with coagulation [37]. On the other hand, the activated carbon powder could provide flocculation centers, which is essential for flocs formation and is beneficial for the removal efficiency of particle colloidal substances.
3.4.3. Coagulant Aid Polyacrylamide
As is depicted in Figure 4c, assisted coagulant PAM is effective in augmenting the decrease of CODcr and UV254, and its optimal dosage is 2 mg/L. PAM’s polymerization degree ranges from 20,000 to 90,000, with a molecular weight between 1,500,000 and 6,000,000. Its strong adsorption for other solids’ surfaces and its bridging between colloidal particles help to promote the coagulation process. When the PAM dosage is relatively low, both adsorption and bridging effects are insignificant. However, when the PAM dosage is too high, itself would dissolve into the water, which increases the CODCr value. PAM could also greatly help to increase the sedimentation velocity of flocs, thus thickening the floc sediments [38].
3.4.4. Secondary Coagulation
As can be seen from Figure 4d, secondary coagulation of the discharged water from coagulation could further enhance its removal efficiency of organics. With a dosage of 1 mmol/L OPASS, CODCr and UV254 dropped to 47.77 mg/L and 0.914, respectively. The CODCr and UV254 removal performance further improved as the dosage was increased. However, the financial costs for the infrastructure construction and management would definitely be increased when secondary coagulation is taken into consideration.
3.5. Coagulation Mechanism Exploration
3.5.1. Ultraviolet Spectrum Scanning
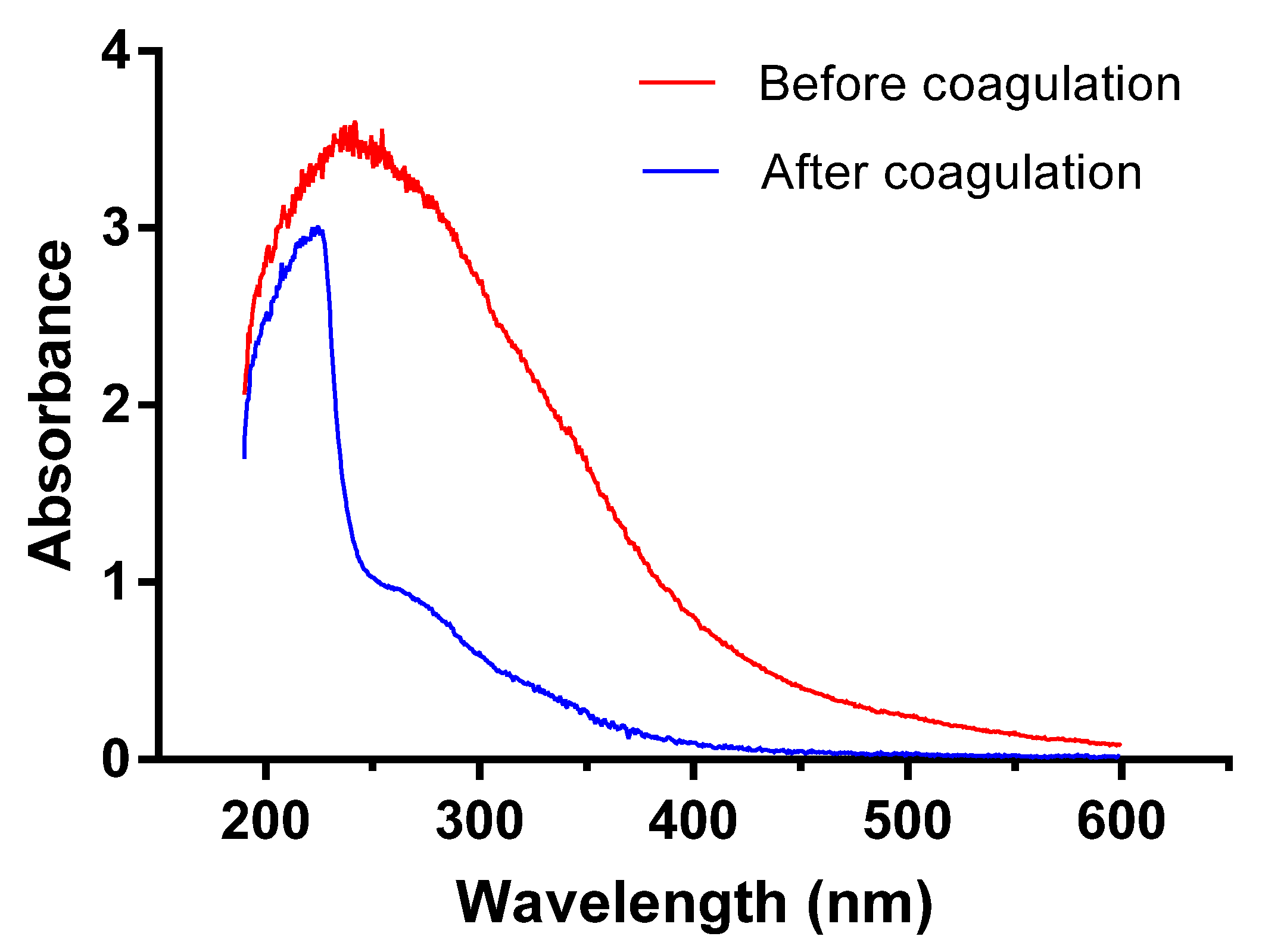
According to spectrum analysis, saturated organic compounds exhibit no adsorption in near ultraviolet band while organics with conjugated double bonds or benzene rings have obvious adsorption in ultraviolet bands ranging from 200 to 400 nm. Specifically, the aromatic compounds with benzene rings adsorb spectrums mainly within 250–260 nm; polycyclic aromatic hydrocarbons (PAHs) exhibit strong adsorption near long wave ultraviolet bands [33]. Thus, ultraviolet spectroscopy scanning could offer clues about the relative concentrations of these organic compounds. As can be seen from Figure 5, ultraviolet spectrum absorbance is relatively lower in discharged wastewater after coagulation than that before treatment, especially in UV spectrums between 250–400 nm. High absorbance of raw water between 200–300 nm suggests a large amount of compounds with conjugated double bonds (such as conjugated diene, unsaturated aldehyde, and unsaturated ketone), or aromatic compounds (such as arene, polyromantic hydrocarbon, and heterocyclic aromatic compounds). Discharged water after coagulation exhibits strong UV absorption between 200–250 nm, suggesting that the coagulation process is not ideal in removing compounds with conjugated double bonds; on the contrary, a sharp decrease in UV adsorption between 250–300 nm indicates the efficient elimination of aromatic compounds.
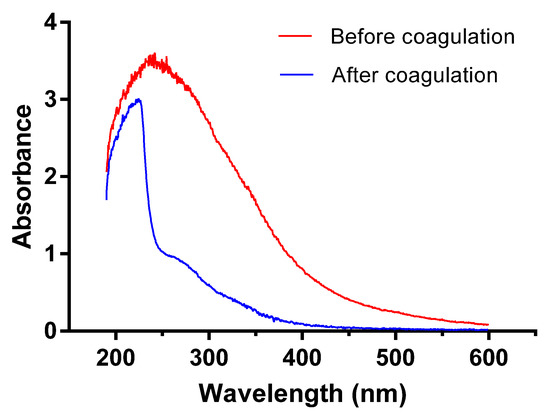
Figure 5.
Ultraviolet spectroscopy scanning results.
3.5.2. Gas Chromatography-Mass Spectrometry (GC-MS)
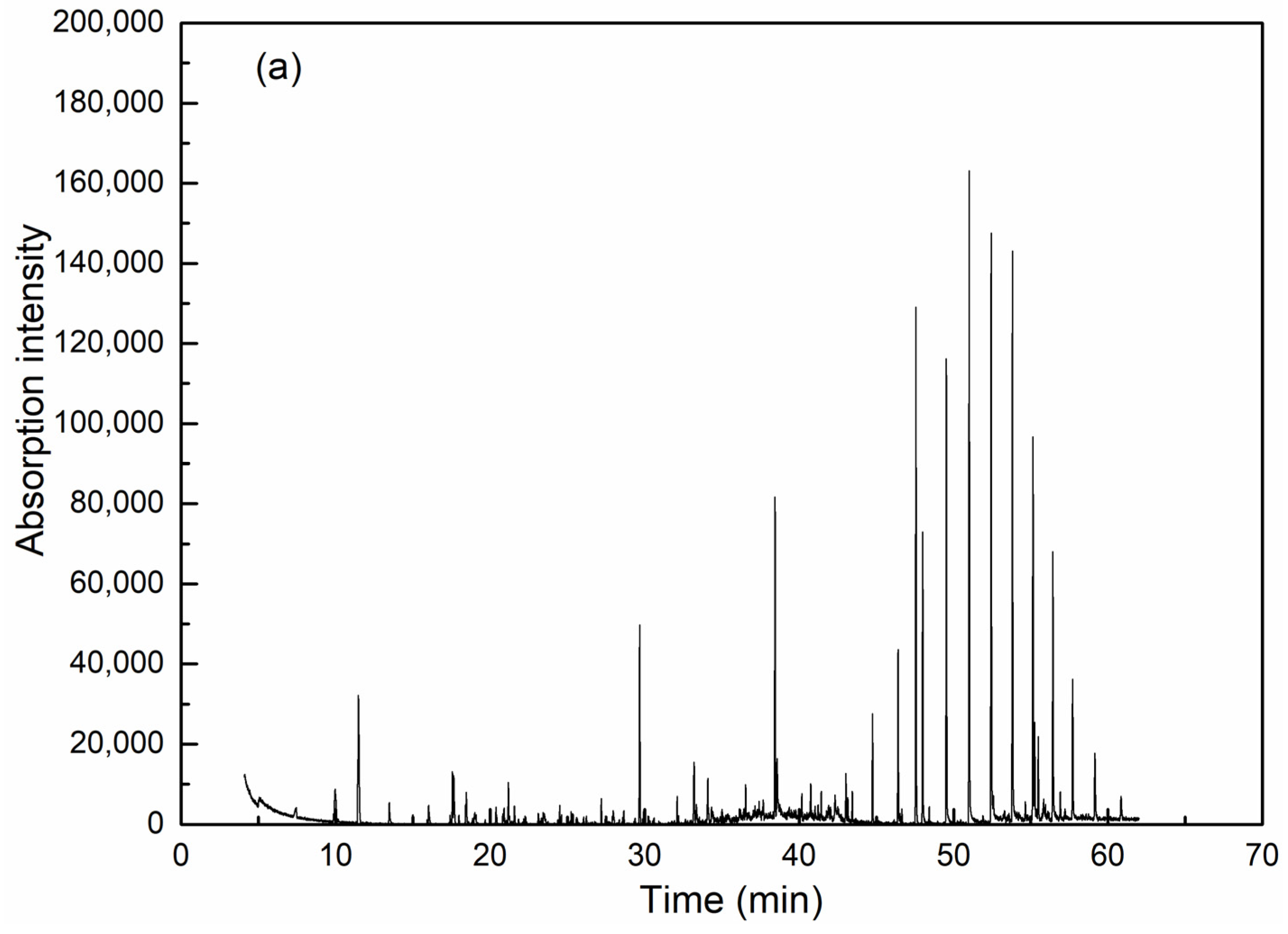
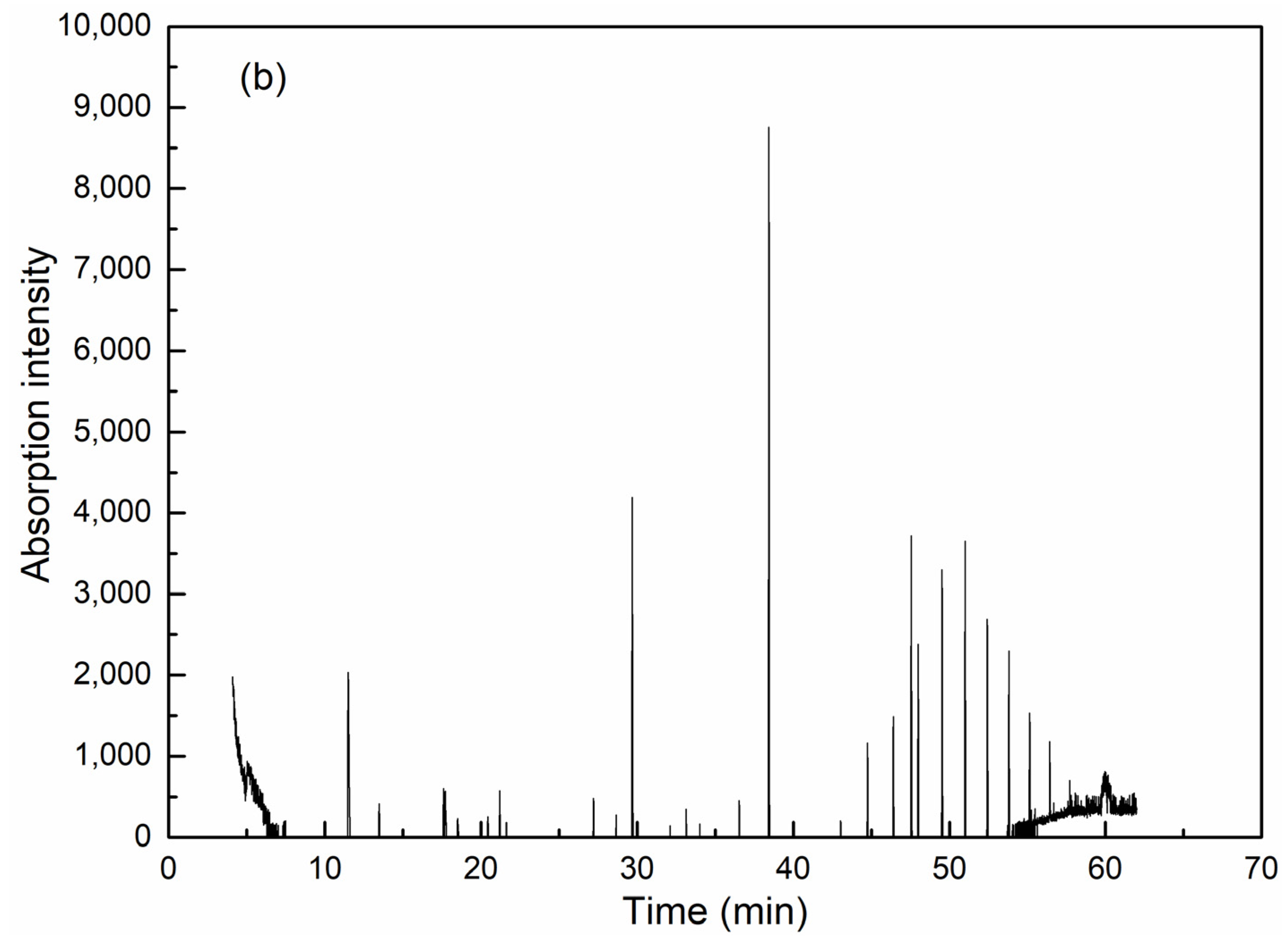
GC-MS is effective for detection and analysis in water quality testing, especially in the isolation, identification and quantification of complicated organic components. As can be seen from Figure 6 and Table 2, coagulation is obviously effective in removing organic compounds. Among the extractable organic compounds by dichloromethane, 19 types of organics with a matching degree over 70 and relative concentration over 0.5% could be detected in raw wastewater, among which there were six kinds of alkane, five heterocyclic compounds, one olefin, one acid, one ester and one phenolic compound. High molecular weight alkanes take up the highest concentration. After coagulation, only six kinds of organics were detected (including two kinds of alkane, one heterocyclic compound, one ester and one phenolic compound) and all have an identical peaking time with the corresponding compounds in raw water, which thereby could be assumed as the exact species from raw water before coagulation.
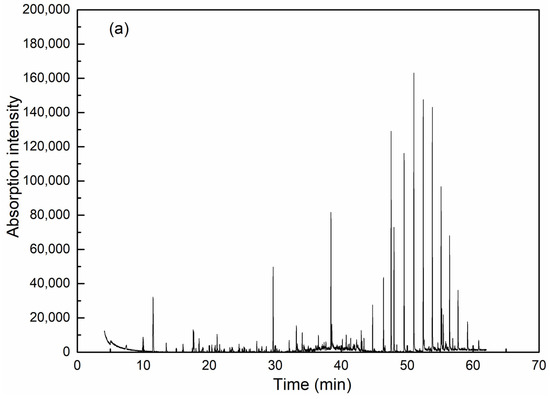
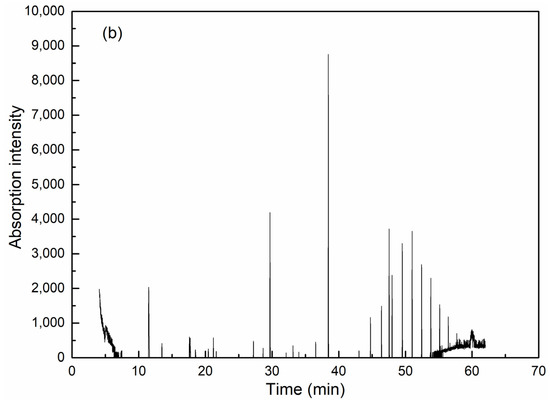
Figure 6.
GC-MS analysis results for wastewater before (a) and after (b) coagulation.

Table 2.
GC-MS analysis of organic compounds in raw and discharged wastewater.
As the concentration of organic compounds was very low in the wastewater after coagulation, the precise GC-MS detection of some trace organics could not be guaranteed. However, considering that the extraction and analytical procedures were the same for both wastewaters before and after treatment, the removal performances of certain organic compounds could still be illustrated qualitatively within the detection range of GC-MS. It could be seen that water after coagulation mainly consists of high molecular weight alkane compounds, mostly heneicosane, however, the removal rate reached as high as 98%. The amount of esters ranks the second in discharged water due to its high stability in water, although only 1,2-benzenedicarboxylic acid, butyl cyclohexyl ester was left with the removal efficiency of 91.08%. For 2-cyclohexen-1-one with the shortest retention time, the removal efficiency reached 94.13%, although the small hydrophilic molecular organic was difficult to capture by coagulants [34]. For heterocyclic compounds, only 1,8-naphthalimide could be detected in the discharged wastewater, indicating the efficient removal of the coagulation. In contrast to the traditional aluminum, the introduction of silica into the hydrolyzing metal promoted the increase of molecular size and enhanced the bridge-aggregating ability to capture and sediment the contaminants in water [25]. Therefore, the removal of organic compounds, especially high molecular weight alkane and heterocyclic compounds was well achieved by PASS. Considering the hazard to the natural water body and to human beings for high toxicity and resistance to biodegradation, coagulation with PASS as an advanced treatment of coking wastewater provides an alternative way to solve this problem.
4. Conclusions
The coagulant polyaluminum silicate sulfate (PASS) was prepared in this study and applied in the advanced treatment of coking wastewater, which showed the best coagulation performance compared with commercial coagulants. At a dosage of 5 mmol/L, the CODCr value after treatment reduced to 86.67 mg/L from 196.67 mg/L. Both single-factor and Taguchi experiments revealed that the optimal conditions for the removal of organics include: dosage of 7 mmol/L, flocculation velocity of 75 r/min, flocculation time of 30 min, pH of 7, and temperature of 20 °C. Under these optimal conditions, the CODCr concentration could be further decreased to 59.94 mg/L. Enhanced coagulation experiments such as pre-oxidation with ozonation, active carbon adsorption, PAM assistance and secondary coagulation could further reduce the CODCr concentration. Ultraviolet spectrum scanning and GC-MS analysis suggest that the coagulation process with PASS played a vital role in the removal of organic compounds, especially for high molecular weight alkane and heterocyclic compounds, providing an alternative for the advanced treatment of coking wastewater.
Author Contributions
Methodology, F.C.; Investigation, J.W.; Writing—original draft, J.W.; Writing—review & editing, F.C. and M.Z.; Supervision, F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the research project of Shanghai Science and Technology Commission (19DZ1204303) and the National Nonprofit Institute Research Grants of TIWTE (TKS20220204).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, M.; Preis, S.; Kornev, I.; Hu, Y.; Wei, C.H. Pulsed corona discharge for improving treatability of coking wastewater. J. Environ. Sci. 2018, 64, 306–316. [Google Scholar] [CrossRef]
- Zhou, N.; Zhao, Z.; Wang, H.; Chen, X.; Wang, M.; He, S.; Liu, W.; Zheng, M. The effects of graphene oxide on nitrification and N2O emission: Dose and exposure time dependent. Environ. Pollut. 2019, 252, 960–966. [Google Scholar] [CrossRef]
- Farris, F.F. Coke oven emissions. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 1. [Google Scholar] [CrossRef]
- Guo, D.; Shi, Q.; He, B.; Yuan, X. Different solvents for the regeneration of the exhausted activated carbon used in the treatment of coking wastewater. J. Hazard. Mater. 2011, 186, 1788–1793. [Google Scholar] [CrossRef]
- Kumar, A.; Sengupta, B.; Kannaujiya, M.; Priyadarshinee, R.; Singha, S.; Dasguptamandal, D.; Mandal, T. Treatment of coke oven wastewater using ozone with hydrogen peroxide and activated carbon. Desalination Water Treat. 2017, 69, 352–365. [Google Scholar] [CrossRef]
- Tian, Z.; Zhou, N.; You, W.; He, D.; Chang, F.; Zheng, M. Mitigating NO and N2O emissions from a pilot-scale oxidation ditch using bioaugmentation of immobilized aerobic denitrifying bacteria. Bioresour. Technol. 2021, 340, 125704. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, N.; Liu, S.; Dang, C.; Liu, Y.; He, S.; Zhao, Y.; Liu, W.; Wang, X. N2O and NO emission from a biological aerated filter treating coking wastewater: Main source and microbial community. J. Clean. Prod. 2019, 213, 365–374. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, L.; Esteban-García, A.L.; Tejero, I. Comparison between a fixed bed hybrid membrane bioreactor and a conventional membrane bioreactor for municipal wastewater treatment: A pilot-scale study. Bioresour. Technol. 2014, 152, 212–219. [Google Scholar] [CrossRef]
- Verma, V.; Joshi, N.; Kushwaha, J.; Singh, S.B.; Ghosh, P.; Chaudhari, P.K. Catalytic thermolysis at atmospheric pressure followed by adsorption in treatment of coking wastewater. Int. J. Chem. React. Eng. 2022, 20, 627–639. [Google Scholar] [CrossRef]
- Yang, X.; Duan, J.; Zhang, X.; Zhang, H.; Liu, X.; Feng, Y.; Zheng, M. Heterojunction architecture of Nb2O5/g-C3N4 for enhancing photocatalytic activity to degrade organic pollutants and deactivate bacteria in water. Chin. Chem. Lett. 2022, 33, 3792–3796. [Google Scholar] [CrossRef]
- Pal, P.; Kumar, R. Treatment of coke wastewater: A critical review for developing sustainable management strategies. Sep. Purif. Rev. 2014, 43, 89–123. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Wu, K.Y.; Zhou, H.T.; Hu, Y.; Sergei, P.; Wu, H.Z.; Wei, C.H. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in the treatment of coking wastewater. J. Environ. Manag. 2018, 224, 376–386. [Google Scholar] [CrossRef]
- Biache, C.; Mansuy-Huault, L.; Faure, P. Impact of oxidation and biodegradation on the most commonly used polycyclic aromatic hydrocarbon (PAH) diagnostic ratios: Implications for the source identifications. J. Hazard. Mater. 2014, 267, 31–39. [Google Scholar] [CrossRef]
- Sharma, N.; Philip, L. Combined biological and photocatalytic treatment of real coke oven wastewater. Chem. Eng. J. 2016, 295, 20–28. [Google Scholar] [CrossRef]
- Ryu, B.-G.; Kim, J.; Han, J.-I.; Kim, K.; Kim, D.; Seo, B.-K.; Kang, C.-M.; Yang, J.-W. Evaluation of an electro-flotation-oxidation process for harvesting bio-flocculated algal biomass and simultaneous treatment of residual pollutants in coke wastewater following an algal-bacterial process. Algal Res. 2018, 31, 497–505. [Google Scholar] [CrossRef]
- Minhalma, M.; de Pinho, M.N. Integration of nanofiltration/steam stripping for the treatment of coke plant ammoniacal wastewaters. J. Membr. Sci. 2004, 242, 87–95. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Zhao, H.; Li, F.; Lei, Z.; Zhang, Z. Highly efficient one-step advanced treatment of biologically pretreated coking wastewater by an integration of coagulation and adsorption process. Bioresour. Technol. 2018, 247, 1206–1209. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Joo, D.; Shin, W.; Choi, J.; Choi, S.; Kim, M.; Han, M.; Ha, T.; Kim, Y. Decolorization of reactive dyes using inorganic coagulants and synthetic polymer. Dye. Pigment. 2007, 73, 59–64. [Google Scholar] [CrossRef]
- Gao, B.Y.; Wang, Y.; Yue, Q.Y.; Wei, J.C.; Li, Q. Color removal from simulated dye water and actual textile wastewater using a composite coagulant prepared by ployferric chloride and polydimeth yldiallyl ammonium chloride. Separ. Purif. Technol. 2007, 54, 157–163. [Google Scholar] [CrossRef]
- Coca, J.; Bueno, J.; Sastre, H. Zeta Potential and the Effect of Coagulants on Coal Slurries. J. Chem. Eng. Jpn. 1983, 16, 335–338. [Google Scholar] [CrossRef]
- Yang, Z.H.; Huang, J.; Zeng, G.M.; Ruan, M.; Zhou, C.S.; Li, L.; Rong, Z.G. Optimization of flocculation conditions for kaolin suspension using the composite flocculant of MBFGA1 and PAC by response surface methodology. Bioresour. Technol. 2009, 100, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D. Flocculant behaviour with coal—Shale slurries. Int. J. Miner. Process. 1974, 1, 243–260. [Google Scholar] [CrossRef]
- Rebah, F.B.; Mnif, W.; Siddeeg, S.M. Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: A Review. Symmetry 2018, 10, 556. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Liu, X.; Du, Z.; Cheng, F. Effect of silicon content on preparation and coagulation performance of poly-silicic-metal coagulants derived from coal gangue for coking wastewater treatment. Sep. Purif. Technol. 2018, 202, 149–156. [Google Scholar] [CrossRef]
- Saravanan, A.; Thamarai, P.; Kumar, P.S.; Rangasamy, G. Recent advances in polymer composite, extraction, and their application for wastewater treatment: A review. Chemosphere 2022, 308, 136368. [Google Scholar] [CrossRef] [PubMed]
- Arnold-Smith, A.K.; Christie, R.M.; Jolicoeur, C. Polyaluminium Silicate Sulphate—A New Coagulant for Potable and Wastewater Treatment. In Chemical Water and Wastewater Treatment II, Proceedings of the 5th Gothenburg Symposium, Nice, France, 28–90 September 1992; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Abujazar, M.S.S.; Karaağaç, S.U.; Abu Amr, S.S.; Alazaiza, M.Y.D.; Bashir, M.J. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, J.; Lin, S.; Zhang, Y. Covalently Bound Organic Silicate Aluminum Hybrid Coagulants: Preparation, Characterization, and Coagulation Behavior. Environ. Sci. Technol. 2009, 43, 2041–2046. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, N.; He, S.; Chang, F.; Zhong, J.; Xu, S.; Wang, Z.; Liu, T. Nitrous oxide (N2O) emissions from a pilot-scale oxidation ditch under different COD/N ratios, aeration rates and two shock-load conditions. J. Environ. Manag. 2021, 280, 111657. [Google Scholar] [CrossRef]
- Zheng, M.; Mu, G.; Zhang, A.; Wang, J.; Chang, F.; Niu, J.; Wang, X.; Gao, T.; Zhao, Z. Predominance of comammox bacteria among ammonia oxidizers under low dissolved oxygen condition. Chemosphere 2022, 308, 136436. [Google Scholar] [CrossRef]
- Cheng, W.P.; Chi, F.H.; Li, C.C.; Yu, R.F. A study on the removal of organic substances from low-turbidity and low-alkalinity water with metal-polysilicate coagulants. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 238–244. [Google Scholar] [CrossRef]
- Lai, P.; Zhao, H.; Wang, C.; Ni, J.-R. Advanced treatment of coking wastewater by coagulation and zero-valent iron processes. J. Hazard. Mater. 2007, 147, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Gong, Z.; Qi, W.; Li, E.; Shen, J.; Li, J.; Zhao, H. Coagulation performance and floc characteristics of poly-ferric-titanium-silicate-chloride in coking wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128413. [Google Scholar] [CrossRef]
- Li, S.; Kang, Y. Impacts of key preparation factors on polymerization and flocculation performance of polyferric silicate sulfate (PFSiS). Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128109. [Google Scholar] [CrossRef]
- Singhania, R.R.; Sukumaran, R.K.; Pandey, A. Improved cellulase production by Trichoderma reesei RUT C30 under SSF through process optimization. Appl. Biochem. Biotechnol. 2007, 142, 60–70. [Google Scholar] [CrossRef]
- Uyak, V.; Yavuz, S.; Toroz, I.; Ozaydin, S.; Genceli, E.A. Disinfection by-products precursors removal by enhanced coagulation and PAC adsorption. Desalination 2007, 216, 334–344. [Google Scholar]
- Gui, Z.; Qian, J.; Li, X.; Zheng, B.Q.; An, Q.F. Study on Chain Deformation of Polyacrylamides in Solutions and Its Flocculation Performance During the Flocculation Process. J. Appl. Polym. Sci. 2010, 117, 2915–2922. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).