A Review on Inflammasomes and Immune Checkpoints in Pre-Eclampsia Complicated with Tuberculosis and Human Immune Deficiency Virus
Abstract
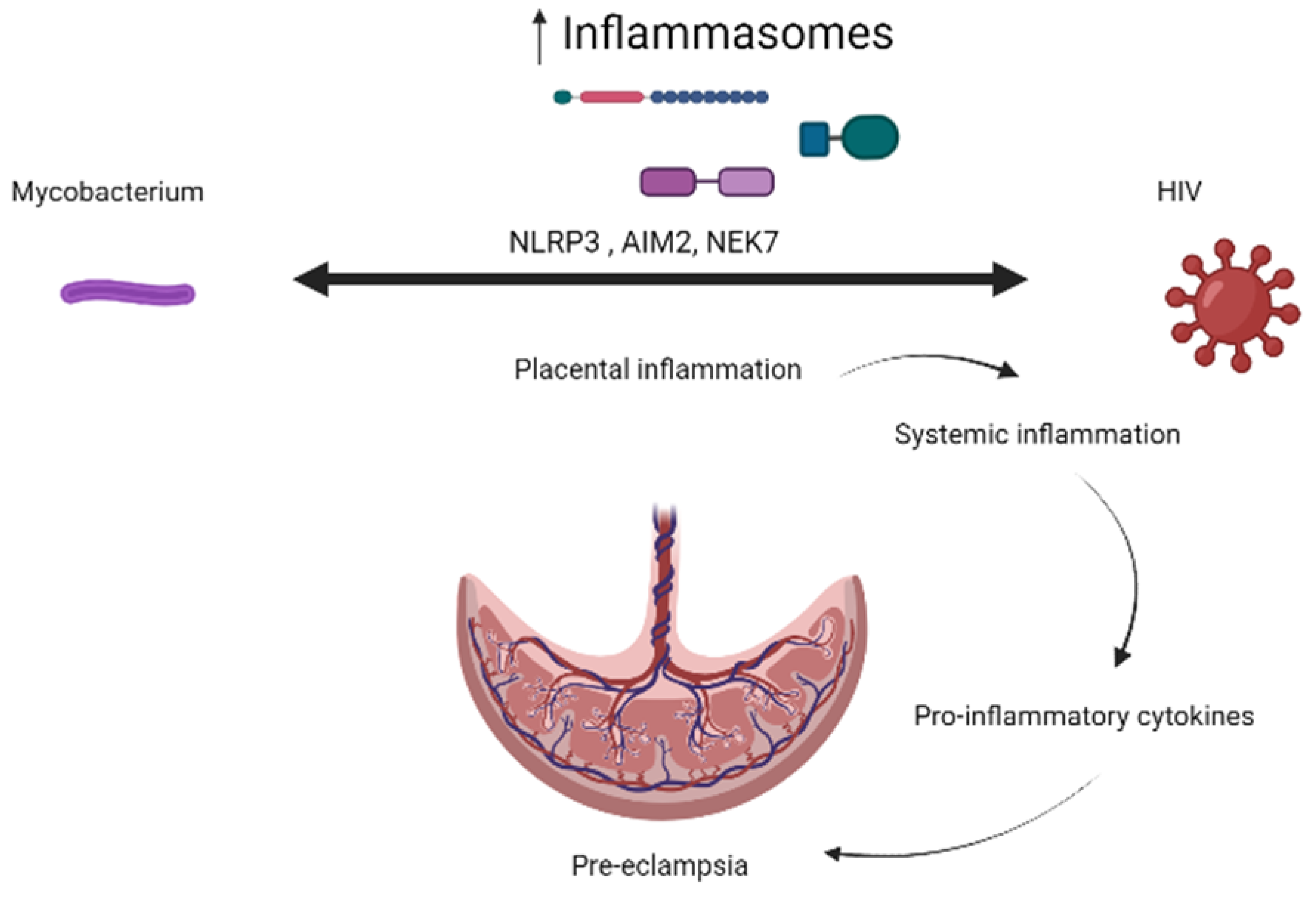
:1. Introduction
2. Inflammasomes and Immune Checkpoint Markers in TB/HIV-Associated Pre-Eclamptic Pregnancies
3. Inflammasomes
3.1. Inflammasomes and Pregnancy Development
3.2. Inflammasomes in Pre-Eclampsia
3.3. Inflammasomes in Tuberculosis
3.4. Inflammasomes in HIV
3.5. Inflammasomes in TB–HIV Co-Infection
4. Inflammasomes in the Presence of HIV and Tuberculosis Treatment
5. Immune Checkpoints and Their Role in Pregnancy
5.1. Cytotoxic T-Lymphocyte-Associated Antigen 4 (CTLA-4)
5.2. The Programmed Death 1 (PD-1) Receptor
5.3. T Cell Immunoglobulin and Mucin-Domain Containing-3 (TIM-3)
5.4. Lymphocyte Activation Gene 3 (Lag-3)
6. Immune Response in Pre-Eclampsia
6.1. Cytotoxic T-Lymphocyte-Associated Antigen 4 (CTLA-4) in Pre-Eclampsia
6.2. The Programmed Death 1 (PD-1) Receptor in Pre-Eclampsia
6.3. T Cell Immunoglobulin and Mucin-Domain Containing-3 in Pre-Eclampsia
6.4. Lymphocyte Activation Gene 3 (Lag-3) in Pre-Eclampsia
7. Immune Checkpoint Inhibitors in Pregnancy
8. Future Recommendations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magee, L.A.; Nicolaides, K.H.; Von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Cushen, S.C.; Ricci, C.A.; Bradshaw, J.L.; Silzer, T.; Blessing, A.; Sun, J.; Zhou, Z.; Scroggins, S.M.; Santillan, M.K.; Santillan, D.A. Reduced maternal circulating cell-free mitochondrial DNA is associated with the development of preeclampsia. J. Am. Heart Assoc. 2022, 11, e021726. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.G.d.; Karumanchi, A.; Sass, N. Preeclampsia: Oxidative stress, inflammation and endothelial dysfunction. Rev. Bras. Ginecol. Obs. 2010, 32, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Redman, C. Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brosens, A. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet. Gynecol. Annu. 1972, 1, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes Mech. Intrauter. Hypoxia Its Impact Fetal Cardiovasc. Syst. A Review. Int. J. Pediatr. 2010, 401323, 2010. [Google Scholar]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Naicker, T.; Ramsuran, V.; Moodley, J. Pre-eclampsia: The role of highly active antiretroviral therapy and immune markers. Inflamm. Res. 2019, 68, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, N.S.; Yeung, E.; Mendola, P.; Hinkle, S.N.; Laughon, S.K.; Zhang, C.; Albert, P.S. Risk factors differ between recurrent and incident preeclampsia: A hospital-based cohort study. Ann. Epidemiol. 2014, 24, 871–877.e873. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.; Sarno, L.; Saccone, G.; Berghella, V.; Maruotti, G.M.; Migliucci, A.; Capone, A.; Martinelli, P. Risk of preeclampsia in human immunodeficiency virus–infected pregnant women. Obstet. Gynecol. 2016, 127, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Sebitloane, H.M.; Moodley, D. The impact of highly active antiretroviral therapy on obstetric conditions: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, A.L. Case Study: Preeclampsia of a Female Patient Late 30s; Pennsylvania State University: State College, PA, USA, 2016. [Google Scholar]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Deets, K.A.; Vance, R.E. Inflammasomes and adaptive immune responses. Nat. Immunol. 2021, 22, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.N.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Stutz, A.; Golenbock, D.T.; Latz, E. Inflammasomes: Too big to miss. J. Clin. Investig. 2009, 119, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Festjens, N.; Cornelis, S.; Lamkanfi, M.; Vandenabeele, P. Caspase-containing complexes in the regulation of cell death and inflammation. Biol. Chem. 2006, 387, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Place, D.E.; Kanneganti, T.-D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Hayward, J.A.; Man, S.M. Molecular mechanisms of inflammasome signaling. J. Leukoc. Biol. 2018, 103, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K. Inflammasome-associated cell death: Pyroptosis, apoptosis, and physiological implications. Microbiol. Immunol. 2020, 64, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Wang, N.; Liu, H.; Ma, B.; Zhao, T.; Chen, Y.; Yang, Y.; Zhao, P.; Han, X. CSF high-mobility group box 1 is associated with drug-resistance and symptomatic etiology in adult patients with epilepsy. Epilepsy Res. 2021, 177, 106767. [Google Scholar] [CrossRef]
- James, J.L.; Whitley, G.S.; Cartwright, J.E. Pre-eclampsia: Fitting together the placental, immune and cardiovascular pieces. J. Pathol. 2010, 221, 363–378. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Wang, X.; Griffin, A.; Morris, R.; Wallace, K. Preeclampsia and COVID-19: The Role of Inflammasome Activation. Curr. Hypertens. Rep. 2022, 24, 341–348. [Google Scholar] [CrossRef]
- Weel, I.C.; Romão-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peraçoli, J.C.; Borges, V.T.; Araujo, J.P., Jr.; Peraçoli, M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, A.; Reis, E.C.; Bricher, P.N.; Vianna, P.; Diniz, S.; Fernandes, K.S.; Chies, J.A.; Sandrim, V. NLRP1 L155H polymorphism is a risk factor for preeclampsia development. Am. J. Reprod. Immunol. (New York, NY) 2015, 73, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Stødle, G.; Silva, G.; Tangerås, L.H.; Gierman, L.; Nervik, I.; Dahlberg, U.; Sun, C.; Aune, M.H.; Thomsen, L.C.V.; Bjørge, L. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP) 3 inflammasome activation in trophoblasts. Clin. Exp. Immunol. 2018, 193, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.L.; Romao, M.; Weel, I.C.; Ribeiro, V.R.; Nunes, P.R.; Borges, V.T.; Araújo, J.P., Jr.; Peracoli, J.C.; de Oliveira, L.; Peracoli, M.T. Endogenous and uric acid-induced activation of NLRP3 inflammasome in pregnant women with preeclampsia. PLoS ONE 2015, 10, e0129095. [Google Scholar] [CrossRef] [PubMed]
- Phoswa, W.N.; Eche, S.; Khaliq, O.P. The Association of tuberculosis mono-infection and Tuberculosis-Human Immunodeficiency Virus (TB-HIV) co-infection in the pathogenesis of hypertensive disorders of pregnancy. Curr. Hypertens. Rep. 2020, 22, 104. [Google Scholar] [CrossRef]
- Bekker, A.; Schaaf, H.S.; Draper, H.R.; Kriel, M.; Hesseling, A.C. Tuberculosis disease during pregnancy and treatment outcomes in HIV-infected and uninfected women at a referral hospital in Cape Town. PLoS ONE 2016, 11, e0164249. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 couples to maitotoxin-and nigericin-induced interleukin-1β release through a dye uptake-independent pathway. J. Biol. Chem. 2007, 282, 2386–2394. [Google Scholar] [CrossRef]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 2009, 5, 487–497. [Google Scholar] [CrossRef]
- Gurcel, L.; Abrami, L.; Girardin, S.; Tschopp, J.; van der Goot, F.G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 2006, 126, 1135–1145. [Google Scholar] [CrossRef]
- Ichinohe, T.; Yamazaki, T.; Koshiba, T.; Yanagi, Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl. Acad. Sci. USA 2013, 110, 17963–17968. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Barber, K.; Barber, D.; Shenderov, K.; White, S.; Wilson, M.; Cheever, A.; Kugler, D.; Sutterwala, F.; Sher, A. Caspase-1 independent IL-1β Production is critical for MyD88-mediated host resistance to Mycobacterium tuberculosis (42.13). J. Immunol. 2010, 184 (Suppl. S1), 42.13. [Google Scholar] [CrossRef]
- Schneider, B.E.; Korbel, D.; Hagens, K.; Koch, M.; Raupach, B.; Enders, J.; Kaufmann, S.H.; Mittrücker, H.W.; Schaible, U.E. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur. J. Immunol. 2010, 40, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, I.; Yamada, H.; Kaneko, H.; Mizuno, S.; Takeda, K.; Akira, S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 1999, 67, 2585–2589. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, I.; Yamada, H.; Hua, S.; Mizuno, S. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol. Immunol. 2001, 45, 743–750. [Google Scholar] [CrossRef]
- Subbarao, S.; Sanchez-Garrido, J.; Krishnan, N.; Shenoy, A.R.; Robertson, B.D. Genetic and pharmacological inhibition of inflammasomes reduces the survival of Mycobacterium tuberculosis strains in macrophages. Sci. Rep. 2020, 10, 3709. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, L.; Wang, G. Advances in research on the role of inflammasomes in Mycobacterium tuberculosis infection. Zhongguo Bingyuan Shengwuxue Zazhi/J. Pathog. Biol. 2019, 14, 857–863. [Google Scholar]
- Van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef]
- Briken, V.; Ahlbrand, S.E.; Shah, S. Mycobacterium tuberculosis and the host cell inflammasome: A complex relationship. Front. Cell. Infect. Microbiol. 2013, 3, 62. [Google Scholar] [CrossRef]
- Wassermann, R.; Gulen, M.F.; Sala, C.; Perin, S.G.; Lou, Y.; Rybniker, J.; Schmid-Burgk, J.L.; Schmidt, T.; Hornung, V.; Cole, S.T. Mycobacterium tuberculosis differentially activates cGAS-and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe 2015, 17, 799–810. [Google Scholar] [CrossRef]
- Liu, X.; Lieberman, J. A mechanistic understanding of pyroptosis: The fiery death triggered by invasive infection. Adv. Immunol. 2017, 135, 81–117. [Google Scholar] [PubMed]
- Law, K.; Weiden, M.; Harkin, T.; Tchou-Wong, K.; Chi, C.; Rom, W.N. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 1996, 153, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Alexander, M.; Kumar, P.; Kulkarni, V.; Deshpande, P.; Yadana, S.; Leu, C.-S.; Araújo-Pereira, M.; Andrade, B.B.; Bhosale, R. Systemic inflammation in pregnant women with latent tuberculosis infection. Front. Immunol. 2021, 11, 587617. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Borzychowski, A.; Sargent, I.; Redman, C. Inflammation and pre-eclampsia. Semin. Fetal Neonatal Med. 2006, 11, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 2010, 12, 1046–1063. [Google Scholar] [CrossRef]
- Mullis, C.; Swartz, T.H. NLRP3 Inflammasome signaling as a link between HIV-1 infection and atherosclerotic cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 95. [Google Scholar] [CrossRef]
- Min, A.K.; Fortune, T.; Rodriguez, N.; Hedge, E.; Swartz, T.H. Inflammasomes as mediators of inflammation in HIV-1 infection. Transl. Res. 2022, 252, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J.-W.; Huang, H.-H.; Fan, X.; Huang, L.; Deng, J.-N.; Tu, B.; Wang, K.; Li, J.; Zhou, M.-J. NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1–infected patients. J. Clin. Investig. 2021, 131, e138861. [Google Scholar] [CrossRef]
- Feria, M.G.; Taborda, N.A.; Hernandez, J.C.; Rugeles, M.T. HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS ONE 2018, 13, e0192845. [Google Scholar] [CrossRef] [PubMed]
- Bosamiya, S.S. The immune reconstitution inflammatory syndrome. Indian J. Dermatol. 2011, 56, 476. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.F.; Stek, C.; Wasserman, S.; Wilkinson, R.J.; Meintjes, G. The tuberculosis-associated immune reconstitution inflammatory syndrome: Recent advances in clinical and pathogenesis research. Curr. Opin. HIV AIDS 2018, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Ravimohan, S.; Nfanyana, K.; Tamuhla, N.; Tiemessen, C.T.; Weissman, D.; Bisson, G.P. Common variation in NLRP3 is associated with early death and elevated inflammasome biomarkers among advanced HIV/TB co-infected patients in Botswana. Open Forum Infect. Dis. 2018, 5, ofy075. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.J.; Mathad, J.S.; Dooley, K.E.; Eke, A.C. Evidence for Implementation: Management of TB in HIV and Pregnancy. Curr. HIV/AIDS Rep. 2022, 19, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Bandera, A.; Masetti, M.; Fabbiani, M.; Biasin, M.; Muscatello, A.; Squillace, N.; Clerici, M.; Gori, A.; Trabattoni, D. The NLRP3 Inflammasome Is Upregulated in HIV-Infected Antiretroviral Therapy-Treated Individuals with Defective Immune Recovery. Front. Immunol. 2018, 9, 214. [Google Scholar] [CrossRef]
- Toribio, M.; Burdo, T.H.; Fulda, E.S.; Cetlin, M.; Chu, S.M.; Feldpausch, M.N.; Robbins, G.K.; Neilan, T.G.; Melbourne, K.; Grinspoon, S.K. Effects of Integrase Inhibitor–Based ART on the NLRP3 Inflammasome Among ART-Naïve People With HIV. Open Forum Infect. Dis. 2020, 7, ofaa459. [Google Scholar] [CrossRef] [PubMed]
- Guerville, F.; Vialemaringe, M.; Cognet, C.; Duffau, P.; Lazaro, E.; Cazanave, C.; Bonnet, F.; Leleux, O.; Rossignol, R.; Pinson, B. Mechanisms of systemic low-grade inflammation in HIV patients on long-term suppressive antiretroviral therapy: The inflammasome hypothesis. AIDS 2023, 37, 1035–1046. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Doba, K.; Barakonyi, A.; Szereday, L. Immune checkpoint molecules in reproductive immunology. Front. Immunol. 2019, 10, 846. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Sun, H.X. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur. J. Immunol. 2020, 50, 160–169. [Google Scholar] [CrossRef]
- Sharma, S. Natural killer cells and regulatory T cells in early pregnancy loss. Int. J. Dev. Biol. 2014, 58, 219. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, J.; Möttönen, M.; Alanen, A.; Lassila, O. Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol. 2004, 136, 373–378. [Google Scholar] [CrossRef]
- Sasaki, Y.; Sakai, M.; Miyazaki, S.; Higuma, S.; Shiozaki, A.; Saito, S. Decidual and peripheral blood CD4+ CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mhr Basic Sci. Reprod. Med. 2004, 10, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.P.; Fan, D.X.; Zhang, T.; Guo, P.F.; Li, D.J. The costimulatory signal upregulation is associated with Th1 bias at the maternal–fetal interface in human miscarriage. Am. J. Reprod. Immunol. 2011, 66, 270–278. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubat, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Kharatyan, E.; Torry, D.S.; Holets, L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am. J. Pathol. 2005, 167, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Chen, L.; Phillips, T.A.; Hunt, J.S. B7 family molecules: Novel immunomodulators at the maternal-fetal interface. Placenta 2002, 23, S95–S101. [Google Scholar] [CrossRef]
- Holets, L.M.; Hunt, J.S.; Petroff, M.G. Trophoblast CD274 (B7-H1) is differentially expressed across gestation: Influence of oxygen concentration. Biol. Reprod. 2006, 74, 352–358. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Z.; Liu, Y.; Li, B.; Zhang, L.; Fang, H.; Song, C.; Wang, X.; Zhang, G.-M.; Feng, Z.-H. Human pregnancy up-regulates Tim-3 in innate immune cells for systemic immunity. J. Immunol. 2009, 182, 6618–6624. [Google Scholar] [CrossRef]
- Van de Weyer, P.S.; Muehlfeit, M.; Klose, C.; Bonventre, J.V.; Walz, G.; Kuehn, E.W. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006, 351, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Su, E.W.; Zhu, C.; Hainline, S.; Phuah, J.; Moroco, J.A.; Smithgall, T.E.; Kuchroo, V.K.; Kane, L.P. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol. Cell. Biol. 2011, 31, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Meggyes, M.; Miko, E.; Polgar, B.; Bogar, B.; Farkas, B.; Illes, Z.; Szereday, L. Peripheral blood TIM-3 positive NK and CD8+ T cells throughout pregnancy: TIM-3/galectin-9 interaction and its possible role during pregnancy. PLoS ONE 2014, 9, e92371. [Google Scholar] [CrossRef] [PubMed]
- Lajko, A.; Meggyes, M.; Polgar, B.; Szereday, L. The immunological effect of Galectin-9/TIM-3 pathway after low dose Mifepristone treatment in mice at 14.5 day of pregnancy. PLoS ONE 2018, 13, e0194870. [Google Scholar] [CrossRef] [PubMed]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; Horton, B.L.; Zheng, Y.; Duan, Y.; Powell, J.D.; Gajewski, T.F. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J. Exp. Med. 2017, 214, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, B.A.; Spradley, F.T.; Drummond, H.A.; LaMarca, B.; Ryan, M.J.; Granger, J.P. Preeclampsia: Linking placental ischemia with maternal endothelial and vascular dysfunction. Compr. Physiol. 2020, 11, 1315. [Google Scholar]
- Prins, J.R.; Boelens, H.M.; Heimweg, J.; Van der Heide, S.; Dubois, A.E.; Van Oosterhout, A.J.; Erwich, J.J.H. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens. Pregnancy 2009, 28, 300–311. [Google Scholar] [CrossRef]
- Toldi, G.; Švec, P.; Vásárhelyi, B.; Mészáros, G.; Rigó, J.; Tulassay, T.; Treszl, A. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet. Gynecol. Scand. 2008, 87, 1229–1233. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Toivonen, S.; Keski-Nisula, L.; Paattiniemi, E.-L.; Helisalmi, S.; Punnonen, K.; Heinonen, S. CTLA-4 polymorphism 49A–G is associated with placental abruption and preeclampsia in Finnish women. Clin. Chem. Lab. Med. 2008, 46, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Dehaghani, A.S.; Doroudchi, M.; Kalantari, T.; Pezeshki, A.; Ghaderi, A. Heterozygosity in CTLA-4 gene and severe preeclampsia. Int. J. Gynecol. Obstet. 2005, 88, 19–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Du, N.; Sun, H.; Chen, L.; Bao, H.; Zhao, Q.; Qu, Q.; Ma, D.; Kwak-Kim, J. Immune checkpoint molecules on T cell subsets of pregnancies with preeclampsia and gestational diabetes mellitus. J. Reprod. Immunol. 2020, 142, 103208. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Xu, X.H.; Jin, L.P. Regulation of the innate immune cells during pregnancy: An immune checkpoint perspective. J. Cell. Mol. Med. 2021, 25, 10362–10375. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhang, Y.; Liu, Z.; Sun, G.; Mor, G.; Liao, A. The PD-1/PD-L1 inhibitory pathway is altered in pre-eclampsia and regulates T cell responses in pre-eclamptic rats. Sci. Rep. 2016, 6, 27683. [Google Scholar] [CrossRef] [PubMed]
- Wafula, P.O.; Teles, A.; Schumacher, A.; Pohl, K.; Yagita, H.; Volk, H.D.; Zenclussen, A.C. PD-1 but not CTLA-4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am. J. Reprod. Immunol. 2009, 62, 283–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Tian, M.; Hu, X.; Wang, L.; Ji, J.; Liao, A. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell. Mol. Immunol. 2018, 15, 710–723. [Google Scholar] [CrossRef]
- Gu, Y.; Morgan, J.; Lewis, D.F.; Cooper, D.B.; Mccathran, C.E.; Wang, Y. Maternal soluble PD-1 levels are significantly increased in women with preeclampsia. Am. J. Reprod. Immunol. 2020, 83, e13193. [Google Scholar] [CrossRef]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef]
- Meggyes, M.; Miko, E.; Lajko, A.; Csiszar, B.; Sandor, B.; Matrai, P.; Tamas, P.; Szereday, L. Involvement of the PD-1/PD-L1 co-inhibitory pathway in the pathogenesis of the inflammatory stage of early-onset preeclampsia. Int. J. Mol. Sci. 2019, 20, 583. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Bogar, B.; Schmitz, N.; Barakonyi, A.; Varnagy, A.; Farkas, B.; Tamas, P.; Bodis, J.; Szekeres-Bartho, J. Involvement of Galectin-9/TIM-3 pathway in the systemic inflammatory response in early-onset preeclampsia. PLoS ONE 2013, 8, e71811. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Sun, F.; Li, M.; Du, M.; Li, X.; Zhang, Y. Involvement of the Tim-3 pathway in the pathogenesis of pre-eclampsia. Reprod. Sci. 2021, 28, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Mittelberger, J.; Seefried, M.; Franitza, M.; Garrido, f.; Ditsch, N.; Jeschke, U.; Dannecker, C. The role of the immune checkpoint molecules PD-1/PD-L1 and TIM-3/Gal-9 in the pathogenesis of preeclampsia—A narrative review. Medicina 2022, 58, 157. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, Y.; Wang, S.; Gu, Y.; Wang, P.; Meng, J. Abnormal Expression of the LAG-3/FGL-1 Signaling Pathway in Patients with Early-Onset Preeclampsia. Med. Sci. Monit. 2022, 28, e937498. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 2019, 176, 334–347.e312. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Schwander, S.K.; Zhu, B.; Van Zyl-Smit, R.N.; Zhang, Y. The immunology of tuberculosis: From bench to bedside. Respirology 2010, 15, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Birku, M.; Desalegn, G.; Kassa, G.; Tegbaru, B.; Howe, R.; Tsegaye, A.; Abebe, M. Pregnancy suppresses Mycobacterium tuberculosis-specific Th1, but not Th2, cell-mediated functional immune responses during HIV/latent TB co-infection. Clin. Immunol. 2020, 218, 108523. [Google Scholar] [CrossRef]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The immune escape mechanisms of Mycobacterium tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2021: Supplementary Material; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef]
- Mofenson, L.M.; Laughon, B.E. Human immunodeficiency virus, Mycobacterium tuberculosis, and pregnancy: A deadly combination. Clin. Infect. Dis. 2007, 45, 250–253. [Google Scholar] [CrossRef]
- O’garra, A.; Redford, P.S.; Mcnab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Wu, M.-F.; Hsu, C.-Y.; Lin, S.-Y.; Chang, Y.-N.; Lee, H.-S.; Wei, Y.-F.; Shu, C.-C. The dynamic change of immune checkpoints and CD14+ monocytes in latent tuberculosis infection. Biomedicines 2021, 9, 1479. [Google Scholar] [CrossRef] [PubMed]
- Zaemes, J.; Kim, C. Immune checkpoint inhibitor use and tuberculosis: A systematic review of the literature. Eur. J. Cancer 2020, 132, 168–175. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Lombardi, A.; Palomba, E.; Bozzi, G.; Ungaro, R.; Alagna, L.; Mangioni, D.; Muscatello, A.; Bandera, A.; Gori, A. Immune checkpoint inhibitors in people living with HIV/AIDS: Facts and controversies. Cells 2021, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Pantazis, N.; Martin, G.E.; Hickling, S.; Hurst, J.; Meyerowitz, J.; Willberg, C.B.; Robinson, N.; Brown, H.; Fisher, M.; et al. Exhaustion of activated CD8 T cells predicts disease progression in primary HIV-1 infection. PLoS Pathog. 2016, 12, e1005661. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, R.; Bakeman, W.; Lawani, M.B.; Khoury, G.; Hartogensis, W.; DaFonseca, S.; Killian, M.; Epling, L.; Hoh, R.; Sinclair, E.; et al. CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 2016, 12, e1005761. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Kaufmann, D.E.; Kavanagh, D.G.; Pereyra, F.; Zaunders, J.J.; Mackey, E.W.; Miura, T.; Palmer, S.; Brockman, M.; Rathod, A.; Piechocka-Trocha, A.; et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007, 8, 1246–1254. [Google Scholar] [CrossRef]
- Jones, R.B.; Ndhlovu, L.C.; Barbour, J.D.; Sheth, P.M.; Jha, A.R.; Long, B.R.; Wong, J.C.; Satkunarajah, M.; Schweneker, M.; Chapman, J.M.; et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008, 205, 2763–2779. [Google Scholar] [CrossRef]
- Garutti, M.; Lambertini, M.; Puglisi, F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: A systematic review. ESMO Open 2021, 6, 100276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Tian, M.; Tang, M.X.; Liu, Z.Z.; Liao, A.H. Recent insight into the role of the PD-1/PD-L1 pathway in feto-maternal tolerance and pregnancy. Am. J. Reprod. Immunol. 2015, 74, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Guleria, I.; Khosroshahi, A.; Ansari, M.J.; Habicht, A.; Azuma, M.; Yagita, H.; Noelle, R.J.; Coyle, A.; Mellor, A.L.; Khoury, S.J. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 2005, 202, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Habicht, A.; Dada, S.; Jurewicz, M.; Fife, B.T.; Yagita, H.; Azuma, M.; Sayegh, M.H.; Guleria, I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J. Immunol. 2007, 179, 5211–5219. [Google Scholar] [CrossRef] [PubMed]
- Poulet, F.M.; Wolf, J.J.; Herzyk, D.J.; DeGeorge, J.J. An evaluation of the impact of PD-1 pathway blockade on reproductive safety of therapeutic PD-1 inhibitors. Birth Defects Res. B Dev. Reprod. Toxicol. 2016, 107, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Müller, L.; Bedussi, F.; Fusaroli, M.; Raschi, E.; Ceschi, A. Immune Checkpoint Inhibitors and Pregnancy: Analysis of the VigiBase® Spontaneous Reporting System. Cancers 2022, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Susok, L. Uncomplicated pregnancy and delivery under ongoing nivolumab therapy for metastatic melanoma. Melanoma Res. 2022, 32, 132–133. [Google Scholar] [CrossRef]
- Le-Nguyen, A.; Rys, R.N.; Petrogiannis-Haliotis, T.; Johnson, N.A. Successful pregnancy and fetal outcome following previous treatment with pembrolizumab for relapsed Hodgkin’s lymphoma. Cancer Rep. 2022, 5, e1432. [Google Scholar] [CrossRef]
- Andrikopoulou, A.; Korakiti, A.; Apostolidou, K.; Dimopoulos, M.; Zagouri, F. Immune checkpoint inhibitor administration during pregnancy: A case series. ESMO Open 2021, 6, 100262. [Google Scholar] [CrossRef]
| Immune Checkpoints | PE | TB | HIV |
|---|---|---|---|
| CTLA-4 |  |  |  |
| PD-1 |  |  |  |
| TIM-3 |  |  |  |
| LAG-3 |  |  |  |
 = decrease and
= decrease and  = increase.
= increase.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoswa, W.N.; Khaliq, O.P.; Eche, S. A Review on Inflammasomes and Immune Checkpoints in Pre-Eclampsia Complicated with Tuberculosis and Human Immune Deficiency Virus. Int. J. Environ. Res. Public Health 2023, 20, 6627. https://doi.org/10.3390/ijerph20176627
Phoswa WN, Khaliq OP, Eche S. A Review on Inflammasomes and Immune Checkpoints in Pre-Eclampsia Complicated with Tuberculosis and Human Immune Deficiency Virus. International Journal of Environmental Research and Public Health. 2023; 20(17):6627. https://doi.org/10.3390/ijerph20176627
Chicago/Turabian StylePhoswa, Wendy N., Olive P. Khaliq, and Simeon Eche. 2023. "A Review on Inflammasomes and Immune Checkpoints in Pre-Eclampsia Complicated with Tuberculosis and Human Immune Deficiency Virus" International Journal of Environmental Research and Public Health 20, no. 17: 6627. https://doi.org/10.3390/ijerph20176627






